Highlights
- AQP4 is expressed in the chicken oviductal parts.
- Starvation decreases AQP4 mRNA in the infundibulum and AQP4 protein in the shell gland.
- Immunoreactivity of AQP4 depends on oviductal tissue and section.
- AQP4 may regulate oviductal function and regression.
1. Introduction
In the domestic hen, an oviduct includes five morphologically and functionally different segments: the infundibulum, magnum, isthmus, shell gland, and vagina. The function of the infundibulum is to engulf the ovulated ovum from the ovary. The infundibulum is also where fertilization occurs and the outer layer of the yolk membrane is formed. The magnum synthesizes and secretes the majority of the albumen by the epithelial and tubular gland cells. In the isthmus, egg-shell membranes are formed. Then, in the shell gland (uterus), fluid with electrolytes is added to the albumen and the calcified eggshell is deposited. Finally, the vagina helps in egg expulsion and is responsible for the storage of spermatozoa in the sperm storage tubules [1, 2, 3].
The functional properties of the avian oviduct undergo dynamic alterations during the reproductive cycle, which includes development, egg laying, and pause in laying. All events related to oviduct activities require a proper balance of water and fluid secretion. Previous research on mammals indicates that membrane proteins known as aquaporins (AQPs) are crucial players in maintaining water availability, and consequent oviductal fluid volume and composition [4, 5, 6, 7, 8].
AQPs are small ( ∼25–35 kDa) integral membrane channel proteins, that are permeable to water and other small, uncharged solutes, such as glycerol or urea. AQPs are divided into three subgroups based on structural and functional properties: classical water channels (AQP0, 1, 2, 4, 5, 6, and 8), aquaglyceroporins (AQP3, 7, 9, and 10), and superaquaporins (AQP11 and 12). It is well established that AQPs are involved in a wide array of reproductive processes in mammals, such as ovarian follicle development, fertilization, and embryonic survival and growth [4, 5, 9, 10]. In contrast, much less is known about participation of AQPs in the regulation of reproductive processes in female birds. So far, Zaniboni and Bakst [11] localized AQP2, 3, and 9 in epithelial cells that form the sperm storage tubules in the turkey vagina. Tiwari and colleagues [12] showed differentially expressed AQP5 in the chicken ovarian tumor cells and in the chicken ovarian cancer cell line, indicating that AQP5 is involved in ovarian tumorigenesis, metastasis, and cell survival. Yang et al. [13] reported an increase in the expression of AQP3 in the ovarian tumors of chickens and revealed that AQP3 is involved in the estrogen-regulated development of the chicken oviduct. Further, previous research by the authors demonstrated the presence of AQP4 in the chicken ovary in relation to follicle development indicating that AQP4 may take part in the regulation of follicle growth [14]. Our recent study revealed the oviductal cell-, part-, and activity-dependent expression of AQP4, suggesting involvement of AQP4 in the functions of the chicken oviduct, mainly the secretion of oviductal fluid [15]. Moreover, a decrease in AQP4 abundance in the oviductal tissues was observed after tamoxifen (estrogen receptor blocker) treatment, suggesting that estrogen may regulate AQP4 expression in the chicken oviduct [15]. Thus, taking into account that during a pause in laying, the oviduct activity decreases along with plasma estradiol and progesterone concentration decreases, we hypothesized that regression of the chicken oviduct during a pause in laying would be accompanied by down-regulation of AQP4 expression. Accordingly, the present study was designed to examine, for the first time, whether AQP4 expression changes during a pause in laying induced by food deprivation.
2. Materials and methods
2.1. Birds and experimental design
The animal experiment was performed according to a research protocol approved by the Local Animal Ethics Committee in Krakow, Poland (approval no. 218/2015). Laying Hy-Line Brown hens were obtained from a commercial farm, and were caged individually under a photoperiod of 14 h light : 10 h dark with free access to commercial food and water.
