1 Introduction
Hindered amines (HALS) are a well-known class of powerful stabilizers that protect plastics from the negative influence of light and heat. Although there are ongoing discussions to explain completely the mechanism of stabilization, radical processes via the nitroxyl radical play an important role, for example, tetramethylpiperidine derivatives act as radical scavengers through transient formation of a nitroxylether [1].
Ciba Specialty Chemicals, with its substantial tradition in piperidine chemistry, has introduced a number of innovative products in the area of HALS chemistry:
- ● oligomeric or polymeric hindered amines and blends thereof contribute as excellent synergists to prolong long-term heat stability, e.g. in polypropylene and polyethylene;
- ● stable aliphatic nitroxyl ethers such as ®Tinuvin NOR 371 combine excellent light stabilizer activity with resistance to aggressive environments;
- ● low basicity is combined with excellent light stability in Tinuvin 123;
- ● nitroxyl radicals such as ®Prostab 5198 and Prostab 5415 are provided as process stabilizers in monomer destillation units and as chain stoppers to terminate polymerization reactions.
The present paper aims at reviewing the benefits of newly developed innovative nitroxyl chemistries beyond stabilization that are tailored as flame retardants, peroxide alternatives for degradation or grafting and initiators for controlled free-radical polymerization (CFRP) processes. Functional materials from CFRP are used for tailor-made modification of polymers and polymer blends.
The broad area of applications accessible by nitroxyl compounds can be adjusted by the structure and the substitution pattern adjoining the functional group. The chemical structure of the nitroxyl ether determines the stability of the N–O–C bond and consequently the thermal stability and the thermally induced split between the N–O or the O–C bond. However, the generation of radicals from nitroxyl derivatives is the key step in all these processes.
2 Nitroxylethers as flame retardants
Hydroxylamine ethers (nitroxylethers, NORs or alkoxyamines), such as Tinuvin 123, were first introduced as non-interacting UV-stabilizers with low basicity. Such stabilizers provide protection for automotive coatings and agricultural films even under severe conditions. In agricultural films pesticides generate acidic species and, therefore, deactivate conventional HALS. This problem is overcome by using NOR stabilizers.
Similarly flame retardants such as halogens or phosphorous compounds generate thermally or photochemically acidic species which result in inferior UV stability. This is solved by using NORs in combination with brominated flame retardants.
Surprisingly, it was found that the nitroxylethers do not only improve the UV stability but also contribute to flame retardancy. Even without any additional flame retardant it was demonstrated that NORs alone can achieve flame retardancy, e.g., in polypropylene fibers and films they pass industrial standards. For example, ®Flamestab NOR 116 provides flame retardancy at a concentration of only 0.5% passing the NFPA 701 fiber test [2] on polypropylene knitted socks [3].
Moreover, the addition of NORs as FR synergist can improve the efficiency of conventional brominated flame retardants as demonstrated in Table 1, where the combination of NOR and decabromodiphenyloxide surpasses the FR alone [4].
NFPA 701 (1996) test results in polypropylene fibers
| Additive | Weight loss (%) | Drip Burn Time (s) | Rating |
| None | > 40 | 50+ | Fail |
| 4% Decabromodiphenyloxide | 0.8 | 7 | Pass |
| 2% Decabromodiphenyloxide | 5 | 10 | Fail |
| 1% Decabromodiphenyloxide 0.25% Flamestab NOR 116 | 0 | 4 | Pass |
Therefore, it is possible to design with NORs flame retardant polyolefin molding compositions with lower levels of halogenated flame retardants and, in addition, to eliminate antimony trioxide, which is often used as FR synergist. Low FR concentrations and no antimony trioxide result in polymers with improved processability, better mechanical properties and reduced smoke density. For example, UL 94 V-2 rating is achieved in PP injection molded plaques at concentrations as low as 3.5% of formulations containing Flamestab NOR 116 [5].
The activity of the nitroxylether as flame retardant is based on the thermolysis of nitroxyl ethers which leads to the formation of either alkoxy and aminyl radicals or alkyl and nitroxyl radicals (Fig. 1). Aminyl and alkoxy radicals are very reactive and cause, on the one hand, degradation of polypropylene (and cross-linking of polyethylene). On the other hand, they are involved in the free radical chemical reactions during the combustion process [4].

Thermolysis of nitroxylethers.
