1 Introduction
α-Amylase (α-1-4 D-glucan glucanohydrolase EC 3.2.1.1) catalyzes endohydrolysis of α-1-4 glucosidic linkages in starch and any related oligosaccharides to make oligosaccharides and glucose. In plants, α-amylase plays a key role in starch degradation during seed germination [1]. Starch hydrolysis by amylases is the basis for several industrial processes such as the preparation of glucose syrups, bread making and brewing. Amylase is instrumental in starch digestion in animals resulting in the formation of sugars, which are subsequently used in various metabolic activities [2].
Generally, the number of identified isoenzymes depends on the cultivars studied and the sensitivity of the resolving method used [3–6]. Several α-amylases have been purified and characterized from different plants by using conventional as well as classical methods. The main problem encountered in the purification of the enzymes from cereals is their occurrence in multiple forms as isoenzymes. However, this problem does not occur in dicotyledonous plants for which the number of isoforms does not exceed two in most cases [7].
Affinity chromatography is one of the best tools for the selective purification of enzymes. For that purpose, substrates, substrate analogs, inhibitors, metal ligands, antibodies are covalently linked to insoluble Sepharose matrix. Selective elution of the loaded enzyme results in highly pure enzyme [8]. Starch adsorption chromatography has been used to purify amylase from malt sorghum [9] and other affinity matrices such as cyclohepto-amylose-sepharose [10] and insoluble starch in the presence of ammonium sulphate [11] Glycogen-Sepharose [12] have also been used in the purification of cereal amylases.
The oleaginous seeds are known to set up during their germination a metabolic process related to degradation of their lipids reserves [13,14]. It is thus often described that lipases are the first hydrolases implied in this process, which is in opposition to cereals and proteo-glucidic seeds where the amylases are the most abundant hydrolases. However, in our laboratory, we showed that oleaginous seeds from sunflower, rapeseed and safflower are endowed with an amylasic activity. Presently, as far as we know, no amylase from oleaginous seeds has been purified. In this work, we purified and characterized one of these oleaginous amylases extracted from safflower germinating seeds
2 Experimental protocols
2.1 Plant material and enzyme extraction
Safflower seeds were supplied from National Institute of Agricultural Research of Tunisia. Seeds were imbibed overnight in water, and then placed to germinate at 27 °C in darkness with a water supply. The 5-d grown seedlings were harvested and kept in −20 °C until use. The cotyledons were homogenised in 4 volumes (w/v) of 0.1 M acetate buffer (pH = 6) containing 1 mM PMSF, 5 mM CaCl2, followed by stirring slowly for 1 h at 4 °C. The homogenate was centrifuged 15 min at 10 000 g at 4 °C. The supernatant was recovered and kept at −20 °C until use.
2.2 Preparation of crude extract
A crude extract was prepared from 20 g of 5-day-old cotyledons as mentioned above. The extract was fractionated by gentle addition of ammonium sulphate up to 20 or 60% concentration of saturation, followed by centrifugation during 15 min at 10 000 g at 4 °C. The fraction 20–60% was recovered, dissolved and dialyzed against 50 mM acetate buffer (pH = 6) containing 5 mM CaCl2.
2.3 Purification of the α-amylase
Affinity chromatography using β-cyclodextrin Sepharose 6B prepared according to the method of Vretblad was performed [15]. To the crude extract 5% ammonium sulphate was added [7] and the solution was poured onto the column (, 40 ml) pre-equilibrated in 50 mM acetate buffer (pH = 6) containing 5 mM CaCl2. After the column was washed with 50 mM acetate buffer (pH = 6) containing 25 mM CaCl2 and 0.5 M NaCl, the elution was effected by 8 mg/ml of β-cyclodextrin in the washing buffer. The active fraction was dialyzed against 50 mM acetate buffer (pH = 6) containing 5 mM CaCl2 and was concentrated by lyophilisation. All purification steps were done at 4 °C.
2.4 Enzyme essay
The hydrolytic activity for soluble starch was measured in the standard reaction mixture consisting of 400 μl of 0.5% soluble starch prepared in 0.1 M of sodium acetate buffer (pH = 6.0) containing 5 mM CaCl2 and 100 μl dilute enzyme with acetate buffer. The reaction was started by adding substrate. The reducing sugar produced in 10 min at 55 °C was measured by DNS (3,5-dinitrosalicylate) method at 540 nm by using glucose as standard reducing sugar. One unit of enzyme activity was defined as the amount of enzyme that produced 1 μmol of reduced sugar per minute under these reaction conditions.