At the age of 32 weeks, the hens were randomly divided into two groups: (1) fed ad libitum (C; N = 6) and (2) subjected to pause in laying by complete food deprivation for 5 days (F; N = 6). Chickens were sacrificed on day 6 of the experiment. The control hens which laid eggs were decapitated 2 h after oviposition. The oviductal parts including the infundibulum, magnum, isthmus, shell gland, and vagina, were separated. Tissue samples, collected from the midportion of each segment, were immediately frozen and stored at − 80 °C for Western blot analysis or were placed into RNAlater (Sigma-Aldrich, Saint Louis, MO, USA) and stored at − 20 °C for later quantitative real-time PCR. The other tissue fragments were fixed in 10% buffered formalin, dehydrated through graded ethanol solutions, cleared in xylene and embedded in paraffin wax. Microtome sections (6 μm thickness) were mounted onto microscope slides and used for immunohistochemical analysis.
2.2. RNA isolation and RT-PCR analysis
Total RNA extraction, reverse transcription (RT) and quantitative real time PCR were performed as described previously [14, 15]. Briefly, RNA was extracted from collected tissues using TRI Reagent (Sigma-Aldrich). Total RNAs (1 μg) were reverse-transcribed with a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Reverse transcriptase reaction mixtures were performed in 10 μL volume including the random primers, dNTP mix and MultiScribe Reverse Transcriptase according to the manufacturer’s recommendations. The obtained cDNA was used in duplex real-time qPCR for AQP4 and 18S rRNA as a reference gene, in a 10 μL volume containing 5 μL of TaqMan Gene Expression Master Mix (Applied Biosystems), 0.5 μL TaqMan Gene Expression Assays with a specific TaqMan MGB-probe and one pair of primers (AQP4, assay ID: Gg03346640_m1, Genbank accession no. NM_001004765.1, amplicon size: 87 bp; Applied Biosystems), 0.5 μl of Eucaryotic 18S rRNA Endogenous Control (pair of primers and TaqMan probe-labeled VIC/TAMRA, cat #4310893E, amplicon size: 187 bp; Applied Biosystems), 3 μL of water and 1 μL of cDNA (10x diluted samples after the RT). Amplifications included an initial denaturation step at 50 °C for 2 min and 95 °C for 10 min and 40 PCR cycles at 95 °C for 15 s and 60 °C for 1 min. Each sample was run in duplicate. Water as a negative control was used in all reactions. The 2−ΔΔCt method was used to calculate relative expression level of AQP4 gene after normalization to 18S rRNA, and calibration to expression in the infundibulum of the control chickens.
2.3. Protein extraction and Western blot analysis
Western blot analysis for AQP4 protein was performed as described recently [15]. The protein concentration in the tissue homogenates was estimated by the Bradford method with a Pierce Detergent Compatible Bradford Assay Reagent (Thermo Fisher Scientific, Rockford, IL, USA). Samples (20 μg of total protein) were mixed with loading buffer and warmed at 99.9 °C for 7 min. After denaturation, samples were loaded into 12% SDS-polyacrylamide gel, and proteins were separated by electrophoresis under reducing conditions. Resolved proteins were transferred from the gel to a nitrocellulose membrane using a semi-dry blotter (Thermo Scientific Pierce G2 Fast Blotter; Thermo Fisher Scientific) in Genie Transfer Buffer (20 mM Tris, 150 mM glycine in 20% methanol) for 7 min at a constant voltage of 25 V. Membranes were blocked for 60 min with 5% non-fat milk in TBST (0.1% Tween-20 in Tris–buffered saline, pH 7.4). After washing, the membranes were incubated overnight at 4 °C with rabbit polyclonal anti-chicken AQP4 primary antibody (custom-made by Operon Biotechnologies, Tokyo, Japan, and its specificity confirmed in the chicken tissues as described previously [14, 15, 16] diluted 1:3000. Membranes were then washed and treated with secondary horseradish peroxidase-conjugated goat anti-rabbit antibody (1:5000, 60 min, RT; cat #R-05072-500, Advansta, Menlo Park, CA, USA). Next, to control for variable amounts of protein, the membranes were stripped and reprobed with mouse monoclonal anti-β-actin HRP-conjugated IgG (1:500; cat #sc-47778, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA). The sites of antibody-antigen reaction were detected using enhanced chemiluminescence (Advansta) and visualized using a ChemiDoc-It 410 Imaging system and VisionWorks Life Science software. The bands representing each sample were densitometrically quantified using ImageJ program (developed at the National Institutes of Health). Relative abundances of AQP4 protein were normalized to the β-actin in each corresponding data point.