In the synergistic combinations NORs can interact with brominated flame retardants and facilitate the release of bromine, consequently increasing the overall FR performance. Additionally, ignition of a polymer creates the formation of volatile combustion products, releasing heat during burning. This heat energy from the flame is then fed back to the polymer to sustain the burning process. It is believed that the thermolysis of NOR significantly reduces the amount of thermal feedback from the flame.
3 Nitroxyls for controlled degradation of PP
Peroxides are the current products of choice for controlled degradation of polypropylene (PP), for cross-linking polyethylene (PE) or elastomers and for grafting reactions. Next to safety and handling aspects, the use of peroxides does influence the material properties not only in the sense of the desired effect, but also leads to side reactions of the involved radicals (degradation vs. cross-linking, disproportionation, combination reactions), especially when high local concentrations of peroxide occur due to inhomogeneous mixing.
A combination of peroxides and nitroxyl radicals moderates the radical reaction, the grafting efficiency of unsaturated molecules is increased [6], and controlled rheology PP with improved mechanical properties is achieved [7]. It is reported, for example, that the impact strength of a controlled rheology PP is retained at a higher level by the combined use of peroxides and nitroxyl radicals.
However, peroxide-free processes are already much more attractive from handling and safety aspects. Tailor-made NOR based radical generators show a very high efficiency at processing temperatures above 250 °C, the common temperature for manufacturing PP fibers. The performance of the radical generator can reach a factor of 4–5 at 290 °C (Fig. 2) compared to peroxide used in this process (e.g., 2,5-dimethyl-2,5-di-tert-butylperoxyhexane = DTBPH). The resulting controlled degraded PP shows identical melt-flow properties with regard to molecular weight and molecular weight distribution basically indicating a similar mechanism as it is known for the peroxide process. As no peroxide is used, several inherent disadvantages of the peroxide process such as discoloration, odor or smoke are eliminated or considerably reduced. In addition, by using NOR radical generators the long-term thermal stability at the same antioxidant level is clearly increased (Table 2) and contributions to light stability are measurable.

Performance of NOR based radical generators in CR-PP manufacturing.
Polymer: Profax PH 350, 0.1% ®Irganox B 225 (stabilizer), 0.05% CaStearate. Extrusion: Twin screw, 100 rpm, 4 kg/h, N2; MFR according to ISO 1133. DTBPH: 2,5-dimethyl-2,5-di-tert-butylperoxyhexane.
Long-term thermal stability of CR-PP manufactured utilizing NOR radical generators
| Additive | MFR (230/2.16) | Days to embrittlement at 135 °C |
| None | 6.1 | 27 |
| 250 ppm DTBPH | 20 | 22 |
| 250 ppm NOR | 28 | 49 |
The proprietary product is supplied as concentrate under the trade name of ®Irgatec CR 76. Most attractive feature of the ready to use concentrate for the applicant is the improvement of the mechanical properties of the resulting meltblown fabrics. Compared to state of the art materials the hydrostatic head, describing the wet barrier of nonwovens [8], can be nearly doubled (Table 3). NOR based radical generators lead to a more uniform and controlled way of degradation, resulting firstly in a narrower molecular weight distribution of the controlled rheology PP compared to peroxidic degradation. Secondly, fibers with more uniform dimensions are manufactured, which results finally in improved mechanical properties of the fabric [9].
Properties of CR-PP and meltblown nonwovens by different processes
| Process | Mw | Mw/Mn | Hydrostatic head of meltblown nonwovens (mm) |
| Starting PP (MFR = 25) | 297 000 | 2.9 | – |
| Commercial peroxide grades | 170 000–195 000 | 4.8–8.6 | 450 |
| Irgatec CR 76 grade | 99 000 | 2.1 | 800 |
Trials using Irgatec CR 76 have proven its advantages in controlled degradation of PP at industrial scale, e.g., in manufacturing meltblown fabrics. Other potential applications using these NOR radical generators include polymerization initiation of vinyl monomers following classical kinetics, polyethylene cross-linking and rheology modification of polyolefins. For example, the combination of these NORs with multifunctional vinyl compounds allows the possibility to tailor a controlled molecular weight increase of PE (Table 4) and even of PP and to combine low melt flow with excellent mechanical properties [10].