2.5 Electrophoresis
2.5.1 Molecular mass determination
SDS-PAGE was performed, as described by Laemmli [16]. The purified enzyme (1 μg) was loaded onto 0.75 mm thick 10% polyacrylamide gel together with molecular size markers. After electrophoresis the gel was stained with silver nitrate solution [17].
2.5.2 Amylase activity staining
Native gel electrophoresis was done in same conditions as SDS-PAGE, but the polyacrylamide gel did not contain SDS and the migration was realised at 4 °C. After electrophoresis the gel was incubated at 55 °C in soluble starch solution (1%) prepared in 0.1 M sodium acetate buffer (pH = 6.0). Subsequently the gel was stained with iodine reagent (KI-I2 solution). The amylase bands were visualized as transparent bands on a dark blue background [18].
2.6 Effect of pH
The effects of pH on soluble starch hydrolytic activity were examined with standard essay conditions, at pH values from 3.0 to 10.0. The pH optimum of purified enzyme was measured by using two different buffers for two different ranges, 0.05 M acetate buffer (pH 3–6.5) and 0.05 M Tris–HCl (pH 7–11). For pH stability, enzyme was kept at 4 °C for 24 h in buffer at different pH values.
2.7 Effect of temperature
The effects of temperature on soluble starch hydrolytic activity were examined with standard essay conditions, but changing the incubation during 10 min in sodium acetate buffer (pH = 6.0) from 4 to 90 °C. For studying the thermal stability, enzyme was kept at variable temperature for 30 min and residual activity was measured by standard essay.
2.8 Effect of metal ions
Purified amylases were incubated with 5 mM and 10 mM solution of salts of metal ions (chlorides of Ca2+, Ba2+, Mg2+, Cu2+, Zn2+ and Zn2+) and their activities were determined. The enzyme activities without metal ions were taken as 100% and relative activities determined in the presence of metal ions were calculated.
3 Results and discussion
3.1 Purification of safflower cotyledon α-amylase
In this study it was found that α-amylase from germinating safflower seeds showed maximum activity after 5 days of growth and then declined rapidly (Fig. 1). From this information, we decided to purify this enzyme activity 5 days following seed imbibitions. The existence of amylase activity in oleaginous seeds testifies the existence of the glucidic reserves, particularly starch. The origin of this starch is to be checked. Does this starch east exist in dry seed or is it synthesized during germination starting from other precursors, particularly the breakdown products of the lipids storage? Earlier work showed that the oleaginous seeds accumulate starch at the beginning of their germination [19,20]. Thus, it was shown that the content of starch in endosperm tissue of castor seed (Ricinus communis) increases from 0.5 to 1.1 mg per seed in 5-day-old seedlings [21]. Recently, another study showed, by proportioning and electronic microscopy, that the cotyledons of yellow lupine seeds (Lupinus luteus L.), which are regarded as leguminous seeds rich in lipids, accumulate starch and that the content of starch increases from 8 to 22 mg/g of dry matter in 4-day-old seedlings [22].
