1 Introduction
The exceptional characteristics of nanoporous materials have prompted their application in different fields such as molecular sieves, sensors, ion-exchangers and catalysis. In this context, zeolites have been the predominant class of open-framework materials so far used. However, in the last two decades, the number of approaches to obtain new porous materials that behaves as zeolites have experimented an spectacular increase. To date, many inorganic-based families [1], such as phosphates [2], nitrides [3], sulfates [4], sulfides [5], selenides [6], halides [7] and cyanides [8] and inorganic/organic-based solids [9] that exhibit fascinating porosity characteristics have been described. Furthermore, such a huge variety of new porous materials has caused an increase of the diversity of elements of the periodic table that compose their inner walls, most of them metal ions with a paramagnetic character. This opens wide perspectives in the design of a novel generation of porous materials, which besides classical applications of zeolites, can offer a wider range of novel chemical and physical properties, such as magnetism, conductivity or chirality. Among them, attainment of nanoporous magnetic molecular materials has attracted considerable efforts in the last few years. The synergism of both, magnetic properties and the exceptional characteristics of nanoporous materials, opens routes to the development of low-density magnetic materials, magnetic sensors and multifunctional materials at the nanometer scale. For instance, magnetic zeotype structures offer excellent conditions to encapsulate different functional materials with conducting, optical, chiral and NLO properties, among others. In consequence, the resulting material combines the magnetic properties of the framework and the inherent properties and applications of the protected functional molecules.
Before 1996, only a few examples describing the magnetic properties of a family of antiferromagnetic porous phosphates and diphosphonates were described [10]. Then, important advances were introduced by Férey, Zubieta and their coworkers who reported an extensive family of porous iron(III) fluorophosphates and phosphates with antiferromagnetic orderings in the temperature range of 10–37 K [11,12]. Furthermore, a first porous ferrimagnet, an hybrid inorganic–organic cobalt carboxylate, was discovered by Livage et al. in 1998 [13]. All these results represented an important breakthrough in the field of magnetic porous materials that was followed by an exponential increase of scientific publications devoted to the study and understanding of magnetism of porous solids showing antiferromagnetic ordering [14], ferri-/ferromagnetic ordering [15], spin-crossover [16], spin-frustation [17] and metamagnetic [18] properties.
In this review, we will give a short overview of a new type of magnetic nanoporous molecular materials developed in our group, based on the use of open-shell organic radicals as building-blocks. To be good candidates for the formation of nanoporous magnetic materials, organic radical building-blocks must fulfill a series of conditions. First, they must combine the correct geometry to induce the formation of open-framework structures. Second, they must show a high persistence with high chemical and thermal stabilities. And third, they should to be able to interact magnetically with transition metal ions or between themselves, which are indispensable conditions to enhance the magnetic interactions of the metal-organic nanoporous material and to build purely organic magnetic nanoporous materials, respectively. To fulfill all three conditions, we have initially designed a series of polychlorotriphenylmethyl (PTM) radicals substituted with two and three carboxylic functions (Scheme 1).
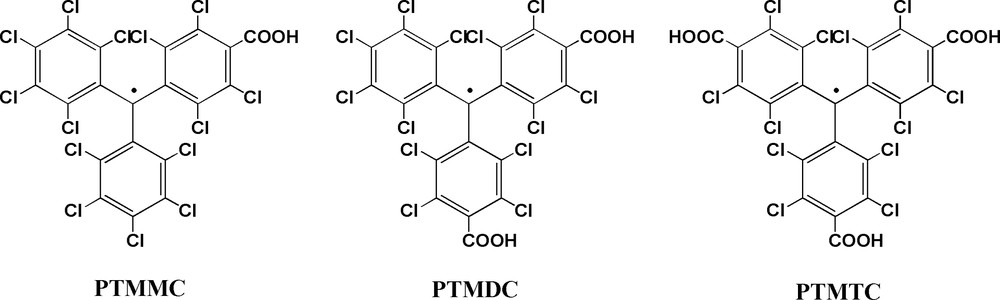
Carboxylic-based radicals: monocarboxylic (PTMMC); dicarboxylic (PTMDC); and tricarboxylic (PTMTC) perchlorotriphenylmethyl radicals.
First, from a geometrical point of view, PTMDC and PTMTC acidic radicals can be considered expanded versions of the isophtalic and trimesic acid molecules, respectively, where the benzene-1,3-diyl and benzene-1,3,5-triyl units has been replaced by an sp2 hybridized carbon atom decorated with two or three 4-substituted 2,3,5,6-tetrachlorophenyl rings. Therefore, according with their related trigonal symmetries and functionalities, PTMDC and PTMTC radicals are expected to yield similar open-frameworks structures than isophtalic and trimesic acid. Second, polychlorinated triphenylmethyl radicals have their central carbon atom, where most of the spin density is localized, sterically shielded by the six bulky chlorine atoms that increase astonishingly its lifetime and thermal and chemical stabilities [19]. Furthermore, the intrinsic molecular bulkiness and rigidity of this family of radicals is also expected to prevent a close packing of molecular units in the solid state. And third, the monocarboxylic PTMMC radical has allowed confirming the excellent conditions of PTM radicals to magnetically interact between them and with transition metal ions through the carboxylic groups. Indeed, a recently reported family of monomeric complexes using PTMMC radical has confirmed the feasibility of this type of carboxylic-substituted radicals to magnetically interact with metal ions [20] (Fig. 1). In the same line, we have also demonstrated the ability of PTM radicals to promote magnetic interactions through hydrogen-bonds, as revealed by the supramolecular arrangement of PTMMC radical, which forms hydrogen-bonded dimers in the solid state (Fig. 2) that endorse the transmission of weak ferromagnetic interactions [21].