2.4. Immunohistochemistry
Immunohistochemical localization of AQP4 was performed routinely as reported previously [15]. Briefly, after blocking of nonspecific binding sites with 5% normal goat serum in TBST, the sections were incubated overnight with primary rabbit polyclonal anti-chicken AQP4 antibody (the same as for Western blot) diluted 1:250 in TBST. The slides were rinsed two times for 5 min in TBS, before incubation with secondary biotin-labelled goat anti-rabbit IgG (1:300, 90 min, room temperature; cat #BA-1000, Vector Laboratories, Burlingame, USA), followed by an avidin-biotin-horseradish peroxidase complex – Vectastain ABC kit (30 min; Vector Laboratories). The color reaction was developed by incubation with diaminobenzidine and H2O2 solution. In addition, sections were stained with hematoxylin QS (Vector Laboratories). Negative control was performed by replacement of the primary antibody with normal rabbit serum or TBST buffer. Slides were examined under an Axio Scope. A1 light microscope with an Axiocam 503 colour camera and Zen 2.3 pro software (Carl Zeiss, Germany). The intensity of the immunoreactivity was estimated as very strong, strong, moderate, weak, and very weak.
2.5. Data analyses
The data were analyzed using different statistical analyses that are specified in the figure legends. The tests included nonparametric Kruskal–Wallis one-way analysis of variance on ranks followed by the Student–Newman–Keuls test. For comparison of the means of the two groups, the nonparametric Mann–Whitney U test or the Student’s t-test were applied. Differences of values were considered to be significant at P < 0.05. Calculations were performed with SigmaPlot_V_13 (Systat Software Inc., USA).
3. Results
3.1. Expression of mRNA and protein for AQP4
The fasted hens stopped egg laying on day 4 or 5 of the experiment. The oviduct weight of the fasted hens was 62.3% lower (P < 0.01) than the control chickens (69.4 ± 4.03 g vs 26.1 ± 0.93 g).
Real-time PCR analysis demonstrated that AQP4 mRNA expression varied by oviductal segment (Figure 1). In the control chickens, the relative mRNA expression (RQ) of AQP4 was the lowest, and least detectable, in the magnum and isthmus (P < 0.001), and the highest in the infundibulum and vagina. In hens subjected to pause in laying, there was a 86% (P < 0.001) decrease in the level of AQP4 mRNA in the infundibulum compared to the control hens.
The differential presence of AQP4 protein in particular segments of the chicken oviduct was also observed using Western blotting (Figure 2). In each segment of the oviduct of both control and fasted birds, a band of approximately 32 kDa was identified (Figure 2A). Image analysis revealed that AQP4 protein density was higher in the infundibulum, shell gland, and vagina than in the magnum and isthmus (data not shown; analysis was performed only on the AQP4 bands without normalization by β-actin density). Figure 2B, C and D show the Western blots and relative expressions of AQP4 in the infundibulum, shell gland, and vagina, respectively, of both examined groups. Fasting caused a 25.2% decrease (P < 0.01) in the relative level of AQP4 protein in the shell gland and an 221% increase (P < 0.001) in the vagina.
