1 Introduction
In Mediterranean countries, rainfall is low and irregular and drought may occur at any period during the growing season, including winter months. Water stress will then affect cereals during their early stages of growth when the plant has between 2–3 and 5–6 leaves and when apex differentiation occurs. Number and size of reproductive organs will be then defined (the number and the size of the primordia, the number of ears and spikelets and consequently the potential number of grains that will be produced) under stress conditions [1,2].
It could have an effect on the duration of the stages for about 20% (5) by altering stem apex temperature and thus plant phenological development [3,4].
In durum wheat and winter wheat [5,6], apical development and rate of leaf appearance are modified, due to the altered thermal statute of apex [2] or leaves [5], leading to contradictory results. These effects of drought on apex temperature seem to be important enough for crop yield and will be considered and discussed here. Thermal time will be used to drive phenology.
In order to measure the thermal response of plants to drought (and to study its use as an indicator of water stress), three water treatments were applied at juvenile stages. Apical temperature and microclimatic conditions were measured in this period. Other parameters, referred to plant growth and development, were measured too.
2 Material and methods
2.1 Growth conditions
Two-pot experiments were carried out at INRAT (in Tunis) with a widely used cultivar of durum wheat in Tunisia, Karim. Seeds have been sown in pots (volume of 2.5 l, five seeds in each pot) that were kept outside and taken under a shelter in case of rain. To explore different edaphic conditions, two substrates were used: in the first experiment, the substrate used was perlite and, in the second, it was a mixture of sand and mould. The perlite was siliceous volcanic sand, composed with granules of 1 to 5 mm diameter and 40 to 150 kg/m3 volume weight, 95.6% total porosity, 74.5% water retention capacity, between 6.5 and 7.5 pH and 1.4% buffer capacity. The mould used for second experiment had a pH of 6, a CEC between 2 and 4, and the particles' diameter was between 0.1 and 10 mm. Nutrients were given through a nutritive solution of Haogland [7].
2.2 Treatments
Three water treatments were applied and soil water content was maintained at 100% (T1), 50% (T2) and 30% (T3) of substrate water capacity. Pots have been weighed every 2 days in the morning, and water has been added until the initial weight of the pots was reached for each treatment. Treatments were applied when plants had between 2–3 and 6 leaves (48 days). During these stages, plants were kept on a moving char placed under shelter each evening or in case of rain, and exposed to the sun every morning. After this stage, the soil's water content has been maintained at the maximum of the substrate's capacity. Each treatment was applied to 15 pots, and the total number of pots was 45 for each sowing.
2.3 Measurements and observations
When water treatments have been applied, air temperature, humidity, wind speed and solar radiation were measured every 15 s and their average values were recorded every 15 min on a data logger: air temperature and humidity were measured by a HMP35A captor (Vaisala Oy, Helsinki, Finland) placed under an aerated shelter; solar radiation was measured with the aid of a pyranometer captor (LI-200, LICOR, Lincoln, Nebraska, USA) placed horizontally; wind speed was measured by an anemometer placed on a char at 2-m height. Captors were placed on the char behind plants day and night, and connected to a data logger (Campbell scientific, LTD CR10 Wiring Panel, Shepshed, Leicester, UK).
Plant apex meristem temperature was measured, by the same token, using thermocouples cupper/constantan of 0.2 mm inserted in it, and recorded at the same frequency as air temperature. Five thermocouples were inserted in apical shoot for each treatment.
Other destructive measurements were done; the plants' numbers of leaves and dry matter of aerial parts were measured; apex's stage and size were also measured using a scale of development stage (inspired from the description by Kirby and Appleyard [8]) on plants taken at each leaf appearance, dissected and observed under the binocular (five main stems of plants for each treatment at least).
2.4 Statistical analysis
ANOVA, comparison test and some linear regressions were made with statistical software GenStat.