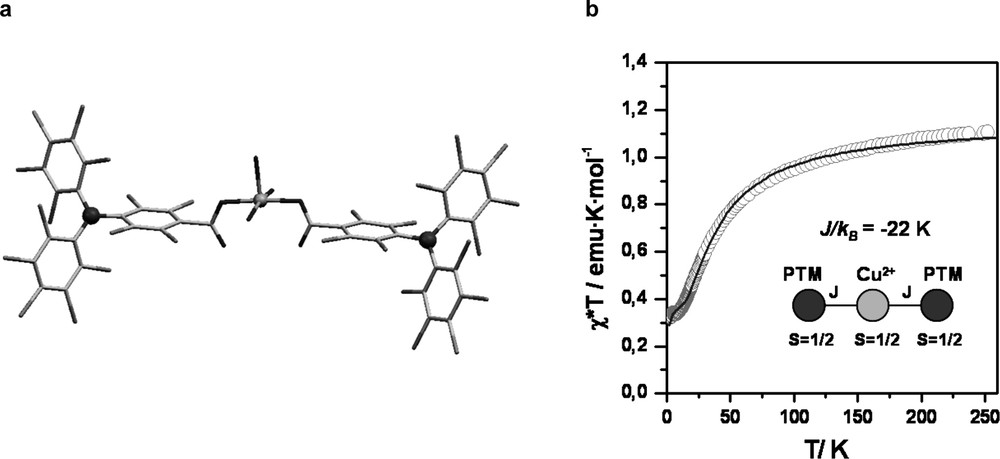
Mononuclear complex Cu(PTMMC)2(H2O)3. (a) Crystal structure. (b) χ·T value as a function of the temperature. Inset, linear trimer arrangement of the metal ion and organic radicals, in which an exchange coupling constant Cu(II)-PTMMC of J/KB = –22 K (H = –2 J S1 S2) was determined.
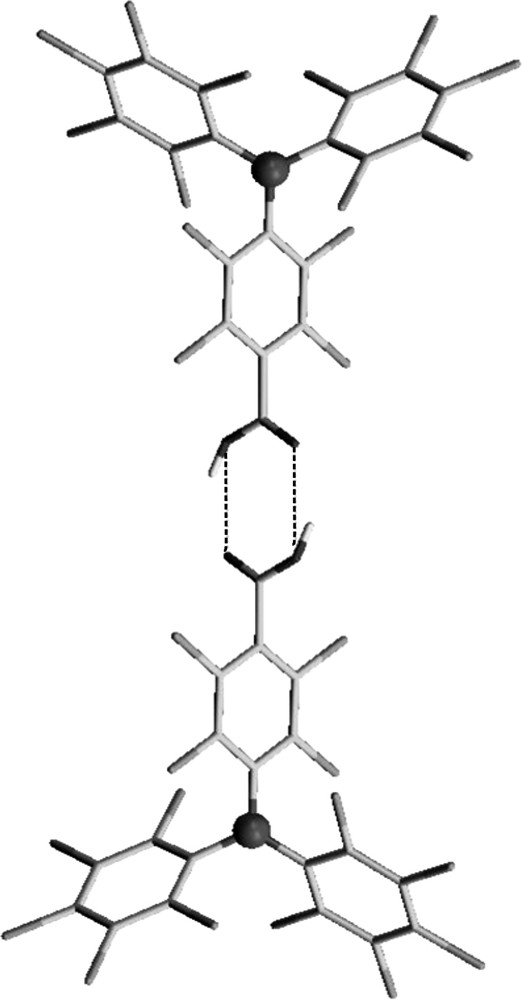
View of the hydrogen-bonded dimer of PTMMC radical in the solid state.
Thus, in this review, we will focus our attention to the recent results on the use of carboxylic-substituted PTM radicals for the obtaining of the first examples of metal-organic and purely organic molecular radical-based materials with combined interesting porosity characteristics and magnetic properties.
2 Paramagnetic organic ligands to design metal-organic radical open-frameworks (MOROF) with magnetic properties
The design of magnetic porous materials with large pore sizes still remains a challenge for chemists today. From a strictly structural point of view, as stated by Cheetham et al. [1], inorganic-based porous systems have basic limitations to show large pores owing to the relatively small sizes of the polyhedral metallic centers and their limited means of connection. This characteristic leads metal-organic molecular materials, made from the connection of 2-D, 1-D or 0-D ‘inorganic’-based components with multitopic organic ligands, to be one of the most promising strategies for the design of nanoporous molecular materials with large pore sizes. In this line, Yaghi et al. have already described a systematic approach for the design of nanoporous coordination polymers based on the use of long organic polyfunctional diamagnetic carboxylate ligands with different lengths and topologies [22]. However, even though this synthetic strategy has allowed the construction of coordination polymers with large pores, from a magnetic point of view, the use of long organic ligands is expected to decrease, or in the worst of the cases disrupt, the superexchange pathway. Indeed, magnetic coupling between transition metal ions in coordination polymers generally takes place through a superexchange mechanism involving the organic ligand orbitals, a mechanism that is strongly dependent on their relative orientation and, specially, in the distance between interacting ions, following an exponentially decreasing law. Therefore, the use of long organic ligands implies long-distances between paramagnetic metal ions, which dramatically lower their magnetic interactions and, in consequence, the overall magnetic properties of the material, becoming just paramagnetic without any magnetic ordering at a significant temperature. For this reason, the difficulty to obtain nanoporous materials with increasing pore size dimensions and simultaneous long-range magnetic properties remains a challenge.
To overcome such an inconvenience, in our group we have recently developed the new synthetic strategy based on the combination of persistent polyfunctionalized organic PTM radicals with COOH groups as polytopic ligands and magnetically active transition metal ions. The resulting structures are expected to exhibit larger magnetic couplings and dimensionalities in comparison with systems made up from diamagnetic polytopic coordinating ligands since the organic radical may act as a magnetic relay. This so-called metal-radical approach-combination of paramagnetic metal ions and pure organic radicals as ligating sites- has already been successfully used by several groups working on the field of molecular magnetism [23]. However, even though a large number of metal-radical systems have been studied, this is the first occasion that this approach is used for the obtaining of magnetic nanoporous materials.