3.2. Immunohistochemical localization of AQP4 protein
Expression of AQP4 mRNA in the chicken oviduct during a pause in laying induced by fasting. The box plot shows median (lines within boxes) and 25/75 percentiles (box sizes). Asterisks indicate significant differences between control (C) and fasted (F) groups (Mann–Whitney U test; ***P < 0.001). Different superscript letters indicate differences (Kruskal–Wallis ANOVA and SNK test; P < 0.05) among control (lowercase letters) and fasted groups (uppercase letters).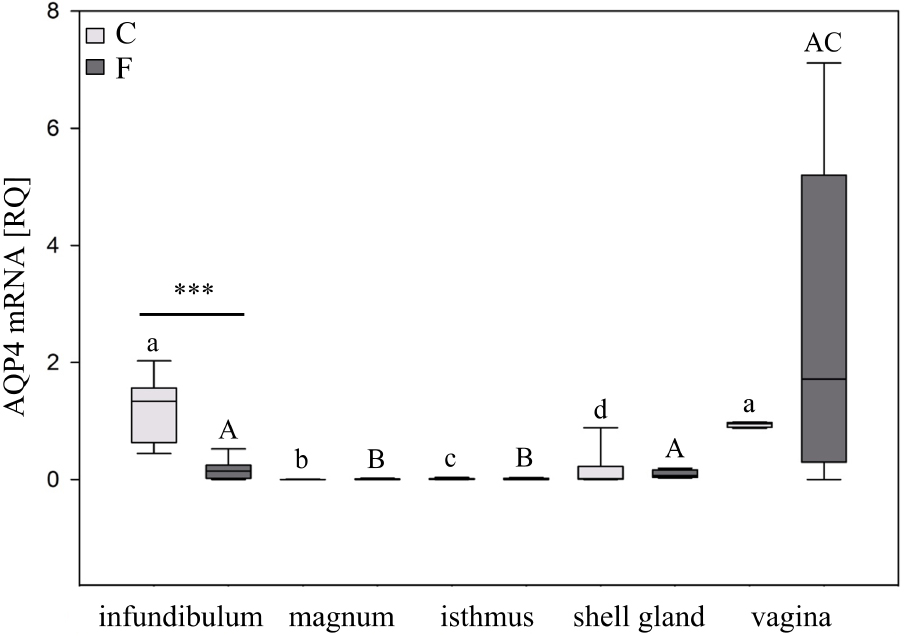
Western blot analysis of AQP4 protein in the chicken oviduct during a pause in laying induced by fasting. A. Representative blot of AQP4 in the oviductal parts of control (C) and fasted (F) groups. B,C,D. The blot and relative expression of AQP4 protein in the infundibulum, shell gland, and vagina, respectively, of control and fasted hens. Values are mean ± SEM (N = 5 or 6) of the ratios of AQP4 to β-actin (Student’s t-test; **P < 0.01; ***P < 0.001). I – infundibulum, M – magnum, Is – isthmus, SG – shell gland, V – vagina.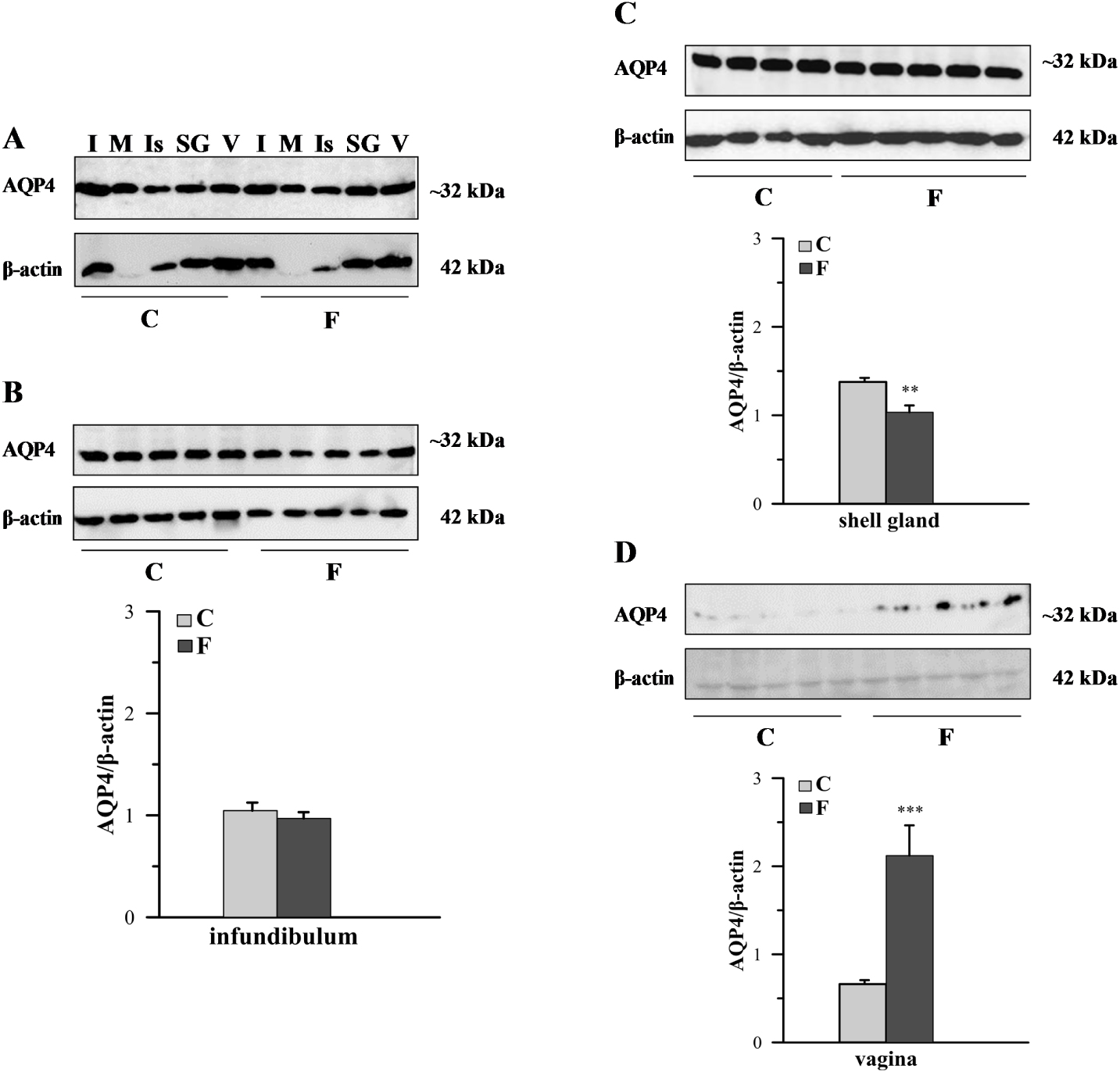
Specific immunoreactivity for AQP4 was found in the wall of all segments of the chicken oviduct of the control and fasted hens. Differences in the intensity of immunoreaction among the segments and within the wall layers of the oviduct were observed (Figure 3). In the control group, the intensity of the immunopositive reaction was as follows: the infundibulum > shell gland > vagina ⩾ isthmus ≫ magnum. A very strong positive reaction for AQP4 was found in the luminal epithelium of the infundibulum. Strong immunoreaction was observed in the muscles located in the stroma of the infundibulum as well as in the luminal epithelium and tubular glands of the shell gland. Moderate intensity of staining was found in the luminal and glandular epithelium of the isthmus and the luminal epithelium and muscles of the vagina. Weak immunoreactivity for AQP4 was present in the luminal epithelium of the magnum, and in the stromal muscles of the isthmus and shell gland. A very weak immunopositive reaction was found in the tubular glands and muscles of the magnum. Moreover, a moderate staining for AQP4 protein was noted in the wall of the blood vessels of the oviductal parts. The immunopositive reaction for AQP4 protein in the oviductal segments was stronger compared for the control hens than for the fasted hens, except in the vagina where no difference in AQP4 immunoreactivity was seen (Figure 3).
