1. Introduction
In 2010, our group described the first nanoparticles of a molecule-based conductor containing a bis(dithiolene) complex, i.e., TTF[Ni(dmit)2]2 (TTF: tetrathiafulvalene, dmit2-: 2-thioxo-1,3-dithiol-4,5-dithiolato) [1]. Nanoparticles were precipitated from an acetone/acetonitrile solution containing an imidazolium-based ionic liquid, acting as growth controlling agent. Thereafter, TTF[Ni(dmit)2]2 nanoparticles exhibiting a mean diameter of 20 nm were obtained by electrodeposition on a platinum wire in the presence of an ionic liquid, acting as both supporting electrolyte and growth controlling agent [2]. In the series of bis(dithiolene) complexes, M(mnt)2 (M = Fe, Co, Ni, Pd, Pt, Cu, Au; mnt2-: maleonitrile dithiolate, Figure 1) have led to a wide variety of molecular conductors. Among them, Per2[Au(mnt)2] (Per: perylene, Figure 1) is a low-dimensional molecular conductor which continues to attract great interest due to unusual physical properties, such as anisotropic field suppression of the charge density wave [3], or superconducting ground state under pressure [4]. Per2[Au(mnt)2] is usually obtained by the electrocrystallization technique leading to single crystals as thin needles (several millimeters long and small cross sections, typically 0.05 × 0.02 mm2). This compound has also been grown as nano-sized needles on highly orientated pyrolytic graphite (HOPG), gold or platinum substrates [5]. At low current density conditions, nano-sized needles grow with the b-axis perpendicular to the substrate surface, following a homogeneous nucleation process, being controlled by diffusion. Using (001)-oriented silicon wafers, our group has reported the preparation of Per2[Au(mnt)2] nanowires [6]. Nanowires were grown in the anodic compartment of an H-type electrochemical cell using perylene and [(n-C4H9)4N][Au(mnt)2] as starting compounds. The oxidation of the perylene was performed at a constant current density of ∼0.30 μA⋅cm−2 at room temperature. Per2[Au(mnt)2] nanowires exhibited a semiconducting behavior in the 100–298 K range and a room-temperature conductivity value of about 0.020 S⋅cm−1.
Formulas for perylene (left) and Au(mnt)2 (right).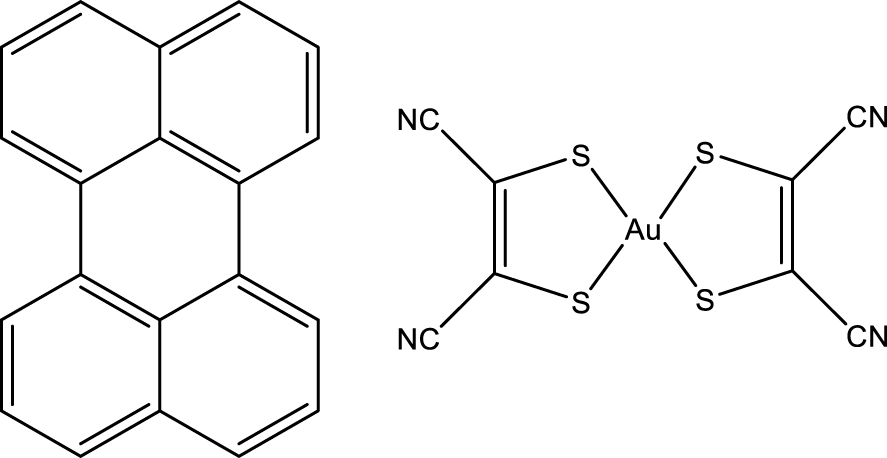
Amphiphilic molecules can control the growth of molecule-based conductors and allow the formation of nanoparticles [7]. In many cases, the molecule-based conductor contains at least one 5-membered heterocycle with two sulfur atoms. The amphiphilic 1-octanamine, N-(2-thienylmethylene) molecule (OATM, Figure 2) can afford two possibilities of interactions with the heterocycle, in favor of a better growth control of the nanoparticles: π–π interactions if the heterocycle contains unsaturation and S⋯S van der Waals interactions. Moreover, the long octyl chain can favor a better dispersion of the nanoparticles due to steric repulsions.