Following this strategy, we have reported the first example of a metal-radical open-framework material, the coordination polymer [Cu3(PTMTC)2(py)6(EtOH)2(H2O)]n referred to as metal-organic radical open-framework (MOROF-1), that combines very large pores with a magnetic ordering [24]. As shown in Fig. 3, the crystal structure of MOROF-1 revealed a two-dimensional honeycomb (6,3) network with the central methyl carbon of the PTMTC ligand occupying each hexagon vertices, similar to those observed for some open-frameworks obtained with the trigonal BTC ligand and a linear spacer [25]. In our case, the linear spacer is composed of Cu(II) centers with a square pyramidal coordination polyhedron formed by two monodentate carboxylic groups, two pyridine ligands and one ethanol or water molecule. The correct arrangement of the honeycomb planes originates the presence of very large one-dimensional hexagonal nanopores, each composed of a ring of six metal units and six PTMTC radicals, which measure 3.1 and 2.8 nm between opposite vertices (Fig. 4). To our knowledge, these are one of the largest nanopores reported for a metal-organic open-framework structure so far. Moreover, complex MOROF-1 shows square and rectangular channels in one perpendicular direction to the nanopore direction, with estimated sizes of 0.5 × 0.5 and 0.7 × 0.3 nm, respectively. This porous structure originate solvent-accessible voids in the crystal that amount up to 65% of the total unit cell volume. This extremely large void volume is responsible of the exotic properties (vide infra) of this magnetic metal-radical open-framework material that behaves as a ‘sponge-like magnet’.
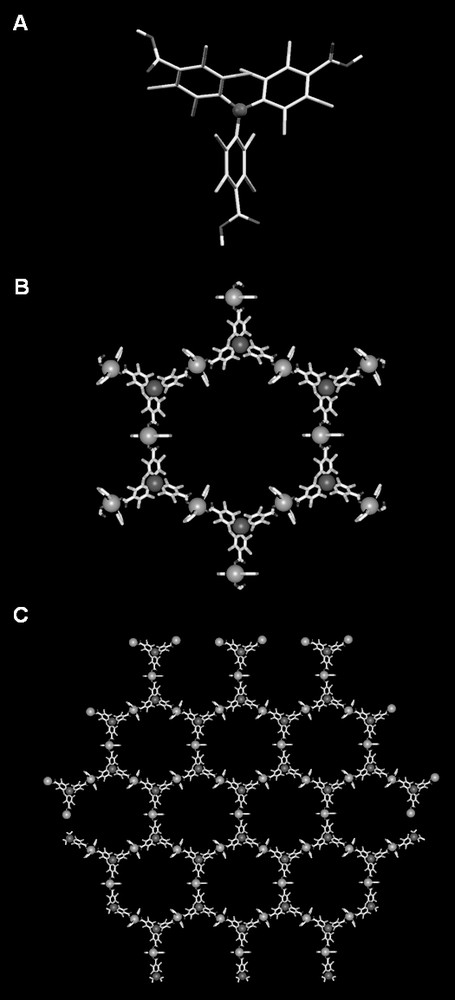
Crystal structure of MOROF-1. (A) Plot of the tritopic PTMTC radical. (B) Hexagonal pore formed by the connection of six PTMTC radical (situated at each vertices) and six Cu(II) ions. (C) 63 honeycomb-like sheet.
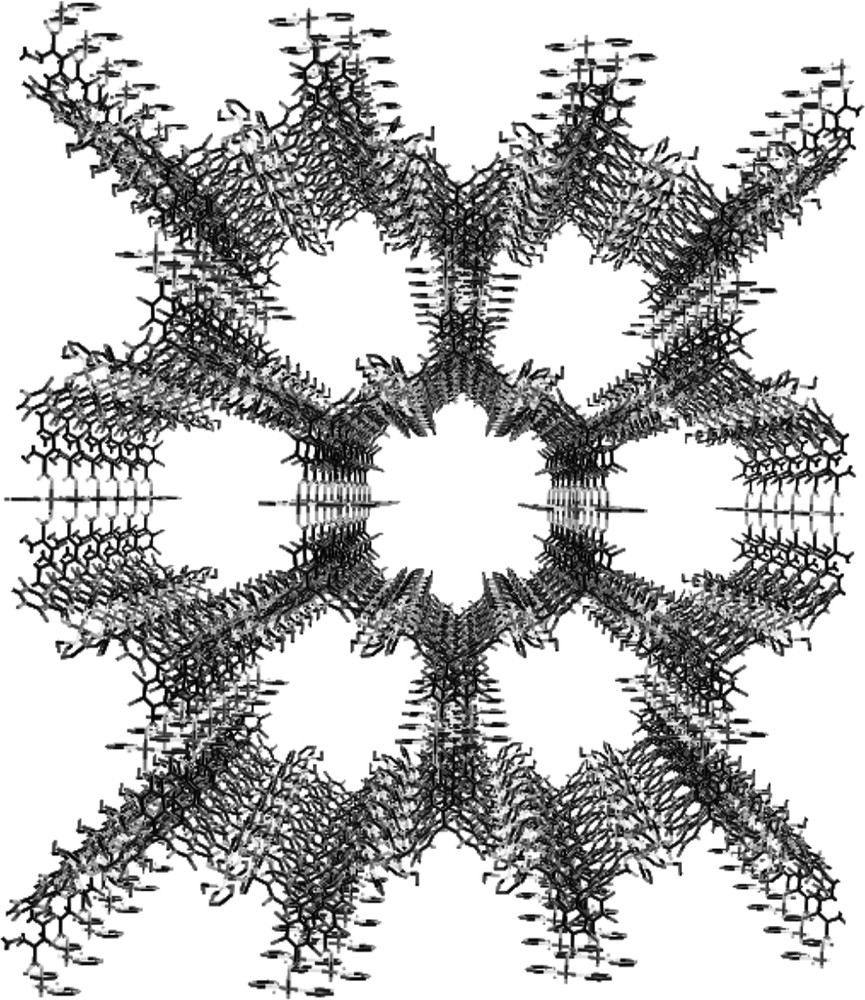
Illustration of the channel-like system of MOROF-1.