Immunohistochemical localization of AQP4 protein in the chicken oviductal parts during a pause in laying induced by fasting. A very strong immunostaining for AQP4 was found in the luminal epithelium (E) of the infundibulum. Strong immunoreaction was observed in the muscles located in the stroma (S) of the infundibulum (A) as well as in the luminal epithelium and tubular glands (TG) of the shell gland (G). Moderate intensity of staining was found in the luminal and glandular epithelium of the isthmus (E) and the vaginal epithelium and muscles (I). Weak staining was present in the luminal epithelium of the magnum (C) and in the stromal muscles of the isthmus and shell gland (E, G). A very weak immunoreactivity was found in the tubular glands and muscles of the magnum. Moreover, a moderate immunoreaction for AQP4 was noted in the wall of blood vessels (bv). In control (C) hens, the immunoreactivity for AQP4 in oviductal parts, except the vagina, was stronger when compared with fasted (F) hens. Scale bar = 50 μm.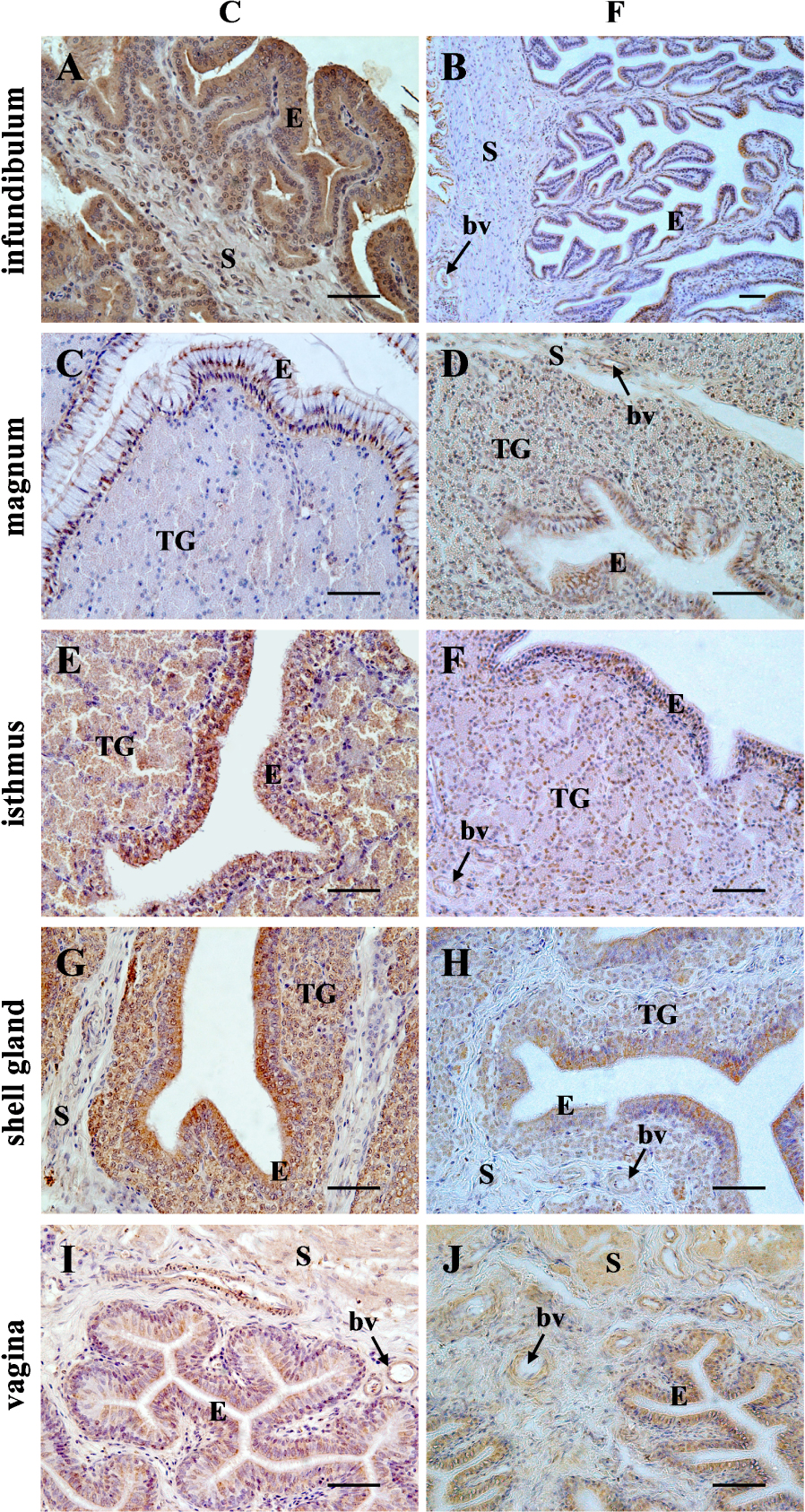
4. Discussion
This study investigated, for the first time, the expression of AQP4 mRNA and protein in the chicken oviductal parts: the infundibulum, magnum, isthmus, shell gland, and vagina during a pause in laying induced by food deprivation. The present results confirm our previous suggestion [15] that AQP4 is involved in the regulation of oviductal fluid volume and composition, and cell volume in the hen oviduct during different physiological states. This was strongly postulated for AQPs in the mammalian oviduct, uterus, or vagina as well [5, 7, 10]. The results obtained also correspond with our recent investigation of the distribution of AQP4 in the chicken oviduct after tamoxifen treatment [15], and are largely consistent with those previously reported in the human and rodent literature [5, 10, 17].
In line with a previous study [15], we observed the highest abundance of AQP4 mRNA and protein in the infundibulum, shell gland and vagina, indicating that these segments are the main places where AQP4 exerts a role. Consequently, the presence of AQP4 mainly in the surface epithelium and stromal smooth muscles of the infundibulum may support fluid secretion by epithelial cells. This helps maintain a proper microenvironment for fertilization and regulate contractility of the infundibulum, particularly