1 Satellite cells in the repair of skeletal muscle
Skeletal muscle undergoes regeneration in response to injury. Mononucleated cells, known as satellite cells, are located under the basal lamina of the multinucleated muscle fibre. When the fibre is damaged these cells become activated, replicate, and then differentiate to form new fibres, thus permitting muscle repair (Fig. 1) [1]. However, in recent years, the role of muscle-derived satellite cells in this process has been challenged and it has been proposed that cells from other sources such as bone marrow may be contributors of adult muscle stem cells [2]. It has now become clear that this is not the case [3] and experiments with purified satellite cells have demonstrated their efficiency in muscle repair as well as their capacity to self renew [4,5]. Satellite cells are marked by the expression of Pax7, and also in many muscles of its paralogue, Pax3. We targeted the mouse Pax3 gene with a GFP reporter sequence, which permitted us to isolate satellite cells by flow cytometry [5]. Clonal analysis demonstrated their myogenicity in culture. Grafting experiments into mdx nude mice, that lack dystrophin and undergo continuous regeneration in an attempt to repair the muscle damage which this entails, resulted in the reconstitution of dystrophin positive fibres. Quantitative estimation of grafting efficiency showed that this was much more efficient than with previously used crude muscle cell preparations (×100) or with purified satellite cells that had been expanded in culture prior to grafting (
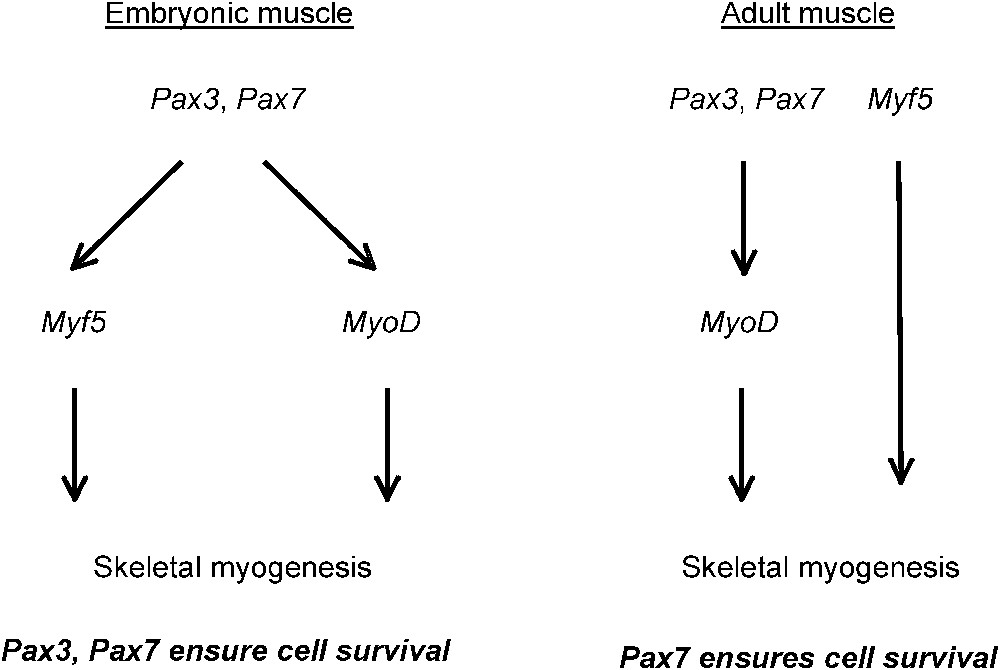
The role of Pax3 and Pax7 in directing skeletal muscle progenitor cells into the myogenic programme and in ensuring progenitor cell survival. Myf5 and MyoD are myogenic determination genes.
2 Pax function in satellite cells
In Pax7 mutant mice, satellite cells are lost and regeneration is compromised. This was first thought to be due to a failure of satellite cell specification [6]. However, some satellite cells are present [7], and we have shown that there is progressive loss of satellite cells after birth, such that at postnatal day two, 80% of the normal number is present whereas by day ten this has fallen to 10% [8]. Postnatal muscle growth is affected, as well as regeneration. Satellite cells undergo apoptosis already at birth, as evidenced by labelling with an antibody to activated caspase 3 on sections of mutant skeletal muscle. Mutant satellite cells in culture also show cell cycle perturbations. The anti-apoptotic role of Pax7 is confirmed by experiments in which a dominant negative form is introduced into normal satellite cells. We had previously shown that Pax3 functions as a transcriptional activator during myogenesis by replacing Pax3 with a PAX3-FKHR allele that encodes the DNA binding domain of Pax3, fused to the strong transcriptional activation domain of FKHR (Foxo1a); this fusion protein saves the myogenic phenotype of the mutant [9]. Furthermore, replacement of Pax3 by a Pax7 coding sequence shows that Pax7 also functions as an activator [10]. We therefore used a sequence encoding a fusion protein with the DNA binding domains of Pax7 or Pax3, followed by the transcriptional repression domain of Engrailed, to produce dominant negative forms of these factors (Pax7-DN, Pax3-DN). These sequences were introduced into adenoviral vectors with a GFP reporter. Infected satellite cells were separated by flow cytometry and tested for propidium iodide uptake as an indicator of cell death. Pax7-DN had a marked apoptotic effect, whereas Pax3-DN did not. This is consistent with the apoptosis of satellite cells in the Pax7 mutant, where expression of Pax3 does not save the phenotype [8].