Paramagnetic Cu(II) ions in MOROF-1 are separated with long through-space distances of 15 Å within the layers and 9 Å between them, for which a non-magnetic coupling between metal ions should be expected. However, each open-shell PTMTC ligand is able to magnetically interact with all three coupled Cu(II) ions, and therefore, to extend the magnetic interactions across the infinite two-dimensional layers. As shown in Fig. 5, the smooth decrease of the χ·T value below 250 K is a clear signature of the presence of an antiferromagnetic couplings between nearest-neighboring Cu(II) ions and PTMTC ligands within a 2-D layer. The minimum of χ·T corresponds to a short-range order state where the spins of adjacent magnetic centers are antiparallel, provided there is no a net compensation due to the 3:2 stoichiometry of the Cu(II) ions and PTMTC radical units. The huge increment of χ·T at lower temperatures indicates an increase of the correlation length of antiferromagnetically coupled units of Cu(II) and PTMTC as randomizing thermal effects are reduced, either via in-plane long-range antiferromagnetic coupling and interplane dipolar–dipolar magnetic interactions. The magnetization curve at 2 K exhibits a very rapid increase, as expected for a bulk magnet, although no significant hysteretic behavior was observed. The magnetization value increases much smoother at higher fields up to a saturation value of 1.2 μB, which is very close to that expected for an S = 1/2 magnetic ground state. Thus, this molecular material can be considered as a ferrimagnet with an overall three-dimensional magnetic ordering at low temperatures (Tc ~ 2 K), which shows a behavior of a soft magnet.
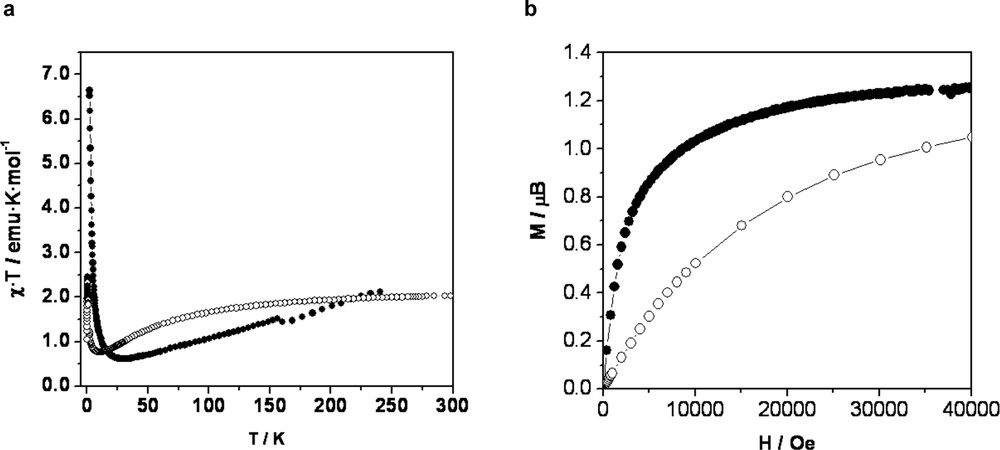
Magnetic properties of MOROF-1. (a) Value of the χ·T as a function of the temperature. (b) Temperature-dependence of the magnetization. As-synthesized (●) and evacuated (○) MOROF-1.
A second remarkable feature of MOROF-1 is the reversible ‘shrinking-breathing’ process experienced by this molecular material upon solvent uptake and release. Indeed, when MOROF-1 is removed from the solution and exposed to air, the crystalline material looses ethanol and water guest molecules very rapidly even at room temperature within a few seconds, becoming an amorphous material with a volume decrease of around 30% (Fig. 6). Even more interesting, the evacuated sample of MOROF-1 experiences a transformation recovering its original crystallinity and up to 90% of its original size after exposure to liquid or vapor EtOH solvent. The quasi-reversible ‘shrinking-breathing’ process experimented by MOROF-1 also occurs with MeOH, but not with other organic solvents, showing, therefore, a large selectivity for such two small alcohols. Thus, apparently MOROF-1 behaves as a sponge-like material that is selective towards MeOH and EtOH.
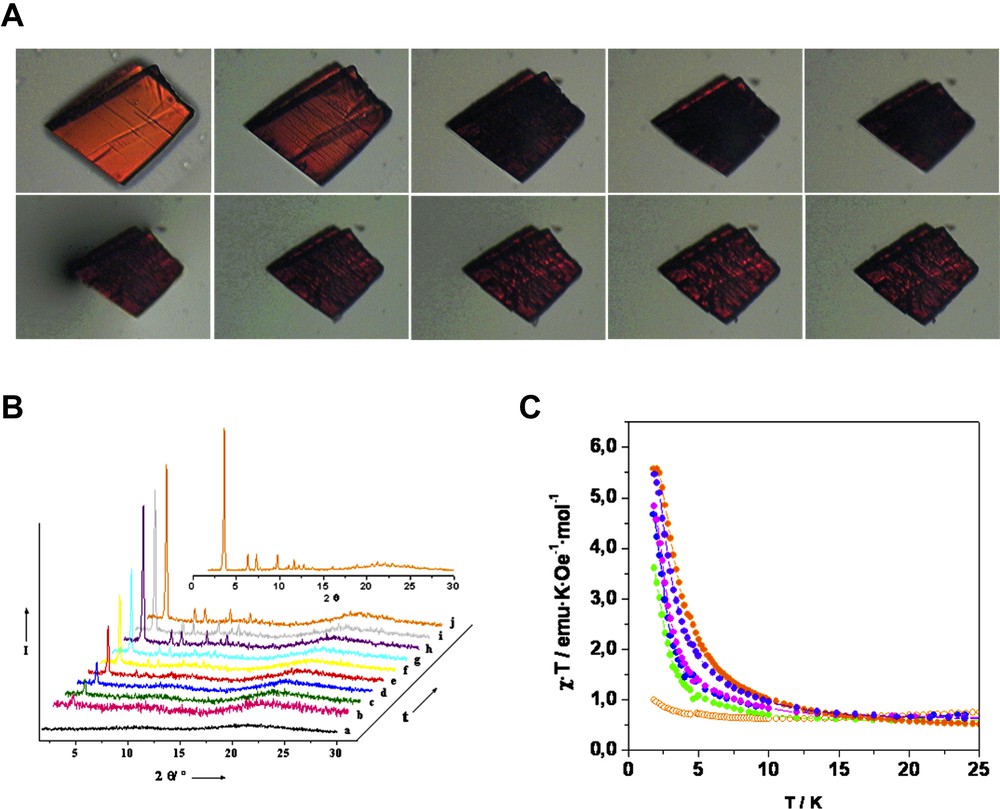
(Top) Real images of a single crystal of MOROF-1 followed with an optical microscope. The top series represents the “shrinking” process, in which a crystal of MOROF-1 exposed to the air experiments a volume decreases of around 30%. In the down series, the same crystal exposed again to ethanol liquid begins to swell. (Bottom left) Powder X-ray diffractograms (XRPD) of MOROF-1, showing the steps of transformation of the amorphous, evacuated phase in contact with ethanol vapors: a, amorphous phase; b, 0; c, 4.5; d, 5; e, 6; f, 8; g, 10; h, 26; i, 28; j, 52 h. Inset, XRPD of the as-synthesized MOROF-1. (Bottom right) Reversible temperature-dependence χ·T value behavior of the amorphous, evacuated phase in contact with ethanol liquid at 1000 Oe; amorphous phase (open orange circle), amorphous phase in contact with ethanol during 5 min. (green filled circle), 12 min. (blue filled circle), 30 min. (pink filled circle), 15 days (violet filled circle) and as-synthesized MOROF-1 (orange filled circle).