3 Results
3.1 Microclimatic conditions
Microclimate conditions were relatively normal for the period of experiments (January and December) and for the bioclimatic layer (Sub humid). Table 1 shows that air temperature and diurnal radiation were included between 6.29 and 15.85 °C, and between 0.94 and 224.04 W/m2, respectively. Relative air humidity was on average 73.77% and finally wind velocity was also moderate for the season (2 m/s).
Microclimate conditions during the period of experiment
| Daily air temperature (°C) | Diurnal radiation (W m−2) Photoperiod ≈ 10 h | Daily relative air humidity (%) | Daily wind velocity (m/s) | |||
| Mean of experiment period | 11.02 | 41.40 | 73.77 | 2.00 | ||
| Max daily mean value | 15.85 | 224.04 | solar noon = 441 | 88.26 | Hr min = 81.35 at noon | 4.13 |
| Min daily mean value | 6.29 | 0.94 | solar noon = 22 | 56.34 | Hr min = 46.44 at 13 h | 0.05 |
3.2 Apex stage and leave stage
It was observed that the number of unfolded leaves (Fig. 1) was related to water treatment. For treatments with water deficit, the number of leaves was reduced and the difference between treatments has increased according to time; this difference was statistically significant for experiment 2, where T1 had 7.33 leaves while T3 had 6 leaves.

Chronologic evolution of leaves number under three water treatments: T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
When plants were fully irrigated again, the difference between the treatments decreased.