Formulas for OATM (above) and BIBS (below).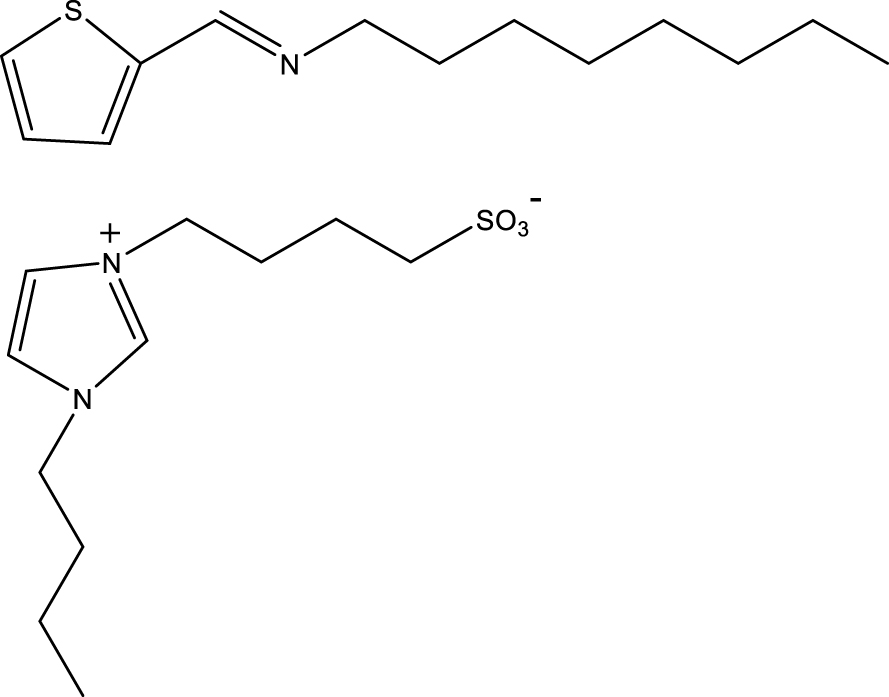
In this paper, we have explored the use of the amphiphilic OATM and the zwitterionic ionic liquid 4-(3-butyl-1-imidazolio)-1-butanesulfonate (BIBS, Figure 2) to control the growth of Per2[Au(mnt)2] as nano-objects.
2. Experimental section
2.1. Materials
Solvents are degassed immediately before use. Perylene, [(C2H5)4N]Br, sodium maleonitriledithiolate (Na2mnt), potassium tetrachloroaurate (KAuCl4), 2-thiophenecarboxaldehyde, octylamine, 1-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4], 1-butyl-3-methylimidazolium hexafluorophosphate [BMIM][PF6], 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl)imide [BMIM][(CF3SO2)2N] and BIBS are commercially available and used without further purification.
2.2. Characterization
Elemental analyses are performed by the Microanalysis Service of LCC-CNRS. Nuclear magnetic resonance (NMR) of OATM is performed in dimethyl sulfoxide-d6 at room temperature on a Bruker Avance 400 spectrometer. All chemical shifts for 1H are relative to tetramethylsilane. Infrared spectra are taken at room temperature (in KBr matrix) on a Perkin Elmer Spectrum GX spectrophotometer. Raman measurements are performed using a LabRAMHR800 (Jobin Yvon) set-up. The spectra are obtained at room temperature using the 632.8 nm line of a He–Ne laser. The incident beam is focused onto the sample through the ×100 optical microscope objective, giving a spot size of ∼1 μm2. The back-scattered light is collected through the same objective, dispersed (single-grating spectrograph, 1800 grooves⋅mm−1, focal length f = 80 cm) and then imaged onto a CCD detector (Andor DU420-OE). Using a laser power density of about 1.7 × 106 W⋅cm−2, no degradation of the material is observed. For transmission electron microscopy (TEM), the samples are sonicated in ether and placed onto a holey carbon-copper grid. TEM experiments are performed on a JEOL Model JEM 1011 operating at 100 kV. Powder conductivity measurements are carried out on pressed pellets of pure powder materials (size: 3.14 mm2 × 1 mm thick) without any grinding. The cylinders used to press the materials play the electrodes role. Resistance data acquisition is achieved using a Hewlett-Packard model 4263A LCR meter. Current–voltage (I–V) curves are acquired on an AFM Smarts SPM 1000 (AIST-NT) in conductivity mode using Au-coated cantilever tips (PPP-NCL Au-10 from Nanosensors, resonance frequency: 146–236 kHz, force constant: 21–98 N⋅m−1, tip radius: ∼10 nm). The particles are dispersed on a gold substrate previously cleaned with acetone, water, and carefully dried.