This chemical, structural and morphological quasi-reversibility is also accompanied by changes in the magnetic properties that are macroscopically detected. Magnetic properties of an evacuated amorphous sample of MOROF-1 shows a similar magnetic behavior to that shown by the as-synthesized crystals of MOROF-1 in the presence of liquid ethanol/water solvent, with two main differences: (a) the displacement of the χ·T minima from 31 K for as-synthesized to 11 K for the evacuated MOROF-1 samples and (b) the magnetic response of the solvent filled MOROF-1 sample at low temperature, where the long-range magnetic ordering is attained, results in a much larger (up to one order of magnitude) magnetic response than that of the evacuated MOROF-1 material. From these results it was inferred that the effective strength of the magnetic interactions for the evacuated sample of MOROF-1 were smaller than those observed for the as-synthesized crystals. According to this fact, the most striking feature exhibited by MOROF-1 is that the structural and chemical evolution of the material in the process of solvent inclusion can be completely monitored either by the magnetic and structural (X-ray) properties. When MOROF-1 is again reimmersed in ethanol solvent, a fast recovery up to a 60% of the signal can be seen during the first minutes, whereupon, the recovery of magnetic signal seems to be linear with the logarithm of time (Fig. 6).
More recently, using the same paramagnetic PTMTC ligand, we have obtained a second example of a magnetic and nanoporous MOROF, the Co(II)-based complex [Co(PTMTC)(4,4′-bpy)(H2O)3·6 EtOH·2 H2O]n (MOROF-2), which shows an unprecedented (63)·(69·81) topology with a paramagnetic behavior (Fig. 8) [26]. Indeed, MOROF-2 is formed by octahedral Co(II) ions linked by 4,4′-bpy spacers to generate monodimensional coordinative chains, where each metal ion is additionally bound to one carboxylate group of PTMTC radical (Fig. 7). Moreover, the remaining carboxylic and carboxylate groups of PTMTC connect each chain through hydrogen-bonds in a three-dimensional mode. This lets an unprecedented (63)·(69·81) topology, in where 63 hexagonal planes (each hexagon is defined by three PTMTC radicals and three octahedral Co(II) centers) are pillared by linear 4,4′-bpy ligands (Fig. 8A).
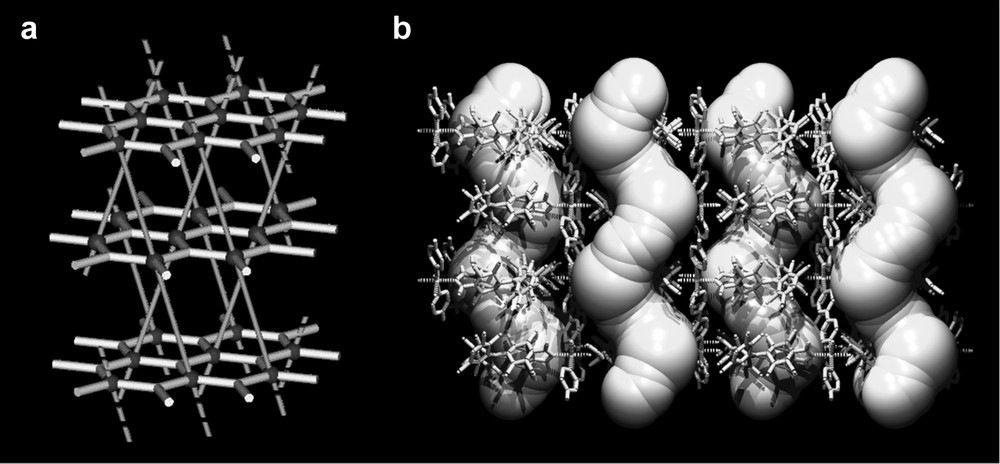
Crystal structure of MOROF-2. (a) Representation of the (63)·(68·81) topology. (b) View of the helical channel-like system.

Coordinative chains of MOROF-2, formed by the connection of Co(II) ions through 4,4′-bypiridine. One PTMTC radical is coordinated to each Co(II) ion.
This topology originates a partial overlap of the two-directional linked hexagonal plane nets and produces surprising helical nanochannels of dimensions 16.6 × 12.9 Å, with an effective size of 13.2 × 9.4 Å when van der Waals radius are considered. The calculated porosity reflects a void volume of 54.5% of the total volume cell (Fig. 8B). Additionally, such a porosity is combined with the existence of antiferromagnetic interactions, attributed to the magnetic interactions between Co(II) ions and PTMTC radicals.
3 Paramagnetic organic building-blocks to design purely organic radical open-frameworks (POROF) with magnetic properties
The excellent conditions of carboxylic-substituted PTM radicals to build nanoporous magnetic metal-organic materials prompted us to exploit the capacity of carboxylic moieties to form hydrogen-bonds for the use of these radicals as open-shell organic tectons. In this manner, it was possible the construction of the first examples of purely organic materials with combined nanoporosity and magnetic ordering. Thus, after notice the ability of PTMMC radical to interact ferromagnetically through hydrogen-bonds, we have used PTMDC and PTMTC radicals to obtain extended H-bonded networks, which, in principle, would show the combination of porosity and relevant magnetic characteristics.
