1. Introduction
Menthol extracted from the leaves of the Mentha species has a typical minty flavor and has been widely used in many fields such as food, pharmacy, and daily chemical products [1, 2, 3, 4]. Menthol exerts its effects on cold receptors by interfering with the movement of calcium across the cell membrane, leading to a sensation of coolness, when it is applied to the skin or a mucosal surface [5, 6]. However, the main limitations on its applications are low water solubility and high volatility [7, 8, 9, 10]. To overcome these drawbacks, many menthol derivatives such as menthol formamide, menthol glyceryl ether, and monomenthyl succinate have been artificially synthesized [11]. However, not all of them are suitable for human use. For example, menthol formamide has a strong stimulating effect on the skin and menthol glyceryl ether loses significantly its function as a cooling agent. Among these derivatives, a well-known alternative to menthol is monomenthyl succinate, which has acceptable solubility and relatively low volatility; it also retains its typical minty flavor [12, 13].
Considering the scarcity of monomenthyl succinate in nature, its artificial synthesis becomes significant. All reported methods are as follows: esterification and alcoholysis (of acyl chloride, amide, and anhydride) [14]. Esterification leads to low reaction selectivity and yield as the succinic acid substrate has two acid radicals, which are easily esterified randomly to simultaneously produce diesters and monoesters. Furthermore, the complicated process of separation is another limitation on its application. Alcoholysis is a type of nucleophilic substitution reaction, and it is widely used for the synthesis of monomenthyl succinate. The potential substituents include succinyl chloride, succinic anhydride, and succinimide. The substituents with lower (succinimide) and higher (succinyl chloride) activity are both not suitable for production. The reaction efficiency of the former is very slow, and that of the latter becomes too active to control. Thus, succinic anhydride is made to react with menthol for the production of monomenthyl succinate due to its acceptable reaction efficiency, low price, and ease of control in the reaction process. So far, many basic organic catalysts have been reported to be used for the synthesis of menthol derivatives including tertiary amine, 4-dimethyl-aminopyridine (DMAP), pyridine, and so on. Among these catalysts, DMAP shows excellent catalytic performance and is the best candidate due to its superior nucleophilicity.
So far, all reported methods have employed free DMAP as the catalyst in homogeneous systems for the production of monomenthyl succinate. After alcoholysis, it is very hard to separate the free DMAP from the reaction mixture, which leads to the contamination of the product. Considering the toxicity of DMAP for human use, its residue in the mixture should be avoided completely to improve the safety of the product [15, 16, 17, 18, 19]. Immobilization technology provides a promising method to permit easy recycling and to simplify the design and performance control of the reactor. Generally, the carriers used in immobilization can be divided into organic and inorganic materials. However, their fragile nature and high cost limit the industrial application of organic carriers. Moreover, the sensitivity of the organic solvent is another significant limitation for the organic materials. On the other hand, the inorganic carriers consist of porous and nonporous materials. In this work, nonporous solids are employed as the carrier for DMAP immobilization to improve mechanical resistance, control the final particle size, and reduce the difficulty in recovery. More importantly, DMAP is immobilized on the outer surface of nonporous carriers, which can minimize internal diffusion problems. Considering that the specific surface area of a nonporous carrier is related to its diameter, the size of the carrier should be controlled at the nanometric scale to obtain a reasonable loading capacity. Nonporous nanoscale silicon dioxide is a cheap nanoparticle with excellent properties including suitable density, large surface area, good mechanical properties, and affinity for organic macromolecules [20, 21].
In this work, DMAP was covalently immobilized on the surface of nonporous nanoscale silicon dioxide by N-alkylation. The variable factors affecting DMAP immobilization were systematically studied. The obtained immobilized DMAP derivative was successfully used in the synthesis of monomenthyl succinate, and relevant reaction conditions were further optimized. Special attention was paid to the effect of the reaction system. A mixed solvent system consisting of cyclohexane and acetone was first introduced and successfully used in DMAP-catalyzed alcoholysis to produce monomenthyl succinate. High-polar acetone was used to improve the solubility of succinic anhydride and less-polar cyclohexane was employed to increase the activity of DMAP. Furthermore, this mixed solvent system was also an environment-friendly system considering its low toxicity and no health-based exposure limit of the solvents used [22, 23]. After the reaction, the immobilized DMAP derivative could be easily collected and reused to prevent its contamination of the product. In addition, a packed-bed reactor was employed to demonstrate the process flow of the continuous production of monomenthyl succinate, which provided a promising method to synthesize monomenthyl succinate in the industrial process.
2. Materials and methods
2.1. Materials
Succinic anhydride, menthol, chloropropyltrime- thoxysilane (CPTMS), and 4-methylaminopyridine (MAP) were obtained from Aladdin Chemistry Co., Ltd. (Shanghai, China). Nonporous nanoscale silicon dioxide (diameter 30 nm) was purchased from Qingdao Haiyang Chemical Co., Ltd. All other reagents and salts were of standard laboratory grade.
2.2. Preparation of chloropropyl-modified nano- scale silicon dioxide
Nanoscale silicon dioxide (4 g) was dispersed in ethanol/water mixture (4:1, v/v, 50 mL). Then, CPTMS (8 mL) was added and the mixture was stirred at 600 rpm for 24 h at room temperature. The resulting chloropropyl-modified nanoscale silicon dioxide was separated by centrifugation and washed at least four times with ethanol and deionized water. Then, the precipitates were dried at 50 °C in vacuum.
