1 Introduction
The composite organization of plant cell walls is the result of a macromolecular assembly that leads to the spatial and temporal development of structures going from the Angstrom scale of the constitutive polymers to the nanometre scale of the supramolecular cell wall complex. The fine description of this organized macromolecular complex and of the changes that occur during cell wall biogenesis must ally high ultrastructural resolution in situ together with identification of the constituents, including their molecular characteristics. In this respect, microscopy techniques offer visualization at high resolution of complex structural organizations and identification of their components. Recent advances in microscope technology and the emergence of new techniques [1] have considerably developed these non-invasive approaches for plant cell wall investigation. In parallel, new probes with high specificity allowing accurate and detailed topochemical description of the plant cell walls have been developed [2]. The most versatile probes for cell wall polymers are by far the immunological probes that can be obtained against almost any cell wall macromolecule or macromolecule fragment. Limited for a long time to cell-wall polysaccharides [3], the immunological probes have more recently been introduced to decipher lignin molecular diversity and topochemical distribution in plant cell walls [4].
The molecular diversity of lignin may be observed at several levels, which are interdependent: first, at the level of its monomeric composition, then at the level of the type of its inter-unit linkages, and finally at the level of the macromolecular conformation. The complexity of lignin immuno-localization relates to its structural diversity. When we started our work on the conception of immuno-probes against lignin [4], we used synthetic lignin polymers (DHPs) because they allowed us to control the monomer composition and, according to the mode of polymerisation in vitro, to favour the formation of prevalent types of inter-unit linkages, which relate themselves to a predominant conformation [5]. With this approach, we were able to raise polyclonal antibodies against various lignin structural epitopes: homo-hydroxyphenylpropane epitopes, homo-guaiacyl condensed and non-condensed epitopes and the mixed guaiacyl–syringyl condensed and non-condensed epitopes, respectively [6]. Chemical analysis describes the lignin of angiosperms as being guaiacyl–syringyl rich, but does not specify whether the intimately mixed S and G units are copolymers or coexisting homopolymer sequences. With a view to establishing the topochemistry of syringyl epitopes in cell walls during xylem formation, and also to assess the impact of genetic manipulation on cell-wall assembly, which often modifies the ratio, we prepared a novel polyclonal antibody exclusively directed against pure syringyl polymer. In this work, we report the preparation of a syringyl-directed polyclonal antibody, the characterization of its specificity, and we show examples of its selectivity in the topochemical distribution of major syringyl epitopes in the model plants, Arabidopsis thaliana, Populus, and tobacco.
2 Materials and methods
2.1 Lignin dehydrogenative polymers (DHPs) and model dimers
The DHPs, homoguaiacyl (DHP-G) and homosyringyl (DHP-S) used in this study were prepared according to [7,8]. The mixed guaiacyl–syringyl DHP with a G/S ratio of 1:5 was prepared by the dialysis method [9] from a mixture of coniferyl alcohol and synapyl alcohol. The peroxidase-catalysed polymerisation product was analysed by nitrobenzene oxidation and pyrolysis-mass spectroscopy [10].
2.2 Model dimers
The various dimer molecules consisting of several combinations of guaiacyl and syringyl moieties (Fig. 1; Table 1) were gifts from Drs Sally Ralph and John Ralph (Madison, WI, USA). The phenyl-guaiacylglycerol-β-guaiacyl ether was a gift from Dr Jean-Pierre Utille (Grenoble, France).

Dimer model compounds used for dot-blot immunoassay.
Reactivity of S-antiserum with synthetic lignin polymers and model dimers
| Model molecules | Structure | Reactivity |
| DHPs | ||
| Condensed | Homo p-hydroxyphenyl propane (H-DHP) | − |
| Non-condensed | Homoguaiacyl (Gzl-DHP) | − |
| Homosyringyl (S-DHP) | + + + | |
| Syringyl/Guaiacyl 5/1a | + + + + | |
| Guaiacyl/syringyl (GSzt-DHP) | + + | |
| Dimers | ||
| Non-condensed | G-β-Gb | − |
| G-β-Sb | − | |
| S-β-Gb | − | |
| S-β-Sb | − | |
| Phenyl-guaiacyl-β-guaiacyl ether | − | |
| Condensed | S-r-Sb (syringaresinol) | − |
| G-r-Gb (pinoresinol) | − | |
| Polysaccharide | ||
| Poplar wood Xylan | − |
a The analytical composition of this compound was ascertained by pyrolysis-mass spectroscopy (unpublished data).
b For structures, see Fig. 1.