These expectations were fully confirmed on the hydrogen-bonded self-assembly of PTMDC by the formation of a robust porous extended network (POROF-1, where POROF refers to as Pure Organic Radical Open-Framework) in the solid state that not only combines the presence of highly non-polar nanocontainers connected by polar narrow windows but also weak antiferromagnetic interactions that are visible at low temperatures [27]. Indeed, the molecular arrangement of PTMDC radical molecules creates a primary structure consisting of two-dimensional hydrogen-bonded sheets formed by two supramolecular synthons: the unusual hexamers of radicals hydrogen-bonded through one carboxylic group [R66(24)] and the typical [R22(8)] hydrogen-bonded dimers (Fig. 9A). These two-dimensional layers are then held together through several weak Cl····Cl contacts, leading a purely organic open-framework structure with one-dimensional nanochannels formed by narrowed polar windows and larger hydrophobic cavities, where a sphere 10 Å in diameter can fit inside them (Fig. 10). The smaller windows with a highly hydrophilic environment, due to the presence of the carboxylic groups at the inner walls, have a diameter of 5 Å, considering van der Waals radius. The combination of supercages and windows gives way to solvent-accessible voids in the crystal structure that amount up to 31% (5031 Å3 per unit cell) of the total volume (16,158 Å3). Furthermore, the fact that POROF-1 is composed of radical species leads a paramagnetic character to this open-framework structure, which shows weak antiferromagnetic interactions between radical units at low temperatures (Fig. 11).
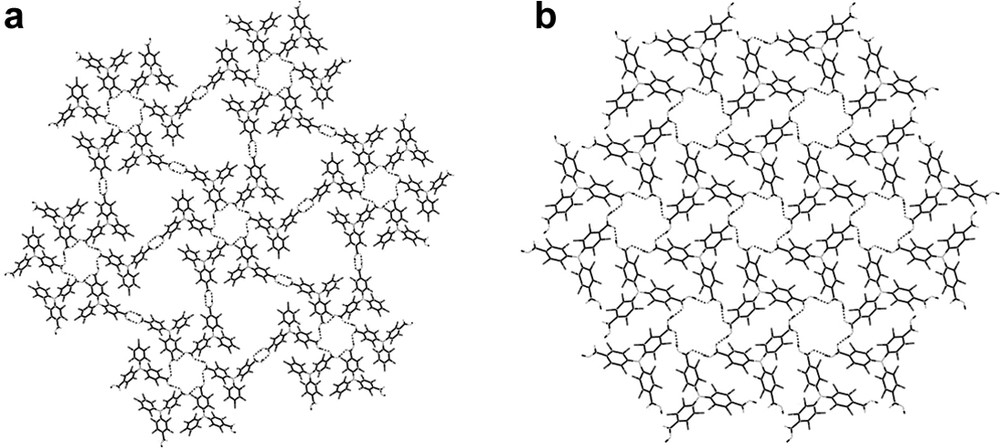
Hydrogen-bonded layers of (a) POROF-1 and (b) POROF-2.
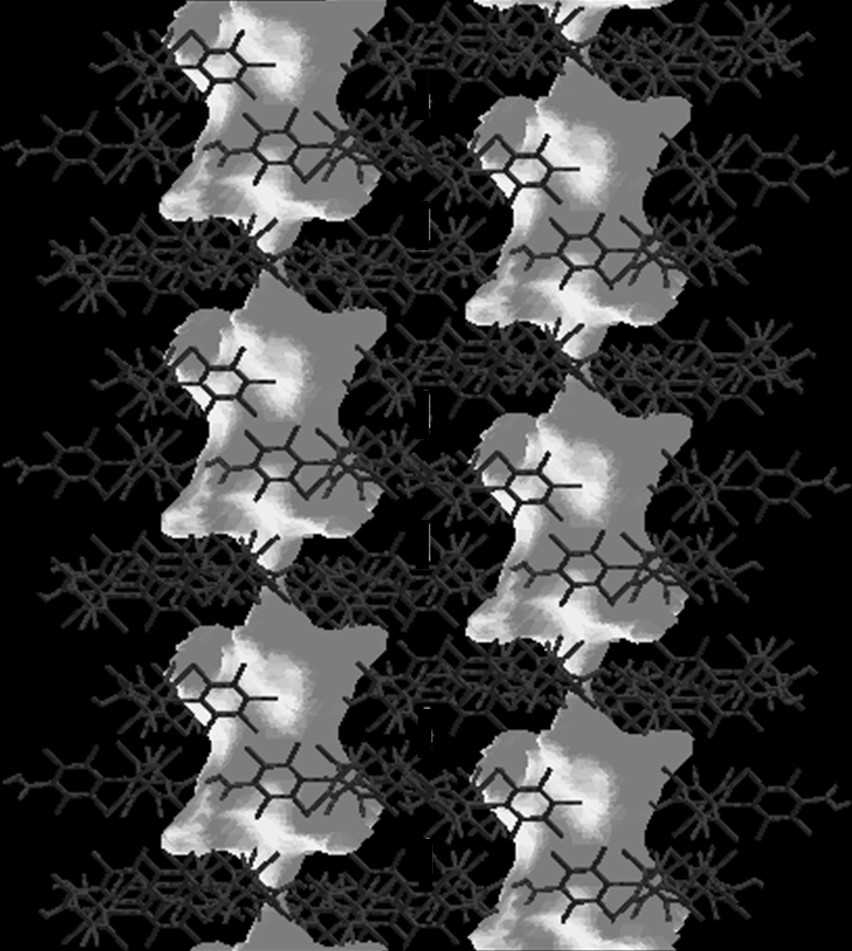
Illustration of the channel-like system of POROF-1, showing the polar nanocontainers separated by hydrophobic narrow windows.

Temperature-dependence of the magnetic susceptibility of POROF-1.
However, the most remarkable feature of POROF-1 is its high thermal stability ascribed to its high structural rigidity that permits to evacuate included solvent molecules without collapsing the packed molecules. Indeed, in the as-synthesized material, the large hydrophobic cavities are occupied by six n-hexane solvate molecules. Accordingly, when an as-synthesized sample of POROF-1 was heated up to 100 °C, the complete loss of all guest n-hexanes molecules was observed without any loss of crystallinity. Beyond this temperature, thermal analysis does not exhibit any significant change up to 275 °C, whereupon an abrupt weight loss, attributed to the decomposition, was observed. Such a thermal stability is highly notable and comparable to those observed for other stable supramolecular systems, such as multi H-bonded aggregates derived from the cyanuric acid and melamine [28].