At each emission of a new leaf, observations on apex size (Fig. 2) and stage (Fig. 3) were made. Apex size was slightly decreased by water stress. Conversely, it increased again when water treatments were removed. On the other hand, it was observed that apex development was slowed down among stressed plants only at the beginning of the water deficit, but it was slightly faster when water deficit lasted.
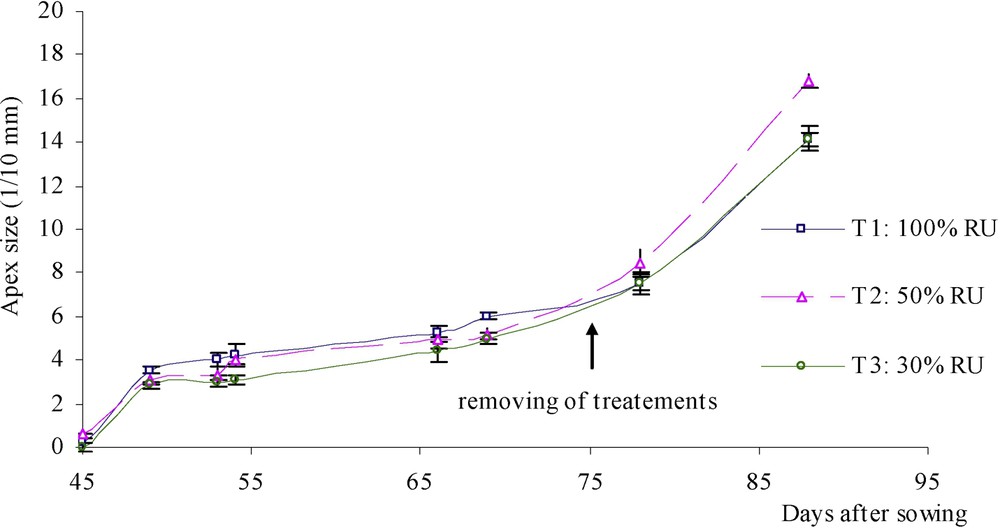
Chronologic evolution of apex size (1/10 mm) under three water treatments: T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
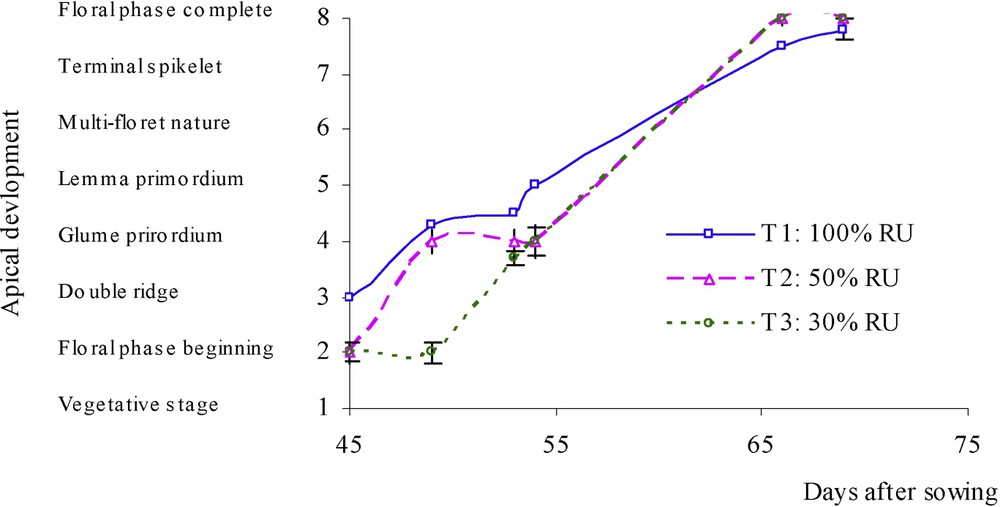
Chronologic evolution of apex development (on a scale of 8 degrees inspired from the description of Kirby and Appleyard [8]) under three water treatments: T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
The relationship between apex stage and the number of leaves was modified with water-deficit treatments (Fig. 4); slope was increased in treatments with water deficit. We can, then, observe a bigger rate of apex development in the case of water stress. The terminal spikelet stage appeared earlier for dryness treatments: hardly stressed plants had 4–5 leaves when the terminal spikelet stage was observed, while fully irrigated plants reached this developmental stage only at 5–6 leaves.
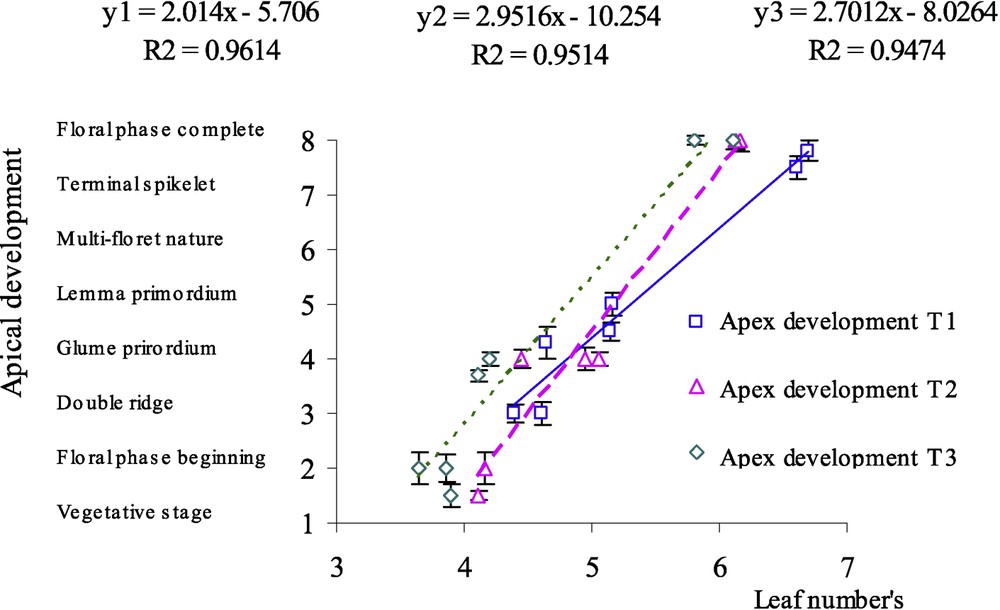
Relationship between apex stage (on a scale of 8 degrees inspired from the description of Kirby and Appleyard [8]) and number of leaves in the case of three water treatments: T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
Table 2 shows that the number of spikelets for fully irrigated plants (10.75), in spite of their apex stage delay (7.67), is higher than the number of spikelets for stressed plants (8.5 and 6.5 for T2 and T3, respectively).