In contrast, Pax3 and Pax7 have similar targets in the context of myogenesis. These factors are key upstream regulators of the myogenic programme (see [11]). Introduction of Pax3-DN or Pax7-DN into satellite cells prevents expression of the myogenic determination gene, MyoD. The other key myogenic determination gene, Myf5, which is already transcribed at a low level in quiescent satellite cells [12], continues to be expressed. This permits activation of myogenin encoding the member of this transcription factor family that controls muscle differentiation, leading to the formation of multinucleated fibres. In satellite cells from
3 The origin of satellite cells: the formation of skeletal muscle
3.1 The onset of myogenesis in the embryo
The skeletal muscles of the trunk and limbs are derived from somites, segments of paraxial mesoderm that form on either side of the axial structures in the vertebrate embryo (see [14]). As somites mature, the dorsal part retains an epithelial structure, known as the dermomyotome, which is the source of myogenic progenitor cells. Initially these cells delaminate from the edges of the dermomyotome to form the first skeletal muscle, the myotome, immediately under the epithelium, or migrate to more distant sites of myogenesis, such as the developing limbs. The latter process is Pax3 dependent in mammals, while the initial formation of the myotome is orchestrated by Pax3 and the myogenic regulatory genes, Myf5 and Mrf4 [13,15]. These are activated by signalling from the surrounding tissues, as exemplified by Wnt and Shh signalling that directly regulate Myf5 in the epaxial somite [16]. Recently, we have shown that Pax3 directly activates Myf5 in the hypaxial somite and limb buds [17].
3.2 Pax3/Pax7 positive progenitor cells in developing skeletal muscle
As development proceeds, the epithelial structure of the central dermomyotome disintegrates and cells that express Pax3 and Pax7, already present in this domain, enter the myotome [18,19]. Such Pax positive cells are present in all developing skeletal muscle masses at late embryonic and foetal stages [19–22]. Most of these cells are proliferating and do not express skeletal muscle markers. However, chase experiments show that they can enter the myogenic programme, with activation of the myogenic determination genes Myf5 and MyoD, followed by their incorporation into skeletal muscle fibres. Lineage tracing experiments, and notably grafts of quail somites into the chick embryo, demonstrate that all these cells in the trunk and limbs derive from somites. Furthermore, as previously suggested [23], the Pax positive satellite cells of postnatal muscle also come from this source [19,22].
In Pax3/Pax7 double-mutant mouse embryos, which lack both Pax3 and Pax7, there is a major deficit in skeletal muscle; only the early myotome and its derivatives are formed [20]. The Pax3/Pax7 positive population of cells do not activate Myf5 or MyoD and fail to enter the myogenic programme. They die, or they become incorporated into other tissues. In this major population of skeletal muscle progenitor cells, prior to birth, Pax3 and Pax7 regulate both myogenic determination genes, and Pax3 as well as Pax7 ensures cell survival.
In the perinatal period, these cells take up a satellite cell position under the basal lamina, which begins to form around muscle fibres. At this stage, many of such quiescent progenitor cells transcribe the Myf5 gene, suggesting that activated, Pax positive-, cells that had begun to enter the myogenic programme revert to a more progenitor-like state, as quiescent satellite cells (Fig. 2). In these cells, Myf5 expression is no longer under Pax control. Furthermore, the anti-apoptotic function of Pax7 compared to Pax3 is now predominant, again perhaps reflecting the shift in progenitor cell status. At earlier stages, the Pax positive cells either self-renew as stem cells or make muscle. The postnatal satellite cells efficiently repair normal damage, but progressively fail to do so when confronted with the degenerative diseases of skeletal muscle. This may reflect the preponderance of progenitor cells that have lost some stem cell like properties, as a result of partial myogenic engagement.
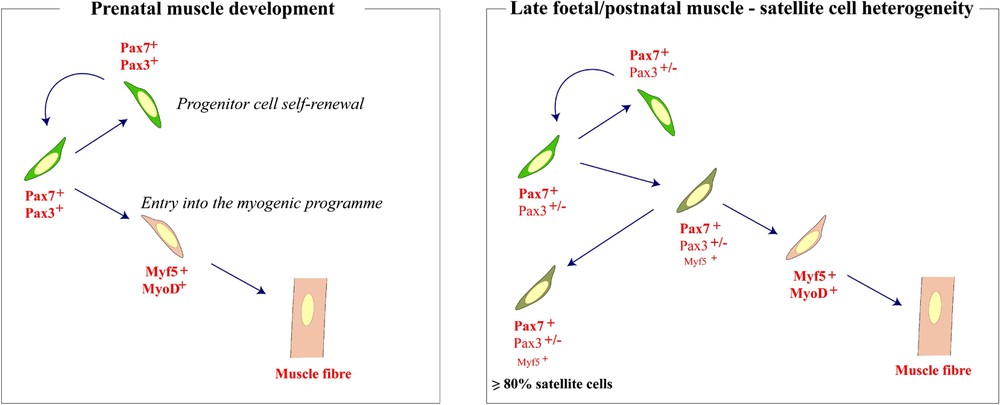
Schematic representation of progenitor cell self-renewal, and myogenic differentiation in the embryo and the adult.
3.3 Regulation of survival and tissue determination in stem cells
Finally, it is important to underline the significance of the regulation of stem cells' survival by the same factors that control the entry of these cells into a tissue differentiation programme. In the absence of such factors, undirected stem cells present a danger for the organism, and this is prevented by their death.
Acknowledgements
The author thanks the members of her laboratory for their contributions to the work discussed here. Work on skeletal muscle in M.B.'s laboratory is supported by the ‘Institut Pasteur’, CNRS, AFM, and the EuroStemCell, Cells into Organs and MYORES integrated projects, and networks of excellence of the E.U.