More recently, the crystal packing of PTMTC molecules has also formed a robust porous extended network (POROF-2), which combines the presence of highly polar nanotubular channels without significant close-fittings and very interesting magnetic properties, such as magnetic ordering at low temperatures [29]. The molecular arrangement of such crystalline radical synthons also creates a primary structure consisting of two-dimensional hydrogen-bonded layers. As shown in Fig. 9B, the repetitive unit consists again of the non typical hexameric hydrogen-bonded motif formed by six molecules of PTMTC. Since every radical unit contains three carboxylic groups, each PTMTC molecule participates in the construction of three identical hexameric units that propagates along the ab plane (Fig. 9B). Remarkable is the fact that the stacking of layers generates a three dimensional structure that exhibits tubular channels, where a sphere of 5.2 Å in diameter can fit inside them (Fig. 12). In addition, such nanochannels are surrounded by a second set of small pores with a diameter of 3.3 Å. The combination of both originates solvent-accessible voids in the crystal structure that amount up to 15% (450 Å3 per unit cell) of the total volume. Interestingly, the location of carboxylic groups at the inner walls of the largest channels furnishes them with a highly polar and hydrophilic environment. This fact may stand for the lack of guest solvent apolar molecules within the nanochannels when crystallized from dichloromethane and hexane, as confirmed not only by X-ray crystallography but also by thermogravimetric studies and elemental analysis. Thermal gravimetric analysis of a few single crystals of POROF-2 showed no weight loss in the temperature range of 25–300 °C. On the contrary, as occurs for POROF-1, POROF-2 remains crystalline and stable up to 300 °C. Indeed, the powder X-ray diffraction pattern of a sample that was heated up to 300 °C shows that the positions and intensities of all lines remain unchanged when compared with the powder X-ray diffraction pattern of an as-synthesized sample. In fact, such stabilities for both purely organic materials result highly remarkable since most nanoporous organic materials so far reported incorporate guest solvent molecules that once eliminated, induce a collapse of the crystalline material.
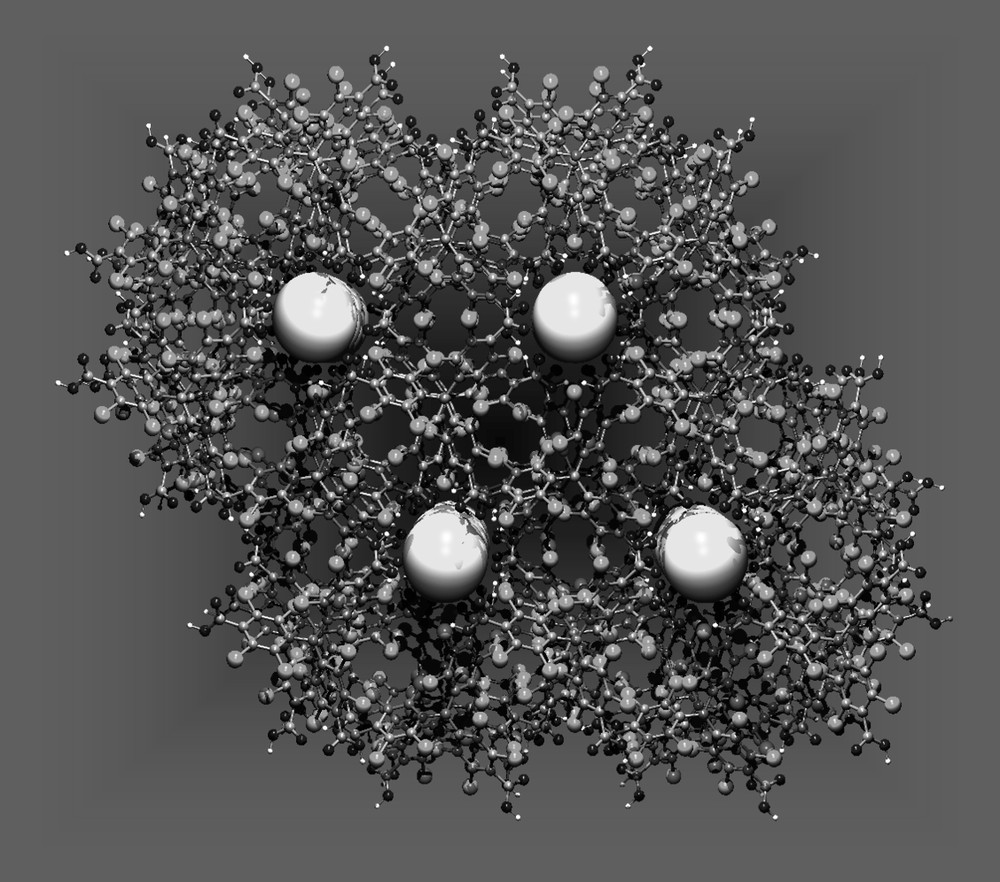
View of the channel-like system of POROF-2 filled with ideal spheres with 5.2-Å diameter.