Number of differentiated spikelets and apex stage for each treatment
| Treatments | Number of spikelets | Apex stage |
| T1 | 10.75 | 7.67 |
| T2 | 8.50 | 8.00 |
| T3 | 6.50 | 8.00 |
We can observe in Fig. 5 that apex development under dramatic water-stress treatment, referred to locally cumulated apex temperature, is accelerated compared to the case of moderately stressed plant, but only when the difference in cumulated temperature becomes relatively important.
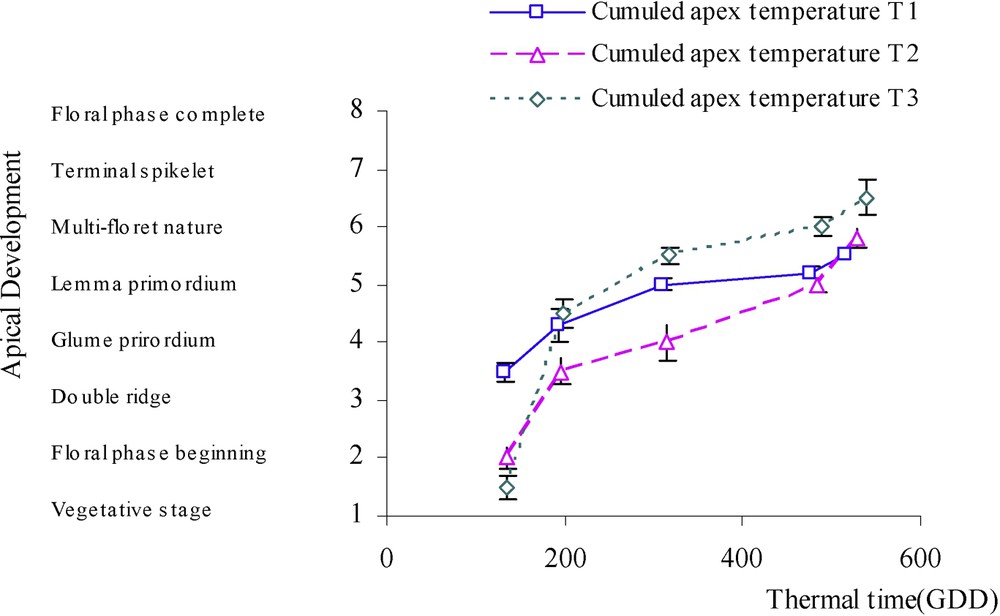
Evolution of apex development (on a scale of 8 degrees inspired from the description of Kirby and Appleyard [8] referred to the thermal time measured locally in shoot apex (GDD, Growing Degree Days) in the case of three water treatments: T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
3.3 Apex temperature
Analysing apex temperature during the experiment as a stress statute indicator amongst climatic variation highlighted the existence of certain days on which differences between treatments were more clearly visible, especially when radiation was high (even if temperature is related positively to radiation) (Fig. 6).