Rheology modification of HDPE be Monomer/NOR combinations
| Additive | MFR (190/21.6) | Tensile impact strength (kJ/m2) | Tensilestrength (N/mm2) |
| None | 7.2 | 300 | 23.5 |
| 0.3% Pentaerythritol-tetraacrylate + 0.05% NOR | 0.6 | 360 | 25.8 |
4 Nitroxyls for controlled grafting onto polyolefins
Grafting reactions are used to attach functional molecules or side chains onto a polymer backbone in order to modify polymer properties such as polarity, adhesion and/or compatibility with other substrates. Free radical grafting is the most common process where combinations of monomers such as maleic anhydride and peroxides are used to manufacture modified PP and PE materials in extrusion processes at industrial scale. The disadvantage of such a process using peroxides is quite obvious as one has to cope with the competing reactions of grafting, degradation or cross-linking. For example, maleic anhydride grafted PP (PP-g-MAA) is obtained by the peroxide process either with a low maleic anhydride content and an acceptable molecular weight or then with a higher maleic anhydride content and a considerably reduced molecular weight. The latter consequently leads to loss of the typical mechanical properties. Lowering the processing temperature reduces the unwanted side reaction of degradation and, therefore, recently a grafting process in the solid state has been proposed in order to reduce the degradation [11]. However, to accomplish grafting of functional molecules by an extrusion process in an exact way and avoiding extensive degradation has still to be realized.
The NOR radical generators combine the advantages of high grafting yield and low degradation in the extrusion grafting process of PP. It results, e.g. in a maleic anhydride content similar or higher than state of the art but, at the same time, allows retention of the molecular weight (Table 5). The resulting grafted PP therefore demonstrates excellent mechanical properties. Processing temperatures as high as 230 °C can be used in this process. The unique advantage of a smooth radical formation at the processing temperature favors the grafting reaction versus degradation of the polymer.
Grafting of maleic anhydride (MAA) onto PP by NOR radical generators
| Grade/additive | MFR | Relative MAA content (%) |
| Commercial PP-g-MAA | > 200 | 100 |
| 0.5% Peroxide + 2.5% MAA | > 500 | 133 |
| 0.5% NOR + 2.5% MAA | 25 | 122 |
5 Nitroxyls for controlled polymerization and modification of polymers
Living free radical polymerization or CFRP combines the advantages of radical processes and the features of anionic polymerization. It opens up a new and versatile route to the synthesis of well-defined polymers and to the design of polymer structures with complex architectures [12–16].
Sterically hindered nitroxylethers (or combinations of radical initiators and nitroxyl radicals) are used to initiate polymerization and, at the same time, to terminate the growing polymer chain in a thermally reversible way, thus avoiding uncontrolled termination reactions. The polymer chain grows stepwise according to the equilibrium between ‘dormant’ chains and free radicals until the monomer is consumed (Fig. 3). A feature of CFRP is that the nitroxyl is present as end group in the oligomer or polymer and, therefore, is available for subsequent reactions (the usual way to synthesize block copolymers).
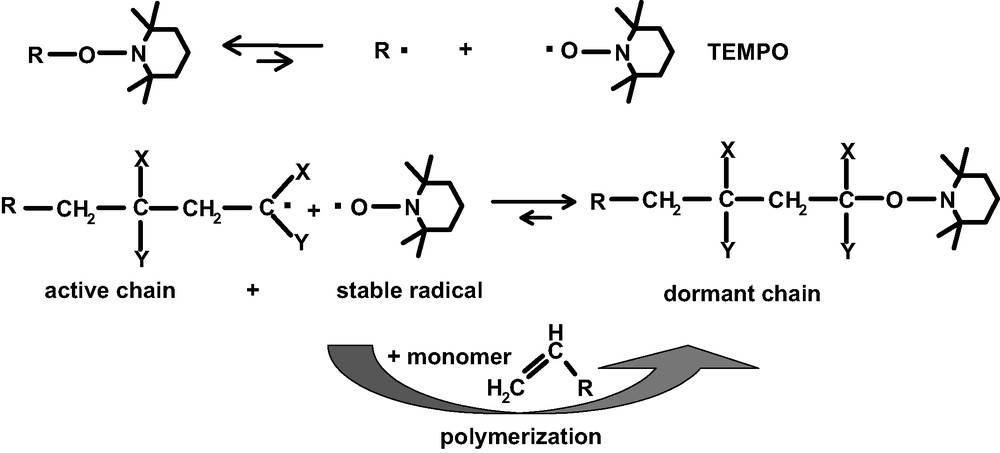
Mechanism of CFRP based on nitroxyl radicals.