2.3 Preparation of the S-antiserum
Two New-Zealand rabbits were injected intradermically a mixture of the S-DHP (C.A. 1 mg) in phosphate buffer saline (PBS) with Freund's complete adjuvant. Booster injections were done with the antigen diluted in complete Freund's adjuvant every two weeks. One week after the last injection, blood was collected, and then centrifuged.
2.4 Dot-blot immunoassay
To reduce the volumes of reagents used, all the immunoassays were carried out in semi-micro disposable cuvettes of 0.5 ml. Dots of the antigen and of the substrates to be tested were deposited (1 μl) at varying concentrations on strips of positively charged nylon membrane (Boehringer; ). The strips were soaked in PBS, and then blocked in PBS non-fat dry milk (5% v/v). Incubation with S-antibody at various dilutions in PBS-milk (3%) was carried out in a sealed plastic bag (room temperature; 3 h, then overnight at 4 °C). The membrane strips were rinsed in PBS and incubated with Protein A diluted in PBS-0.1% fish gelatine (pA-G20, BioCell; 5h, room temperature). After rinsing in PBS, then water, the strips were fixed in 2% glutaraldehyde and rinsed in water before silver enhancement (SEK 250 BioCell).
2.5 Immunolabelling in transmission electron microscopy
Plant samples were fixed in a freshly prepared mixture of 0.1% glutaraldehyde and 0.2% paraformaldehyde in 0.05 M phosphate buffer, dehydrated through a graded series of ethanol and embedded in London Resin White (hard mixture) and polymerised for 24 h at 50 °C. Immunolabelling was done on ultra-thin transverse sections (500 Å) floating on plastic rings according to Ruel et al. [8]. The sections were floated on the antiserum diluted – in 10 mM Tris buffer saline (500 mM NaCl). The secondary marker was Protein A-gold (pA G5, BioCell). The gold particles were further enhanced using a silver-enhancing Amersham kit. Finally, the thin sections were transferred onto copper-grids, post-stained with 2.5% aqueous uranyl acetate and examined with a Philips CM 200 Cryo-TEM at an accelerating voltage of 80 kV.
3 Results and discussion
In order to obtain a polyclonal antiserum exclusively directed against the S units of lignin, a pure syringyl dehydrogenative polymer (DHP) was synthesized. The products resulting from the peroxidase-catalysed polymerisation of sinapyl alcohol in the presence of hydrogen peroxide were fractionated on a Sephadex LH 60 column, further purified by ethyl ether precipitation, and characterized by FTIR spectroscopy [7]. The purified fraction was inoculated to rabbits in complete Freund's adjuvant [4]. The specificity of the antiserum was assayed by dot blotting technique using a collection of model compounds corresponding to various structural motives commonly found in native lignins. A first series consisted of various DHPs, either made from a single monolignol or from a mixture of guaiacyl and syringyl monomers, the structure of which had been partly induced by the mode of addition of the reagent during the polymerisation experiment [11]. A second series consisted of dimers differing either in their monomer composition or the type of inter-monomeric linkages (Fig. 1, Table 1).