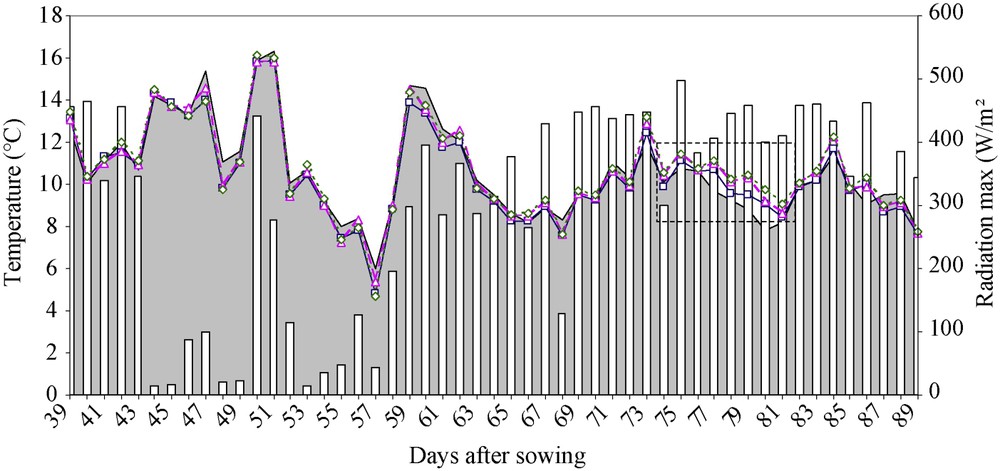
Air temperature (grey area), apical temperature (°C) under three water conditions: T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants) and radiation (histogram) (W/m2) during the experiment. We can observe in the indicated area that the difference between the treatments was more important when radiation was high.
A typical day was then chosen with relative high radiation to illustrate the difference between treatments during a day (Fig. 7).
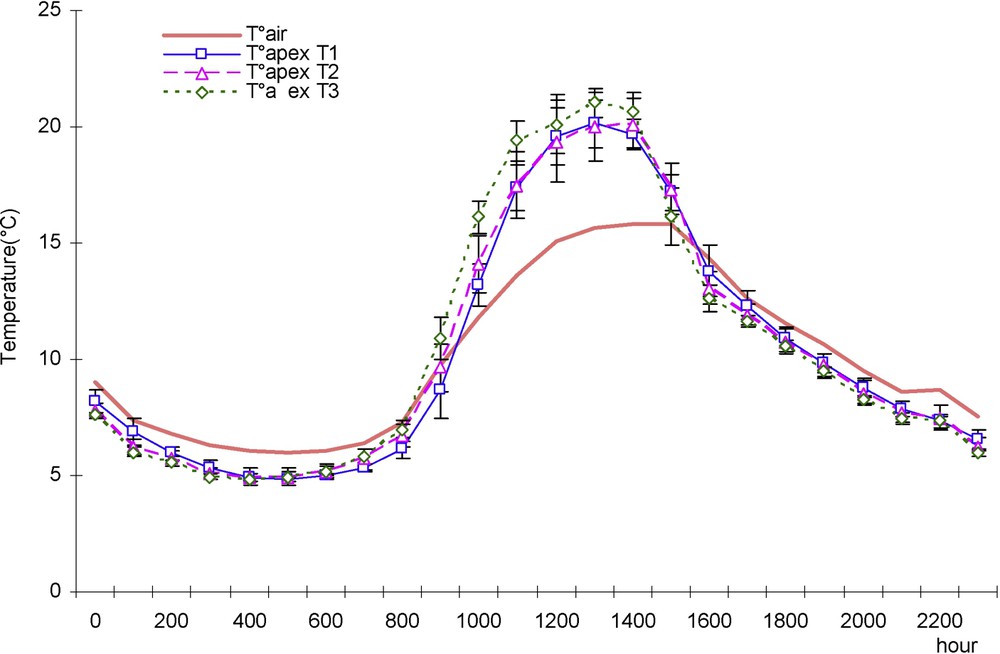
Evolution of air temperature and apex temperature (°C) in a typical day with high radiation in case of three water treatments: T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
- – During the day, apex temperature is higher than air temperature (Fig. 7); drought treatments had higher apex temperature. The difference with air temperature can reach up to 4 °C, as the difference between treatments can be as high as 2 °C.
- – On the other hand, during the night, apex temperature does not depend on the treatment and is slightly lower than air temperature.