around the time of ovulation [18]. In the shell gland, AQP4 was localized to the luminal and glandular epithelium. This may be essential for the regulation of fluid release and absorption. The role of AQP4 in the shell gland seems to be especially crucial because in this oviductal segment, the volume and biochemical composition of fluid significantly changes during eggshell formation. It elevates within the first 6 h after the egg enters the shell gland and 8 ml of water containing electrolytes is added into the egg albumen. During the last 6 h of eggshell calcification, the fluid volume in the shell gland declines and the rate of fluid uptake decreases to 0.15 ml/8 h or less [1]. Moreover, during egg formation, the concentration of K+ and glucose increases, while the concentration of Na+ and Cl- decreases in the shell gland fluid [1]. AQP4 may also regulate the subcellular localization of other membrane proteins. Interactions between transient receptor potential vanilloid 4 (TRPV4; a volume sensitive calcium channel) and AQP4 constitute a molecular system that regulates astroglial volume by integrating osmosensing, calcium signaling, and water transport [19]. Similar regulation may exist in the shell gland where fast biomineralization occurs, resulting in approximately 6 g of calcium carbonate being deposited into the eggshell. In the vagina, AQP4 is abundant in the luminal epithelium and muscles. This may be attributable to the regulation of water homeostasis that is associated with spermatozoa storage, release, and movement. A similar role was previously presumed for AQP2, AQP3, and AQP9 in the turkey oviduct because they are localized to the epithelial cells of the sperm storage tubules located at the utero-vaginal junction [11]. The current results provide further evidence suggesting that AQP4 participates in regulation of water and ion balance in the chicken oviduct.
In addition to water and ion transportation, AQPs are also responsible for transporting gases, such as CO2, O2, NO, and NH3 [20]. Although AQP4 was identified as a gas channel for NO and O2 [21] it seems likely that this AQP maintains CO2 exchange as well. Therefore, it can be assumed that AQP4 is involved in the regulation of gas transport in the hen oviduct, especially in regards to CO2 in the shell gland where it is required for CaCO3 synthesis for deposition in the eggshell.