2.3. Syntheses
[(C2H5)4N][Au(mnt)2] is prepared by addition of [(C2H5)4N]Br to a water solution of Na2mnt and KAuCl4 at room temperature. Recrystallization is performed in a 1:2 (vol./vol.) mixture of acetone and isopropanol (yield: 35%; elemental analysis, calculated: C 31.7%; H 3.3%; N 11.6%, found C 31.7%; H 3.2%; N 11.4%). OATM is prepared as a pale yellow oil by the condensation reaction of 2-thiophenecarboxaldehyde and octylamine in toluene (yield: 91%; 1H NMR (ppm): 0.84 (3H, t), 1.23–1.30 (10H, m), 1.56 (2H, tt), 3.48 (2H, t), 7.12 (1H, dd), 7.43 (1H, dd), 7.62 (1H, dd), 8.43 (1H, s)).
The synthesis of Per2[Au(mnt)2] nanoparticles is performed in a classical H-shaped electrocrystallization cell equipped with two platinum wire electrodes (length L = 1 cm, diameter d = 1 mm). The cathodic compartment is filled with [(C2H5)4N][Au(mnt)2] (20 mg, 0.03 mmol) solubilized in a mixture of 8 mL dichloromethane and 4 mL acetonitrile. The anodic compartment is filled with perylene (30 mg, 0.12 mmol), [(C2H5)4N][Au(mnt)2] (40 mg, 0.07 mmol) and OATM (70 μL, 0.30 mmol, i.e. 2.5 molar eq. vs. perylene) [or BIBS, 78 mg, 0.30 mmol] solubilized in a mixture of 8 mL dichloromethane and 4 mL acetonitrile. The electrolysis is conducted at room temperature under galvanostatic conditions (∼10 μA⋅cm−2). The anodic solution is vigorously stirred during the entire electrolysis procedure ( ∼3.5 days). The air-stable black powder of Per2[Au(mnt)2] is collected by filtration from the anodic compartment (Scheme 1). Yield: 40%. Elemental analysis, calculated: C 58.7 %; H 2.5 %; N 5.7 %, found C 58.4%; H 2.2%; N 5.8%. IR (cm−1): 3055 (νCH ethylenic for perylene), 2222 and 2208 (νCN for mnt2- ligands), 1536 and 1512 (νC=C), 813 (νC−S).
Electrosynthesis of Per2[Au(mnt)2] nanoparticles (anodic reaction).
3. Results and discussion
The electrocrystallization technique under galvanostatic conditions and low current densities is the method of choice to grow single crystals of molecule-based conductors which are suitable for physical studies [8, 9]. In the absence of stirring of the solution, the crystallographic quality of the product is much better. When the electrosynthesis is conducted in the presence of an amphiphilic molecule or an ionic species bearing bulky long carbon chains, the electrode can be rapidly passivated. A vigorous stirring is then necessary to favor the access of the electroactive species to the electrode.
We have performed the oxidation of perylene in the presence of [(C2H5)4N][Au(mnt)2], acting as both reactant and supporting electrolyte, at 10 μA⋅cm−2 under stirring for 3.5 days. Transmission electron micrographs for the Per2[Au(mnt)2] shiny black powder collected from the anodic compartment evidence micro-sized crystals (Figure 3). We therefore note that the absence of a growth controlling agent in the solution is accompanied by the absence of size control of the crystallites at the nanometer scale.