3.1 Dot-blot immunoassay micro-test of the S-antibody
A difficulty in testing the specificity of antibodies directed against lignins is the low solubility in conventional solvents of the substrates to be assayed. The nature of the solvents compatible with the synthetic lignin models tested in the immunoassays prevents the use of several types of membranes. To reduce the amount of antibody used, assays were performed on a microscale on small strips of nylon membrane. In order to visualise the fixation of the antibody on the molecules adsorbed on the membrane, gold-conjugate secondary marker was used with silver enhancement technique [12]. The reactivity of the antiserum was first tested against the S-DHP, antigen using various concentrations of antigen and various dilutions of antiserum. The coloration decreased as the concentrations of antigen and antiserum decreased, respectively. No coloration was observed when the antiserum was replaced by the pre-immune serum, or when the antiserum was used in the absence of antigen (Fig. 2). Among the synthetic DHPs tested, the S-DHP, purely made up of syringyl residues, and S enriched GS-DHP (zt) gave strong positive reactions. In particular, a strong positive response was given with syringyl-rich DHP having a composition of five syringyl for one guaiacyl residue. The pure S polymer and the S-rich (G/S, ) DHP gave the highest responses. On the other hand, DHPs totally devoid of syringyl units, such as p-hydroxyphenyl-propane and guaiacyl homopolymers were not recognized by the antiserum (Table 1). This behaviour of the antibody according to the composition of the DHPs, points out to its selectivity for syringyl sequences.
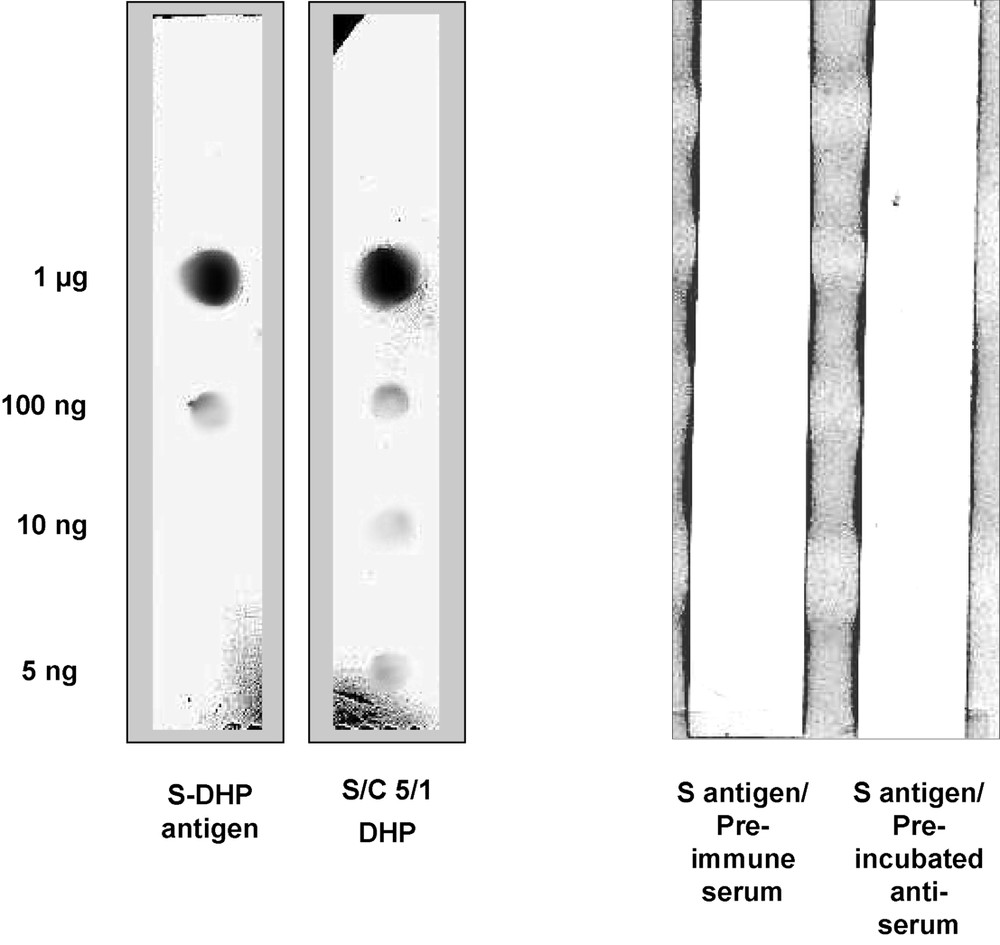
Dot-blot immunoassay micro-test on membrane strips. The reactivity of S-antiserum with its DHP-antigen and syringyl-enriched DHP-5/1. Negative responses were observed with the pre-immune serum and the antigen pre-incubated serum.