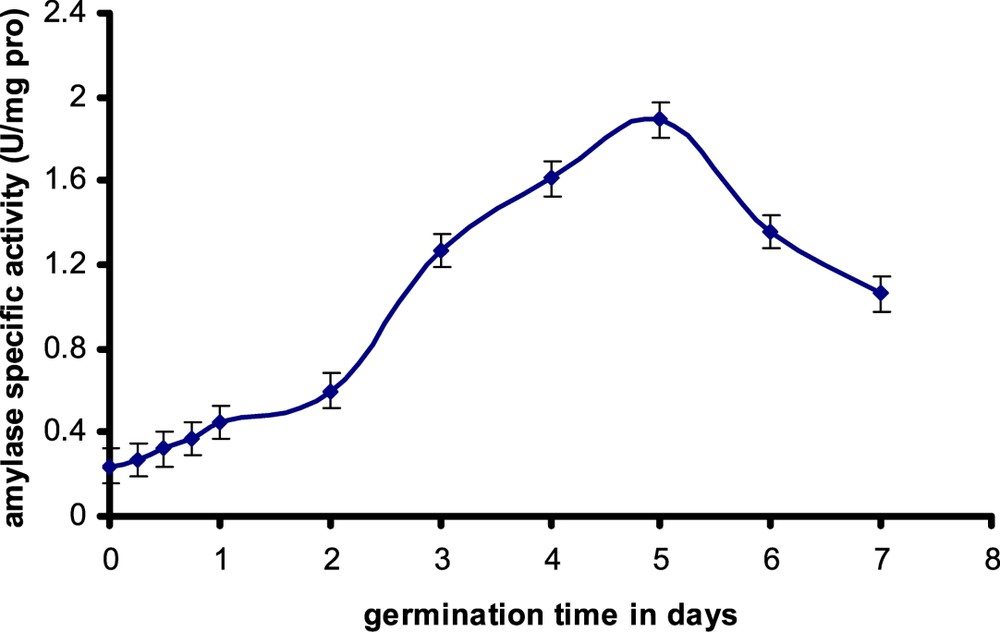
Evolution of α-amylase activity during safflower seeds germination. Each value is the average of three tests.
A summary of the purification procedure that was used for this cotyledonary α-amylase is presented in (Table 1). We have obtained 0.14 mg of purified α-amylase. Crude extract for 20 g of safflower cotyledons for 5-day-old seedlings of germination was fractionated by ammonium sulfate precipitation and separated into two fractions (0–20% and 20–60%). More than 95% of the activity was obtained in the 20–60% fraction. Ammonium sulphate fraction 20–60% was loaded onto an affinity chromatography column. After elution by 8 mg/ml cyclodextrin solution, amylase activity and proteins were found in one peak (Fig. 2). Safflower amylase was purified more than 131.52 folds with an 81.25% activity yield and its specific activity equaled 238 U/mg of protein (Table 1). The elution pattern of the Sepharose 6B-β-cyclodextrin column chromatography showed one peak of protein and of amylase activity (Fig. 2). The affinity chromatography technique used in this study has also been used successfully for amylases purified from Vigna mungo cotyledons [23,24], from Arabidopsis thaliana leaves [25], and from roots and cotyledons of Pisum sativum [26]. These results are in accordance with similar purification procedures used by others [23,25]. It can be observed that the enzymatic activity was eluted in one peak, which coincided with the peak of protein. Fractions of this peak (46–49) were collected and concentrated.
Summary of the purification of α-amylase from safflower germinating seeds.
| Purification steps | Proteins mg | Total activity U | Specific activity U/mg | Purification yield | Purification factor |
| Crud extract | 22.6 | 42.7 | 1.89 | 100 | 1.0 |
| Ammonium sulphate precipitation | 18.7 | 41.1 | 2.19 | 96.25 | 1.16 |
| Affinity chromatography | 0.14 | 34.8 | 248.57 | 81.5 | 131.52 |
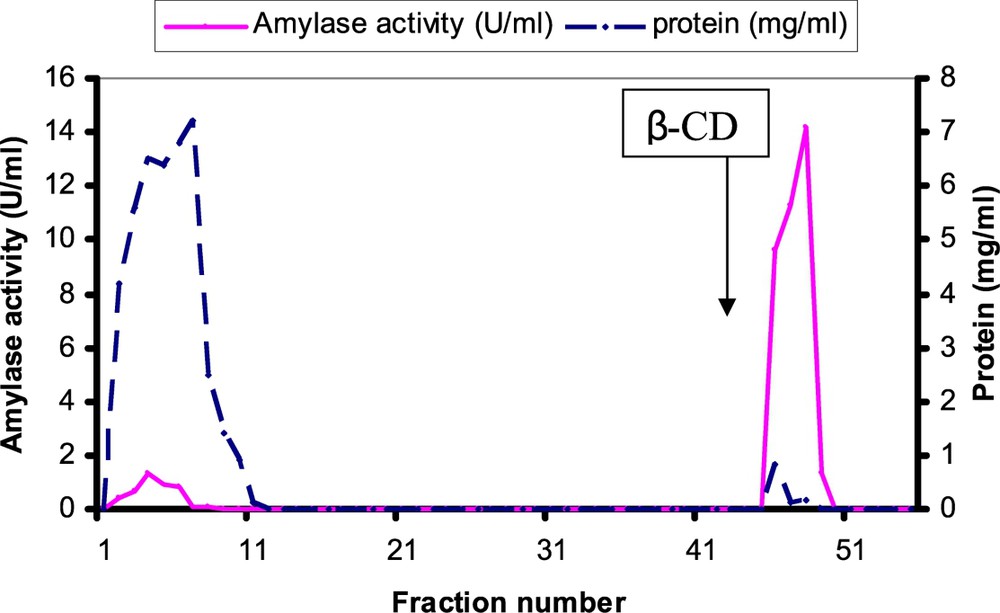