Electron micrograph for Per2 [Au(mnt)2] in the absence of growth controlling agent (bar = 500 nm).
When the oxidation of perylene is conducted in the presence of [(C2H5)4N][Au(mnt)2] and OATM (2.5 molar eq./perylene) at 10 μA⋅cm−2 under vigorous stirring for 3.5 days, a black powder of Per2[Au(mnt)2] is isolated in the anodic compartment (see Section 2). Electron micrographs evidence nanocrystals exhibiting irregular shapes and sizes in the 35–100 nm range (Figure 4). Similar results are obtained for 5 molar eq. of OATM vs. perylene. The OATM molecule thus plays a crucial role in the control of the growth of Per2[Au(mnt)2] as nano-objects. We assume that a π stacking can occur between the thiophene group of OATM and the AuS2C4 ring of the mnt2- ligand. Furthermore, OATM molecules can adsorb onto the platinum electrode surface. Therefore, the germination process of Per2[Au(mnt)2] is more closely controlled at the electrode-solution interface, and the growth process is quickly blocked due to steric hindrance, leading to well dispersed nanocrystals. Finally, for higher current densities, namely, 40 μA⋅cm−2, both well-dispersed nanocrystals and aggregates are observed. A faster growth rate thus implies a poorer control of the state of dispersion of the nanocrystals.
Electron micrograph for nanocrystals of Per2[Au(mnt)2] grown in the presence of OATM (bar = 500 nm).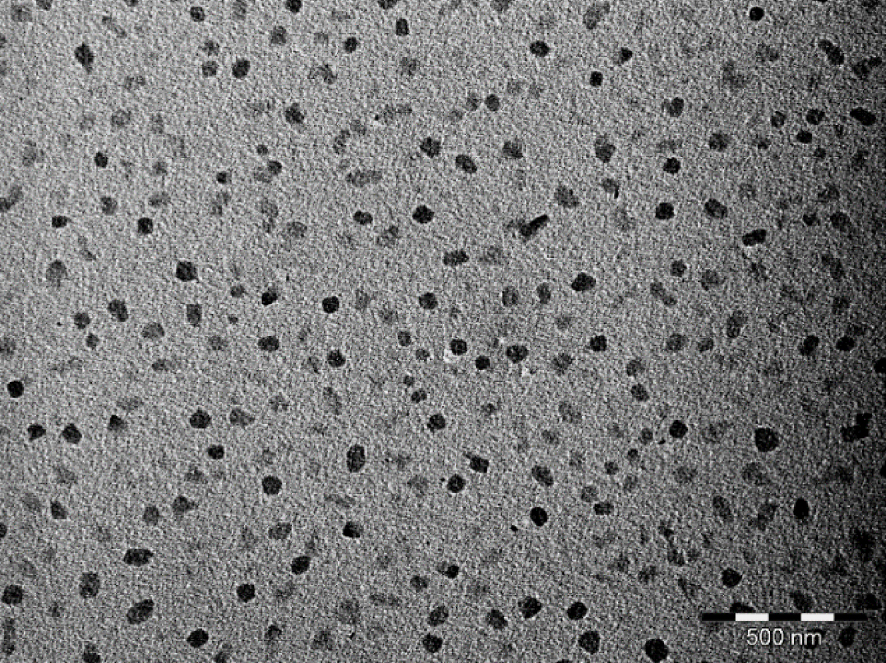
As said in the introduction, ionic liquids containing the 1-butyl-3-methylimidazolium cation, [BMIM]+, are the most often used as growth controlling agents for the preparation of molecule-based conductors as nanoparticles. At low temperatures, the ionic liquid organized into nonpolar microdomains in which the confinement of the growing species is more efficient, affording small particles. The electrochemical oxidation of perylene in the presence of [(C2H5)4N][Au(mnt)2] and [BMIM][X] where X- stands for , or (CF3SO2)2N- leads to Per2BF4, Per2PF6 or Per2[(CF3SO2)2N]. The counter anion of BMIM+ prevails over the [Au(mnt)2]- anion in the final product. To overcome this problem, we have therefore evaluated the use of the zwitterionic ionic liquid, i.e. BIBS (Figure 2), as both supporting electrolyte and growth controlling agent in the electrosynthesis of Per2[Au(mnt)2].