The nitroxyl compounds used in CFRP such as 2,2,6,6-tetramethyl-piperidine-N-oxyl (TEMPO) are mainly based on sterically hindered piperidine derivatives. Moreover, the structure of the nitroxyl/nitroxylether plays a crucial role in the success of controlled polymerization. Bulkiness of substituents in the NO neighborhood makes the C–O bond considerably longer and weaker. Steric factors increase the reaction entropy. Polar factors can influence the bond dissociation energy [17].
By combining nitroxyl CFRP with grafting reactions, and the subsequent formation of comb polymer structures, a versatile method exists to modify polymer properties—either via radical or via condensation processes.
The grafting reaction of nitroxyl radicals onto unsaturated polymers, e.g., on EPDM by extrusion and subsequent polymerization in the presence of monomers has been previously demonstrated [10].
The grafted nitroxyl can be used for (a) re-initiation (macroinitiator) and for the subsequent synthesis of comb polymers or (b) polymer modifications simply by adding a second monomer in order to modify unpolar polymers like SBS or EPDM through polar molecules such as maleic anhydride or acrylates. The synthesis of comb polymers can be carried out either in the usual processes like bulk polymerization or by using the extruder in the sense of a cascade reaction depending on final requirements (monomer, length of the side chains).
As the CFRP mediated by nitroxyl radicals is based on the mentioned equilibrium between dormant species and active radicals, usual polymerization temperatures are in the range between 110 and 140 °C, depending on the nitroxyl derivative, the monomer and the process. At extrusion processing temperatures of 180 °C or above, the equilibrium between dormant species and free radicals is shifted to a high radical concentration which is sufficient for a high yield of grafting onto an unsaturated polymer, allowing the manufacturing of comb structures in a rather simple way (Fig. 4).
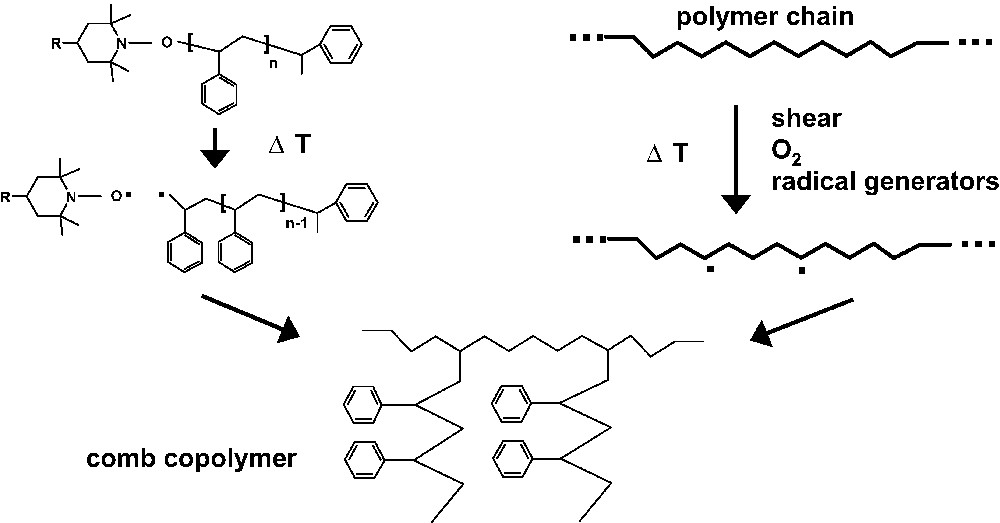
Formation of comb polymer structures from nitroxyl terminated oligomers/polymers by extrusion process.
As an example, a blend of EPDM and 10% of nitroxyl terminated polystyrene (Mn = 6400) is extruded (200 °C, 50 rpm, twin screw). During this process the molecular weight Mn of the starting EPDM is increased from 88 000 to 122 000 proving, together with extraction experiments, the formation of chemical bonds between EPDM and PS side groups. In comparison, the extrusion of EPDM and a commercial polystyrene containing no nitroxyl groups shows a decrease of the molecular weight of EPDM from 88 000 to 63 000.
A further advantage of a synthetic process for comb polymer structures via CFRP is that the length of the side chains can be adjusted as required (oligomers or polymers) in addition to possessing a narrow molecular weight distribution. Furthermore, a variety of random and block copolymers can be synthesized with nitroxyl termination and, therefore, used as potential reaction partners.