Affinity chromatography elution profile of safflower α-amylase on the β-cyclodextrin Sepharose 6B column. 20 ml of the 20–60% fraction saturation of ammonium sulphate in 50 mM acetate buffer pH = 6 containing 5 mM CaCl2 and 5% ammonium sulphate was loaded onto the affinity column (1.6 x, 40 ml). After the column washing by 20 mM acetate buffer containing 25 mM CaCl2 and 0.5 M NaCl pH = 6, the elution was started with 8 mg/ml of β-cyclodextrin in same buffer in the position indicted by an arrow β-CD. The column elution was effected at flow rate of 1 ml/mn at 4 °C. Fractions were collected by a collector fraction and fraction size was 5 ml. Fractions 46–49 was pooled, dialysed and concentrated.
3.2 Isoform and molecular mass
This purified cotyledonary α-amylase from 5-day-old safflower seedlings appeared to be present as only one isoform in the zymogram of α-amylase and protein band pattern of the purified enzyme (Fig. 3). Similarly Vigna mungo cotyledons from 4-day-old seedlings had only one α-amylase that exists mainly as a monomer that partially aggregates to form dimer, trimer and further multimers [23]. In contrast, cotyledonary amylases preparations obtained from Pisum sativum seedlings exhibit from two [27] to seven [28] α-amylase isozymes.

Polyacrylamide gel electrophoresis under native conditions of α-amylase activity from safflower seed at different moments of purification procedure: lane 1: 1 μl of pure α-amylase from affinity chromatography; lane 2: 10 μl of crude extract.
The molecular mass of this purified cotyledonary α-amylase was found by SDS-PAGE to be 35 kDa (Fig. 4). This value is very close to that obtained for the cotyledonary α-amylase purified from pearl millet α-1-amylase (31 kDa) [29], from Vigna mungo (43 kDa) [23], Azuki bean Amy1 (47 kDa) and Amy2 (44 kDa) [7], and Vigna Ungularis (45 kDa) [30] (Table 2).
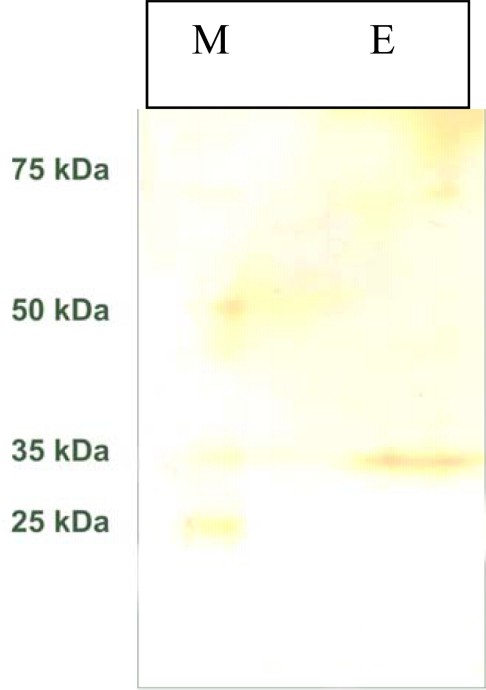
Polyacrylamide gel electrophoresis under denatured conditions SDS-PAGE. Lane E: amylase active fraction obtained by affinity chromatography; lane M: marker proteins. The gel was stained with silver nitrate solution.