Variable temperature magnetic susceptibility data for a crystalline sample of POROF-2 was obtained on a SQUID susceptometer, under a temperature range of 2–300 K and an applied magnetic field of 200 Oe (Fig. 13). The χ·T value at 300 K is 0.38 emu·K·mol–1, which is in agreement with the theoretical value expected for a non-interacting S = 1/2 spin in each molecule. Upon cooling, the χ·T value remains constant down to 5 K, whereupon the χ·T value increases according with the presence of weak ferromagnetic interactions. This behavior was fitted to the Curie–Weiss law with a Weiss constant of θ = +0.2 K. To investigate the existence of magnetic ordering at very low temperatures, variable temperature magnetic susceptibility experiments down to 70 mK were performed in a dilution cryostat (Fig. 13A). A considerable increase of the χ·T value up to a maximum peak around 110 mK was observed on cooling down below 2 K, which reveals the transition to a ferromagnetic ordered state at very low temperatures. The intensity of the peak decreases whereas its maximum shifts slightly to higher temperatures on increasing the external applied magnetic field. For instance, for an applied magnetic field of 20 Oe a value of 2.2 emu·K·mol–1 was obtained, whereas for an external field of 50 Oe the value is reduced to 1.4 emu·K·mol–1. This behavior originates in the saturation of magnetization for fields of few hundred Oe. Magnetization curves were measured above and below the critical temperature and are illustrated in Fig. 13. At 1.35 K, POROF-2 remains in the paramagnetic region, and therefore, the magnetization curve has a slight gradient. On the contrary, the curve at 80 mK, even if it is very close to the critical temperature, traces a hysteretic loop characteristic of a soft ferromagnet. The magnetization is almost saturated at about 400 Oe, and though the coercitive force is of the order of 50 Oe (see inset of Fig. 13B), the remanent magnetization at zero field is of about 35% of the saturation value.
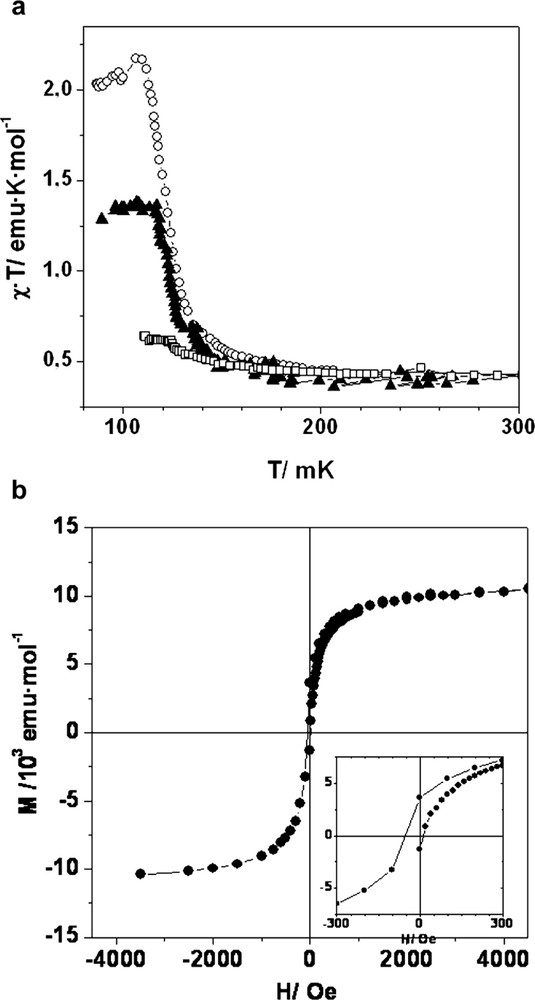
Magnetic properties of POROF-2. (A) χ·T as a function of temperature for different applied magnetic fields: empty circles (◯), H = 200 Oe; filled triangles (▲), H = 500 Oe; and empty squares (□), H = 1000 Oe. (B) Magnetization curves as a function of the applied magnetic field measured at 80 mK. Inset, detail of the hysteresis curve at 80 mK.
4 Concluding remarks
In this short review, we have demonstrated that perchlorinated triphenylmethyl radicals, properly functionalized with more than one carboxylic function, are excellent organic building-blocks for the preparation of magnetic nanoporous molecular materials. Three are the most important features of these radicals for such a purpose. First, carboxylic groups on these radicals have been shown as good superexchange pathways for attaining magnetic couplings. Second, taking advantage of this ability, their use as open-shell organic linkers has allowed to overcome the apparent contradiction in combining interesting magnetic properties and large pore sizes in metal-organic nanoporous materials. And third, the intrinsic good geometry and paramagnetic character of carboxylic-substituted PTM radicals give the opportunity to generate magnetic nanoporous zeolite-like materials with a purely organic nature.
The synergism of magnetic properties and nanoporous materials together with the molecular characteristics of coordination polymers and purely organic materials opens a new route to the development of Multifunctional Molecular Materials. For instance, along with the possibility to act as zeolite-like materials, magnetic POROF and, specially, MOROF materials with very large pores offer excellent conditions to encapsulate different functional systems with conducting, optical, chiral and NLO properties, among others. In consequence, the resulting material combines the magnetic properties of the framework and the inherent properties and applications of the protected functional molecules. Another field of research where this type of materials will have an enormous interest in the near future arises from their use as magnetic sensors. Indeed, for example, the enhanced magnetic response of a solvent filled sample of such material, when compared to that exhibited by an evacuated sample of MOROF-1, together with the reversible solvent-induced structural changes experienced by this sponge-like material, open the door to the possible development of magnetic sensors based on open-framework molecular magnets. Moreover, from more than 15 different solvents so far used, including several alcohols, such a reversible behavior has only been observed for EtOH and MeOH solvents; a fact that enhances the selectivity of the sponge-like magnetic sensor. Also one can imagine molecular (drug) delivering devices activated by external magnetic fields.
Actually, further work on this synthetic approach is underway in our laboratory. Further experimentation aimed not only at the generation of new magnetic and nanoporous molecular materials using PTMDC and PTMTC radicals but also at the obtaining of new organic building-blocks by the chemical modification of perchlorotriphenylmethyl skeleton. Thus, the use of both radicals with new metal ions and/or new additional ligands is expected to allow the design of novel architectures with interesting porosity characteristics, magnetic properties and surprising topologies. Furthermore, the possibility to functionalize different positions of the three phenyl rings of the PTM skeleton may allow the design of a whole range of carboxylic-substituted PTM radicals. In conclusion, such new radical units, along with PTMDC and PTMTC radicals, are expected to increase the new family of magnetic metal-organic (MOROF) and purely organic (POROF) radical open-frameworks.
Acknowledgments
This work was supported by Programa Nacional de Materiales of the Dirección General de Investigación (Spain), under project MAGMOL (MAT2003-04699) and Generalitat de Catalunya (2001SGR00362). D.M. is grateful to the Generalitat de Catalunya for a predoctoral grant. N.D. is also grateful to the Ministerio de Educación Cultura y Deportes (Spain) for the predoctoral grant No. AP2000-1842 of the FPU program.