Preferred polymers for modifications by grafting oligomers/polymers via nitroxyl termination are materials with unsaturated units which already show a high grafting yield under mild conditions.
Polymers without unsaturation (PP, PE) can also be taken as substrate. However in this case adding additional radical generators improve the grafting yield.
The CFRP initiated and controlled by NOR molecules offers also the introduction of chemical functionalities, which is attractive if the functionality is located at the R group, so that end-functionalized oligomers and polymers of tailored molecular weight are accessible.
With an epoxide functionalized NOR (CGX PR 774) the polymer chain contains through the initiation step the epoxide group at one end (and the nitroxyl group at the other end). The epoxide terminated oligomer/polymer of defined molecular weight (to be calculated from initiator concentration and monomer conversion) and defined molecular weight distribution is reactive towards different chemical groups such as anhydride, acid or amine.
These end-functionalized oligomers, e.g. based on polystyrene, can be used per se as compatibilizers in polymer blends during compounding. It could be shown that, compared to unfunctionalized materials of the same type, the epoxy functionalized polystyrene leads to a decisive improvement of the mechanical properties of the blend. For example, the energy to break is improved by 250% and the elongation by more than 100% in a PPO/PA blend. As the reactive group of the polystyrene oligomer can react with the amino or acid functionality of polyamides the resulting chemical bond is believed to be responsible for the modification of the blend characteristics. Scanning electron microscopy pictures prove the influence of the reactive function on the morphology and clearly reveal the improved dispersion and smaller phase size resulting finally in the measured mechanical improvements [18,19].
Epoxy functionalized oligomers from CGX PR 774 can also be used in polymer analogous reactions to synthesize comb polymer structures of a fixed block length and of narrow molecular weight distribution. The reaction of maleic anhydride grafted PP with an epoxide terminated polystyrene takes place under extrusion conditions in a precise way and under mild conditions (Table 6). The molecular weight of the starting PP-g-maleic anhydride is increased as expected (and with increasing functionalized PS concentration). From the measured molecular weight increase it can be calculated that approximately three polystyrene side chains per PP backbone are grafted (and 4–5 grafted side chains at the higher functionalized PS concentration). The properties of the hybrid polymer reflect features of the parent polymers, e.g. improved stiffness compared to PP in the instance when PS is used as the reaction partner. The reaction of epoxide terminated styrene-acrylate random copolymers, instead of polystyrene homopolymers, introduces elastomeric properties and improves considerably impact values (Table 7, [20]).
Polymer analogous grafting of functionalized oligomer
| Mn | Mw/Mn | |
| PP-g-MAA starting material | 46 000 | 2.5 |
| PP-g-MAA + 10% epoxide terminated PS | 63 000 | 2.0 |
| PP-g-MAA + 20% epoxide terminated PS | 73 000 | 1.9 |
Polymer analogous grafting of functionalized oligomers
| MFR (190, 1.2) | Relative tensile impact strength | Relative tensile strength | Relative elongation | Relative E-modulus | |
| PP-g-MAA | 103 | 1 | 1 | 1 | 1 |
| PP-g-MAA + epoxide PS-co-PBuA 1 | 103 | 1.72 | 0.93 | 15.2 | 0.99 |
| PP-g-MAA + epoxide PS-co-PBuA 2 | 102 | 2.15 | 0.91 | 5.7 | 0.94 |
6 Conclusions
There are numerous applications of tailor-made hindered amines beyond the traditional stabilization areas in which the unique radical generator behavior of NOR molecules is decisive. Selected NORs have proven to be efficient flame retardants and flame retardant synergists in polyolefins. NOR based radical generators are superior alternatives to peroxides at high processing temperatures in controlled rheology polypropylene manufacturing. Nitroxyl radicals control degradation and polymerization processes efficiently and offer new ways to synthesize block polymers, comb polymers and complex polymer architectures. CFRP through functionalized NORs give access to new types of efficient compatibilizers and polymer modifiers.
7 Chemical structures
®Ciba ®Flamestab NOR 116.
®Ciba CGX PR 774 (R1 = proprietary).
®Ciba ®Irgatec CR 76: proprietary NOR compound.
Acknowledgements
The author wishes to thank the excellent scientists and technicians involved in generating and developing data presented in this review and supervising projects at Ciba Specialty Chemicals. Explicitly mentioned should be Jochen Fink and Michael Roth in Lampertheim, Germany and Nikolas Kaprinidis in Tarrytown, NY, USA.