We have performed the oxidation of perylene in the presence of [(C2H5)4N][Au(mnt)2] and BIBS (2.5 molar eq./perylene) at 10 μA⋅cm−2 under stirring for 3.5 days. A black powder of Per2[Au(mnt)2] is isolated in the anodic compartment (see Section 2). Electron micrographs evidence well dispersed roughly spherical nanoparticles with sizes ranging from 10 to 40 nm (Figure 5). In comparison with OATM, the BIBS allows a better control of the morphology of the particles (spherical vs. irregular shapes). Furthermore, smaller particles are grown (10–40 nm vs. 35–100 nm). Per2[Au(mnt)2] nanoparticles obtained in the presence of BIBS are very similar to those previously described for another bis(dithiolene) complex, i.e., TTF[Ni(dmit)2]2 grown with [BMIM][X], as growth controlling agent [2]. The BIBS could play a double role in the controlled growth of Per2[Au(mnt)2] as nanoparticles. As for OATM, π–π interactions between the imidazolium cycle of the BIBS and the AuS2C4 ring of the mnt2- ligand can occur. Furthermore, at the electrode surface, the BIBS could stabilize the Per+ ion via electrostatic attraction with the sulfonate group.
Electron micrograph for nanoparticles of Per2[Au(mnt)2] grown in the presence of BIBS (bar = 200 nm).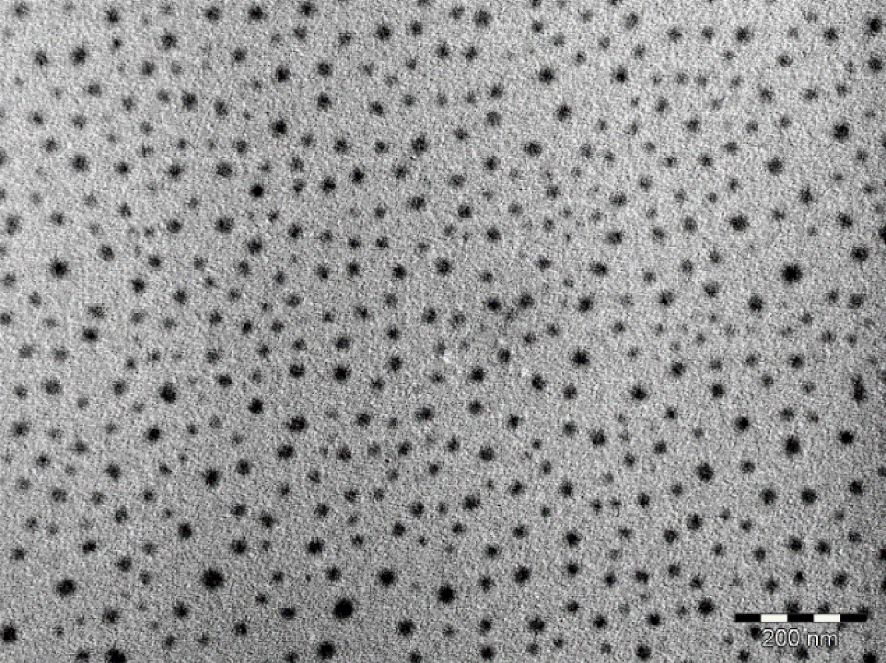
The Raman spectrum for Per2[Au(mnt)2] nanoparticles (Figure 6) is dominated by the C=C stretching modes for both the perylene molecules and the dithiolate ligands (in the 1320–1600 cm−1 range). Low frequency bands at 370, 518, and 800 cm−1 are assigned to Au–S stretching mode, AuS2C2 ring deformation mode, C–S stretching mode, respectively [10].