To get a better insight into the specificity of the antiserum, model compounds consisting of synthetic dimers with various combinations of guaiacyl and syringyl units linked together by different types of inter-monomeric bonds (Fig. 1) were assayed. The negative responses observed with molecules harbouring no syringyl residue confirmed the lack of recognition of the corresponding DHPs. More surprising were the results given by the dimers containing one or two syringyl residues, since none of the molecules tested were recognized by the S-antibody (Table 1). Altogether, these results indicate that the structural unit recognized by the antibody requires a sequence of more than two contiguous syringyl monomers. This conclusion is substantiated by the positive reaction observed with the S-enriched DHP (S/G ratio: 5), whose composition and structure, confirmed by pyrolysis-mass spectroscopy analysis (data not shown) [10] implies that it necessarily contains sequences of three or more consecutive syringyl residues linked together.
Taking these results together, it can be deduced from the dot blot immunoassays that the recognition selectivity of the S-antibody is directed against polymeric forms of syringyl monomer displaying sequences of more than three consecutive S units.
3.2 Ultrastructural localization of S epitopes in plant cell walls
Three plants, conventionally used as Angiosperm model plants, were investigated for the distribution of syringyl lignins by transmission electron microscopy (TEM). The application of the S-antiserum to ultra-thin sections gave the results shown in Fig. 3. In A. thaliana, the labelling was principally in fibres with the highest concentration in the inner part of the wall, which corresponded to the developing S2 layer. Virtually no labelling could be seen in S1 nor in the middle lamella and cell corner areas. The vessel showed only weak labelling, indicating that the S epitopes were almost absent. In tobacco, an intense labelling was observed in fibres. Here, the highest concentration of the epitopes was localized in the outer part of S2, corresponding to the older part of the fibre secondary wall. S1, middle lamella and cell corner were only weakly labelled. The labelling of vessels was clearly positive, but weaker than that observed in fibres. In Populus wood, the distribution pattern of the S epitopes was comparable to that in Tobacco, with more homogeneity in S2, in which no zonation appeared in the intensity of labelling between the outer and inner S2. As for the other model plants, S1 was only weakly labelled.

Immunogold labelling of syringyl epitopes with S-antiserum. In Arabidopsis thaliana, Tobacco and Populus, the reactivity of fibres displayed similar localisation of the S epitopes in S2 with lower or no labelling in S1. Note that the vessel in Arabidopsis was almost void of labelling.
4 Conclusion
The results of dot-blot immunoassays revealed that the S-antiserum was selective of the syringyl nucleus. An interesting attribute of this new antiserum is that it shows a particular affinity for sequences of three or more contiguous S units. The demonstration of the occurrence of such sequences in angiosperm lignins is consistent with the results of thioacetolysis [13] and thioacidolysis [14], indicating that S units were more released as monomer than dimmer fractions. Since these methods proceed by cleavage of β-O-4 bonds, the predominant release of monomer suggests that they occurred in lignin as sequences consecutively linked by β-O-4 bonds.
As expected in angiosperm plants, the antibody globally labelled fibres with more intensity than vessels [15]. The difference in labelling patterns observed between the three model plants investigated indicates that the distribution of the S epitopes recognized by the antiserum varies slightly between species, both in intensity and topology. Such variations are consistent with the observation that the S lignin biosynthetic pathway differs among angiosperm species [16], therefore resulting in variation in S lignin deposition. The particularly low intensity of immunolabelling in S1 layer of all plants examined reveals that syringyl lignins are deposited at a later stage of secondary wall formation. It is worth noting that another S antiserum raised from a different DHP fraction gave different labelling patterns. This suggests that the two DHPs prepared by different procedures should have differences in their bonding patterns and macromolecule conformations. This may have affected the nature of the epitopes, respectively recognised by the polyclonal antibodies. Work is in progress to assess the specificity of this antibody and to develop new probes to better depict in situ the lignification patterns of secondary cell walls and thereby the biogenesis of lignified cell walls.
Acknowledgments
The authors are indebted to Drs. Sally Ralph and John Ralph and Dr. Jean-Pierre Utille for the gift of reference model dimers.