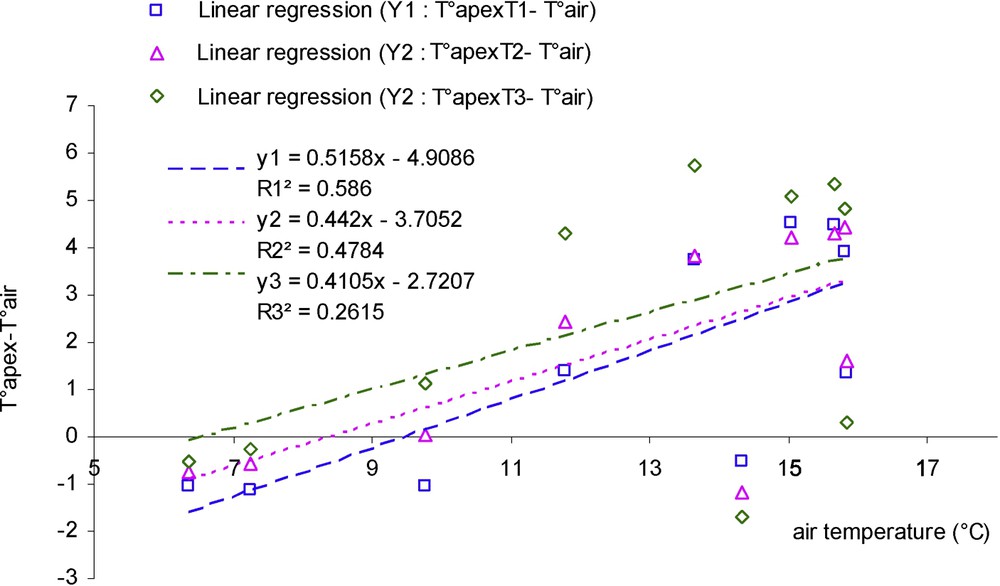
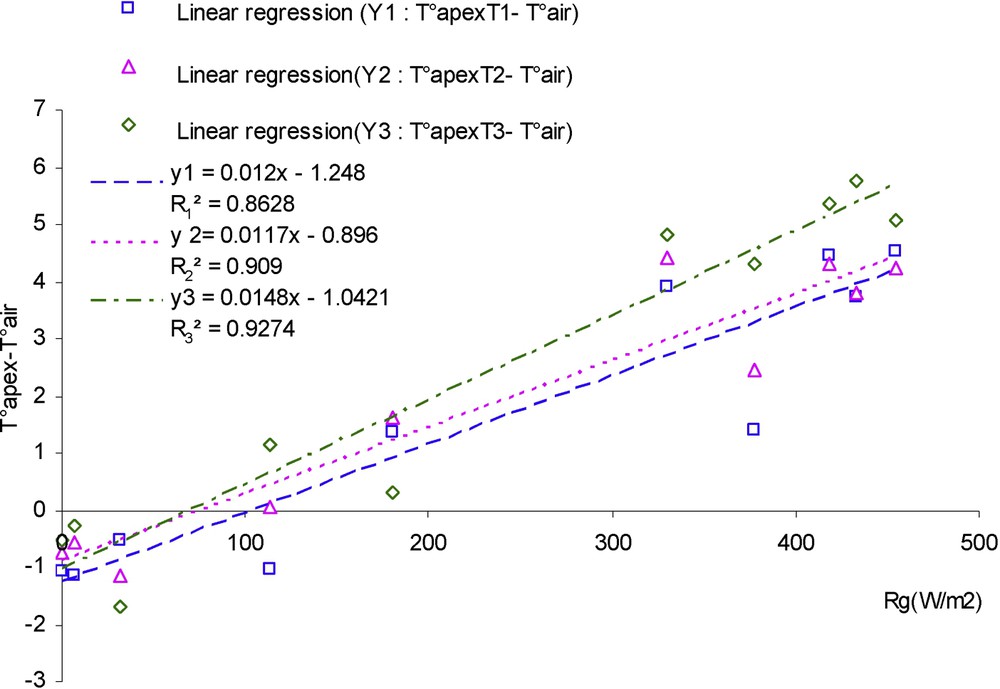
A graph displaying apex temperature related to the different treatments minus air temperature on this same day (Fig. 8) shows that differences between treatments are more visible at midday, when radiation is maximal. These differences between apex temperature and air temperature for the three treatments were then related to air temperature and to radiation (in case of sunny day) (Figs. 9 and 10), the slopes of linear regressions of drought treatments are lower than treatment fully irrigated for the relationship with both air temperature and especially radiation. However, the best regressions are obtained in the case of the relationship with radiation in this same sunny day (, , ).