3.3 Effect of pH and temperature
The effect of pH on this enzyme activity was examined at constant temperature using sodium acetate and Tris–HCl buffers. As shown in (Fig. 5), purified safflower α-amylase showed an optimum pH value of 6.0. This optimum pH value is consistent with data reported for other amylases (Malted wheat α-1, Malted barley α-1, Malted finger millet α-2 [31,32]) and it is close to the optimum pH of azuki bean amylases: VAAmy1 (5.3) and VAAmy2 (5.2) (Table 2).
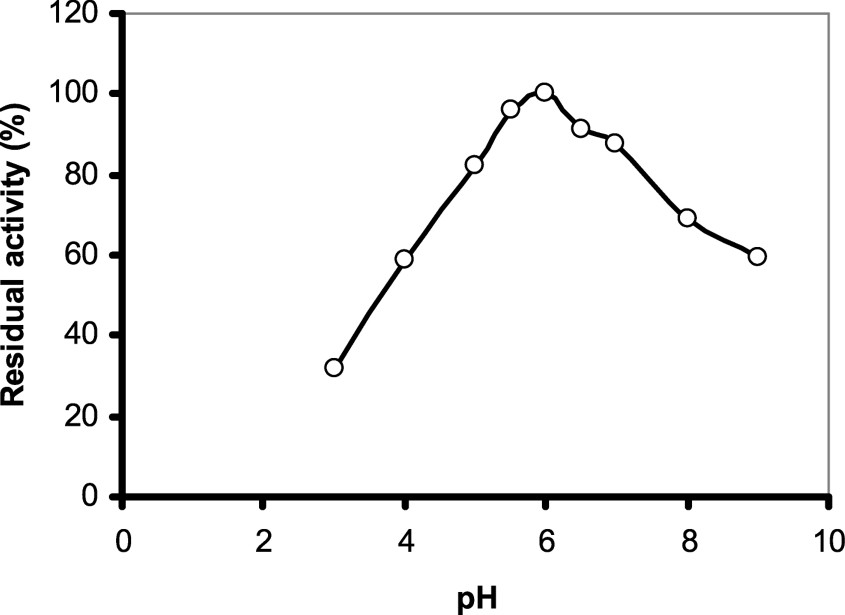
Determination of pH optimum of the enzyme: the pH optimum of purified enzyme was measured by taking two different buffers for two different ranges, 0.05 M acetate buffer (pH 3–6.5) and 0.05 M Tris–HCl (pH 7–11). Activity unit for the enzyme is defined in the text. The enzyme activity at pH 6.0 was taken as 100%.
The range of pH stability, where the presently purified amylase conserved more than 60% of original activity after 24 h of incubation at 4 °C, was from 4.0 to 9.0 (Fig. 6). At values higher than 9, however, enzymatic activity dropped sharply to 50% of maximal activity.
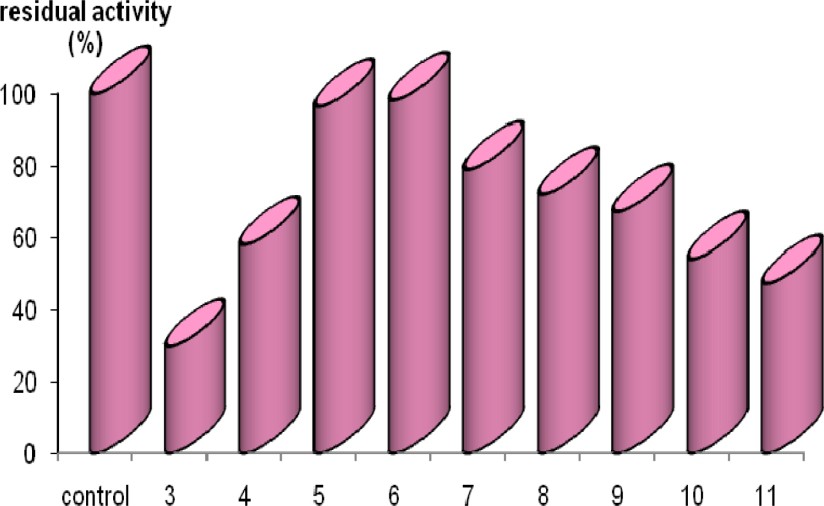
pH-stability: The enzyme solution (25 μl) was mixed with 75 μl of 50 mM acetate buffer of various pHs and incubated at 4 °C for 60 min. Then 400 μl of 0.5% soluble starch prepared in 50 mM acetate buffer (pH = 6) were added and the activity was measured under the standard conditions given in Materials and Methods.