Raman spectrum for Per2[Au(mnt)2] nanoparticles.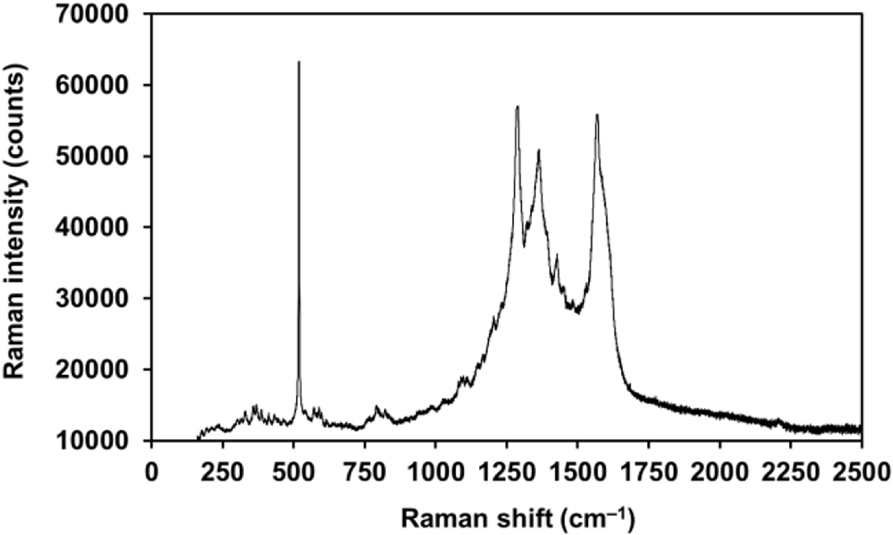
Per2[Au(mnt)2] single crystals present an electrical conductivity of 700 S⋅cm−1 along the stacking axis (b) at 25 °C [11]. For Per2[Au(mnt)2] nanoparticles described in this paper, the conductivity at room temperature is found to be of about 0.025 S⋅cm−1. This value is in agreement with those reported for TTF[Ni(dmit)2]2 nanopowders prepared in the presence of BMIM-based ionic liquids (in the 0.01–1 S⋅cm−1 range) [1]. The conductivity of a compressed pellet of a nanoparticle powder is the result of two main contributions: the conductivity of the particles (exhibiting random crystallographic orientations) themselves and the conductivity of the boundaries between them. The absence of a preferential crystallographic orientation of the particles within the nanopowder and the resistive boundaries between the particles explain a much lower conductivity value than that for single crystals. Figure 7 shows the I–V curve of an individual 40 nm high nanoparticle using conductive atomic force microscopy (sample prepared in the presence of BIBS). The deviation from the linear ohmic behaviour that would be expected for a metallic nanoparticle arises from the involved boundaries, such as tip-nanoparticle and substrate-nanoparticle. The observed energy gap is about 0.26 eV. A least-squares fit of the region corresponding to positive bias voltages (from 0.00 to 0.50 V) is shown on Figure 7. The fit is obtained using the Shockley diode equation:
Topographic image for Per2 [Au(mnt)2] nanoparticles prepared with BIBS (top), I–V curve for the particle indicated by the arrow (middle), least-squares fit of the region corresponding to positive bias voltages (down).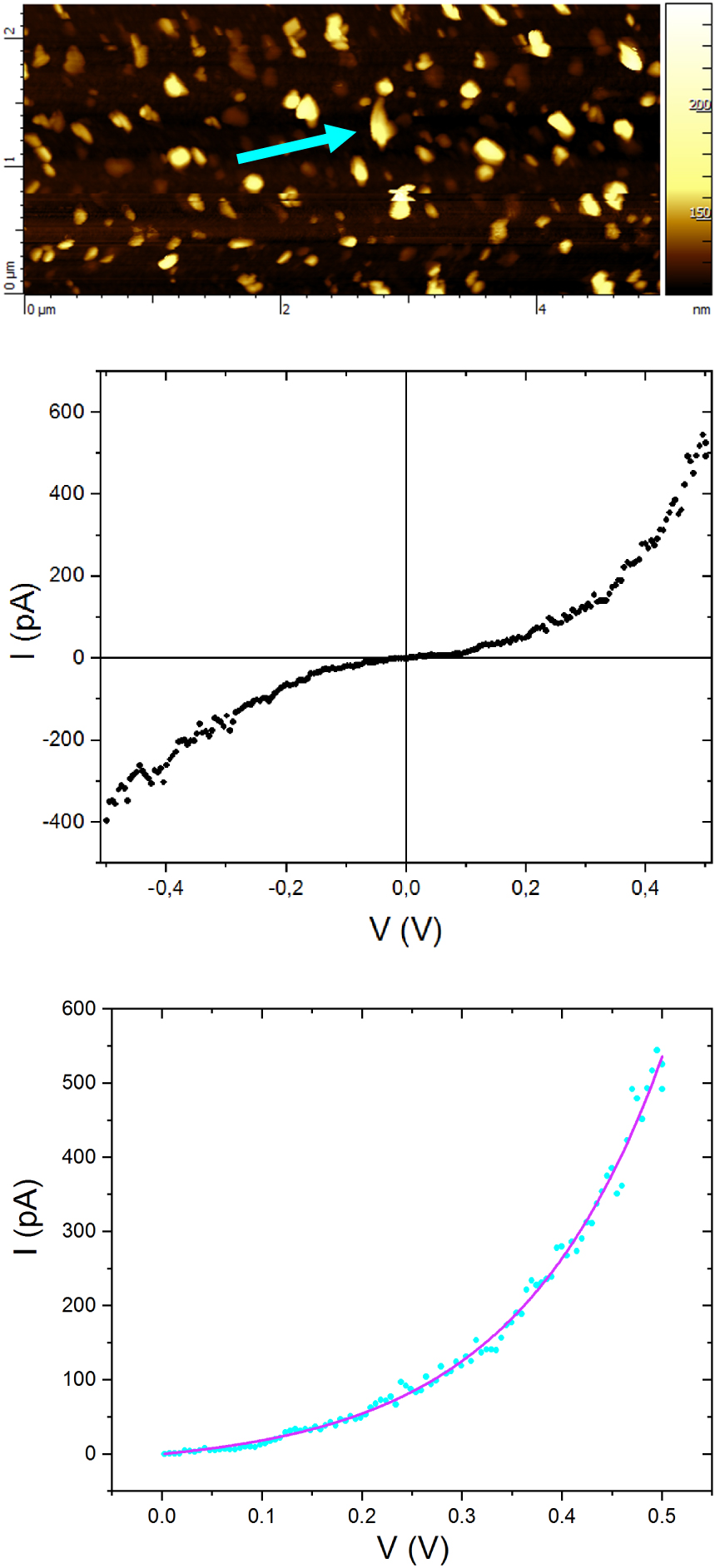
4. Conclusion
In this paper, we have described the first nanoparticles of the metal-organic conductor Per2[Au(mnt)2]. The growth controlling agents evaluated in this study, namely, OATM and BIBS, prevented the preparation of Per2[Au(mnt)2] as micro-sized crystals. Irregularly shaped nanocrystals are grown in the presence of OATM, whereas spherical nanoparticles are obtained if the zwitterionic ionic liquid BIBS is used. It has been reported that Per2[Au(mnt)2] exhibits a fairly good thermopower value of S = 40 μV⋅K−1 [12]. Moreover, the thermal conductivity 𝜆 is very low for organic-based conductors as nanoparticles [13]. As the thermoelectric efficiency of a material is evaluated by its figure of merit ZT in which S2 is in the numerator and 𝜆 in the denominator, Per2[Au(mnt)2] nanoparticles described in this paper could be interesting as one of the components for organic-based thermoelectric devices. Furthermore, our study opens the way to the potential preparation of nanoparticles of Per2[M(mnt)2] systems in which MIII is a magnetic center such as NiIII, PdIII or PtIII. Such systems as nanoparticles could be interesting for fundamental physical studies.



 CC-BY 4.0
CC-BY 4.0