Evolution of the ‘apex–air’ temperature difference (°C) during a day with high radiation in the case of three water treatments: T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
Difference between apex temperature and air temperature (°C) in relation with radiation (day with high radiation, case of three water treatments): T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
Difference between apex temperature and air temperature (°C) in relation with air temperature (°C) (day with high radiation, case of three water treatments): T1 (□), witness plants; T2 (▵), moderately stressed plants; T3 (◊), much stressed plants.
4 Discussion
The number of unfolded leaves is decreased by water stress. Actually, Ben Haj Salah [9], Malvoisin [10] and Muchow et al. [11] had found that water deficit decreases the gear of leaf lengthening (cellular expansion) in maize, wheat, bean and others plants [10,11]. In the same time, Tivet and Siband had proposed for Oryza sativa a curvilinear response of leaf emission rate to meristem temperature with an optimum between 25 and 28 °C [12].
Development and growth of apex depend on duration and intensity of water stress. Apical development is, in fact, more important when water deficit lasts for a long time. In contrast, apical growth is slower: apex size and differentiated spikelet number are reduced (even if the apex size succeeds in recovering delays when treatments are removed). This result confirms the next work made by McMaster et al. [13].
McMaster et al. [13] proposed that temperature and photoperiod are the primary factor controlling phenological development rates, which are being important for crops too [11,14]. Water availability is generally an important factor, but it could often exceed threshold values before influencing phenology. We had, in fact, found that the phenological development of only plants subjected to severe water stress was really affected.
Therefore, the co-ordination between the apex and leave stages [10] cannot be adapted in conditions of early water deficit. Slafer and Rawson [15] had already refuted this. Using morphologic appearance as apical development stage indicator cannot, then, be adopted in this case (water deficit).
Otherwise, increase in apex temperature caused by lower transpiration of stressed plants can lead to cumulate sums of temperature higher than in the case of fully irrigated plants. This is one of the reasons that cause accelerated apical development in case of water stress in spite of low leaf growth, development being proportional to sums of temperature (thermal time) [16]. When water deficit occurs, one of the plant's strategies is to close stomata under ABA regulation to limit water loss by transpiration [17], which is responsible for the organism's temperature regulation. Likewise, sums of temperature of stressed plants became more important, mainly when climate conditions (especially radiation) are severe during a day (generally at noon), first, and during the season, too. In fact, the rate of increase of apical temperature relatively to air temperature was more sensitive to water stress in case of days with high radiations. This could be possibly explained by the relationship between radiation and temperature and also a logically great photosynthetic activity in case of high radiation occurring under water deficit, water being an important element of the photosynthesis mechanism. So, therefore, we can observe true stress behaviour in these climatic conditions.
Then again, the optimum temperature for the reproductive phase is included between 9.3 and 11.9 °C [16], and apex temperatures of stressed plants had often risen beyond this interval. A number of differentiated spikelets had then been affected. Johnson and Kanemasu [18] had also demonstrated that high temperatures in kernel development decrease the number of spikelets and the grains number in each spikelet, too. Slafer and Rawson [19] had also found that apical differentiation is highly sensitive to temperature.
5 Conclusion
As a result of the experiment, it seems that when drought was applied, the apex development was accelerated, but that the amount of leaves decreased as a result of growth reduction. Apex temperature measurements showed a radiation effect on apex temperature and the acceleration of apex development could be related to apex temperature increase.