The optimum temperature for safflower α-amylase activity is 55 °C (Fig. 7). This value is consistent with data reported for other plant amylases (wheat α-1 (55 °C), pearl millet α-1 (55 °C) [29]) and smaller than that for azuki bean amylase (70 °C) [7]. This enzyme was not stable up to 70 °C. For example, the enzyme lost 50% of its activity after 10 min incubation at 70 °C (Fig. 8), and was completely denatured at 100 °C.
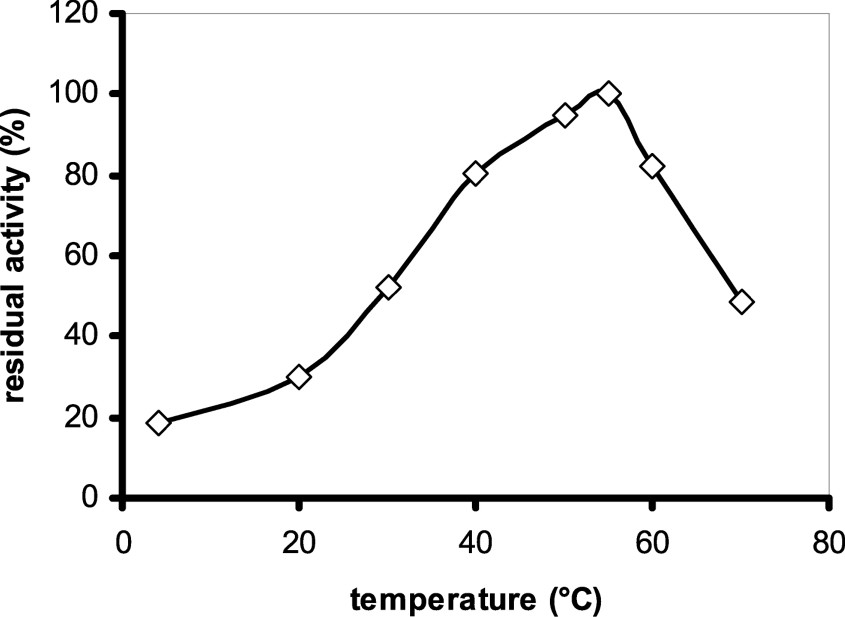
Determination of optimum temperature: 0.1 ml of the purified enzyme was incubated with 0.4 ml of the substrate (50 mM sodium acetate (pH = 6.0), 0.5% soluble starch) at different temperatures for 10 min.
3.4 Effect of metal ions
Various metal ions such as Ca2+, Mg2+, Zn2+, Fe2+ and Cu2+, at 5 and 10 mM concentration were tested for amylase activation/inhibition. The results are given in Table 3. Ca2+ was found to have both activating and stabilizing effects as indicated by increased activity whereas Mg2+ had negligible effect on amylase activity. However, the enzyme inactivation in the presence of Zn2+ and Cu2+ was found to be partial. In contrast, Fe2+ completely inactivated this amylase. The inactivation by this metal may be due to its binding to either catalytic residues or by deplacing the Ca2+ from the substrate binding site of the enzyme. The role of Ca2+ in maintaining the stability and structure of the α-amylase is well documented [33].
Effect of metal ions (5 and 10 mM) on purified safflower α-amylase activity (represented as % activity).
| Salts | 5 mM | 10 mM |
| CaCl2 | 125 | 128 |
| FeCl2 | 0 | 0 |
| MgCl2 | 98 | 95 |
| CuCl2 | 102 | 99 |
| ZnCl2 | 36 | 22 |
Enhancement of amylase activity by Ca2+ ions is based on its ability to interact with negatively charged amino acid residues such as aspartic and glutamic acids, which resulted in stabilization as well as maintenance of enzyme conformation [34]. In addition, calcium is known to have a role in substrate binding [35]. It has also been documented that binding of Ca2+ to amylase is preferred to other cations such as Mg2+ [36].
To our knowledge, this is the first oleaginous α-amylase that has been purified. This purification opens many horizons to us to comprehend the germination metabolisms of these types of seeds, the physiological significance of which calls for study in future works.