While AQPs are important in the regulation of fluid secretion and absorption, and cell volume maintenance, they are also involved in other processes not directly connected to water transport. These processes include cell adhesion, migration, proliferation, and death [22]. In birds, induction of pause in egg laying is accompanied by a low rate of cell proliferation [23] and a high rate of epithelial and glandular cell apoptosis in the oviductal wall [24, 25]. In this study, AQP4 was lower in large part of the oviduct of the fasted hens. This may, at least in part, be attributable to the occurrence of apoptotic cell death and reduction in cell proliferation. Findings within the rodent literature provide support for this speculation. An increase in the apoptosis of neurons and astrocytes was observed following intracerebral hemorrhage in mice with AQP4 deletion [26]. Additionally, increased basal apoptosis was observed in adult neural stem cells obtained from AQP4 knockout mice [27].
It is becoming increasingly evident that AQPs are regulated by sex steroid hormones [8, 9, 17, 28, 29, 30, 31, 32]. On the other hand, the oviduct of birds is a steroid hormone responsive organ and it is known that the concentration of steroids in the blood plasma and oviductal tissues dramatically decreases during natural or food withdrawal-induced pause in laying [33]. Therefore, in the study, we examined whether food deprivation would affect AQP4 expression concomitantly with oviduct regression and pause in oviduct activity. We found visible reduction in AQP4 expression at mRNA or protein levels in the infundibulum, magnum, isthmus, and shell gland of food deprived hens when compared to control hens. It seems that there are two possible explanations for decreased AQP4 expression in most of the oviductal segments for the fasted hens. First, there was a decrease in plasma and tissue estradiol and progesterone concentrations as a result of fasting, which under normal conditions may stimulate AQP4 synthesis. Second, there was a reduction in functional activity of oviductal cells as a consequence, at least in part, of steroid action limitation. These assumptions are supported by previous studies showing a reduction in plasma estradiol and progesterone concentrations in chickens during a pause in laying induced by food deprivation [33, 34]. These findings are also in line with previous finding that there is a marked reduction in AQP4 expression in the oviduct of hens treated with tamoxifen, an estrogen receptor blocker [15]. The present results support our earlier hypothesis about the involvement of sex steroid hormones in the mechanisms orchestrating AQP4 expression in the chicken oviduct.
Interestingly, in the vagina, there were no changes in AQP4 mRNA expression, and even an increase in AQP4 protein abundance in the fasted hens. However, after tamoxifen treatment, AQP4 expression was markedly reduced [15]. These results may suggest a slightly different mechanism controlling AQP4 synthesis in the chicken vagina than in the other oviductal segments. Moreover, it should be noted that in female birds, the spermatozoa can be stored for prolonged periods of time in the vagina. Thus, a high level of AQP4 abundance detected in the vagina, also during a pause in laying provoked by food deprivation may be attributable to maintaining a proper environment for sperm residing within the sperm storage tubules, especially during adverse physiological conditions.
5. Conclusions
In conclusion, the results of this study showed, for the first time a reduction in AQP4 expression at mRNA or protein levels in the infundibulum, magnum, isthmus, and shell gland of hens subjected to a pause in laying induced by food deprivation. On the other hand, no changes in AQP4 mRNA expression with concomitant increase in AQP4 protein abundance in the vagina of fasted hens were observed. These data provide support for our previous assumption that the AQP4 mRNA and protein expression depend not only on the activity of the chicken oviduct, but also on the physiological state. Based on our results, it seems likely that AQP4 is essential in regulating water transport required for creating a proper microenvironment for fertilization and egg formation in the hen oviduct. Since AQP4 might participate in the cell volume decrease that is necessary for apoptotic events, we propose that AQP4 may take part in the apoptotic cell death that is frequently occurring in the regressing oviduct during a pause in laying. Additionally, a relationship between steroid action and AQP4 gene and protein expression is suggested.
Acknowledgements
This work was supported by the Ministry of Sciences and Higher Education, Poland (grant number 0215000000-D204).
Conflict of interest
None of the authors have any conflict of interest to declare.
Author contributions
JKS performed the research. AH wrote the manuscript. MG, NS and AS revised the manuscript. AH designed the research study.




 CC-BY 4.0
CC-BY 4.0