2.3. Preparation of immobilized DMAP derivative
The MAP (0.375 mmol) was dissolved in o-xylene (5 mL). Then, chloropropyl-modified nanoscale silicon dioxide (0.2 g), KI (0.12 mmol), and K2CO3 (0.29 mmol) were added to react at 130 °C for 20 h at 500 rpm in nitrogen atmosphere. Samples (20 μL) were taken from the reaction solution at regular time intervals to monitor the immobilization process by high-performance liquid chromatography (HPLC) [24]. After the reaction, the precipitates were collected by centrifugation and washed at least four times with ethanol, o-xylene, and deionized water. The obtained precipitates were dried at 60 °C for 12 h in vacuum.
2.4. Synthesis of monomenthyl succinate
Monomenthyl succinate was synthesized using the immobilized DMAP derivative in the mixed solvent. The cyclohexane/acetone mixture solvent (3:2, v/v, 5 mL), menthol (0.21 mmol), succinic anhydride (0.33 mmol), and immobilized DMAP derivative (100 mg) were added to react at 50 °C and 500 rpm for 20 h in nitrogen atmosphere. Samples (30 μL) were periodically withdrawn from the reaction mixture and centrifuged at 3000 r/min for 5 min. The samples were analyzed by high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS) [25]. The crude product was purified by recrystallization using absolute alcohol. After the reaction, the catalyst was recycled by filtration and drying.
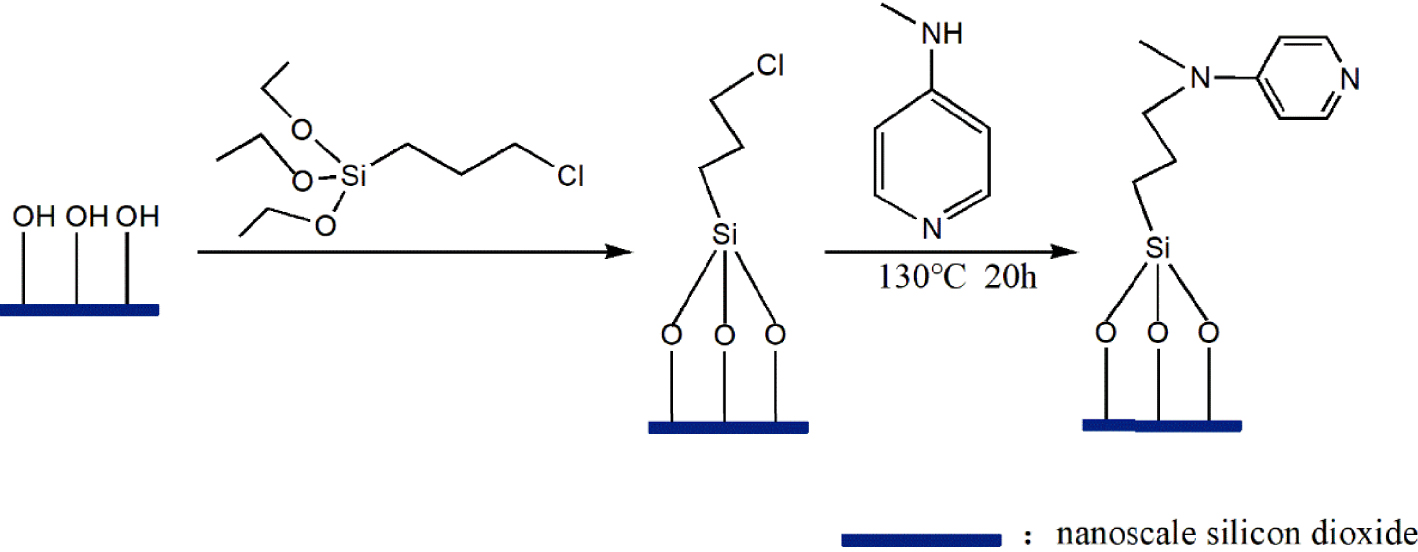
Route for the preparation of immobilized DMAP derivative.
2.5. Continuous synthesis of monomenthyl succinate
A process flow for the continuous production of monomenthyl succinate was systematically investigated using a packed-bed reactor. The immobilized DMAP derivative (200 mg) was added to the packed-bed reactor with inner diameter 20 mm (containing cotton). Menthol and succinic anhydride were dissolved in the cyclohexane/acetone mixture (3:2, v/v) at specific concentrations (0.11 mol/L and 0.192 mol/L), which were pumped into the reactor and cycled 60 times. After the reaction, the packed-bed reactor was washed by an eluent. The concentration of monomenthyl succinate was measured as described previously and the yield of monomenthyl succinate was calculated.
2.6. Catalyst characterizations
The immobilized DMAP derivative was characterized by thermogravimetric analysis using a thermogravimetric analyzer (STA 449 F3, Netzsch, Germany). This detection was carried out in nitrogen atmosphere at a consistent heating rate of 10 °C∕min from room temperature to 800 °C. Fourier transform infrared (FT-IR) spectroscopy was recorded on a Fourier transform infrared spectrometer (Frontier, PerkinElmer, America) in the range from 400 cm−1 to 4000 cm−1.
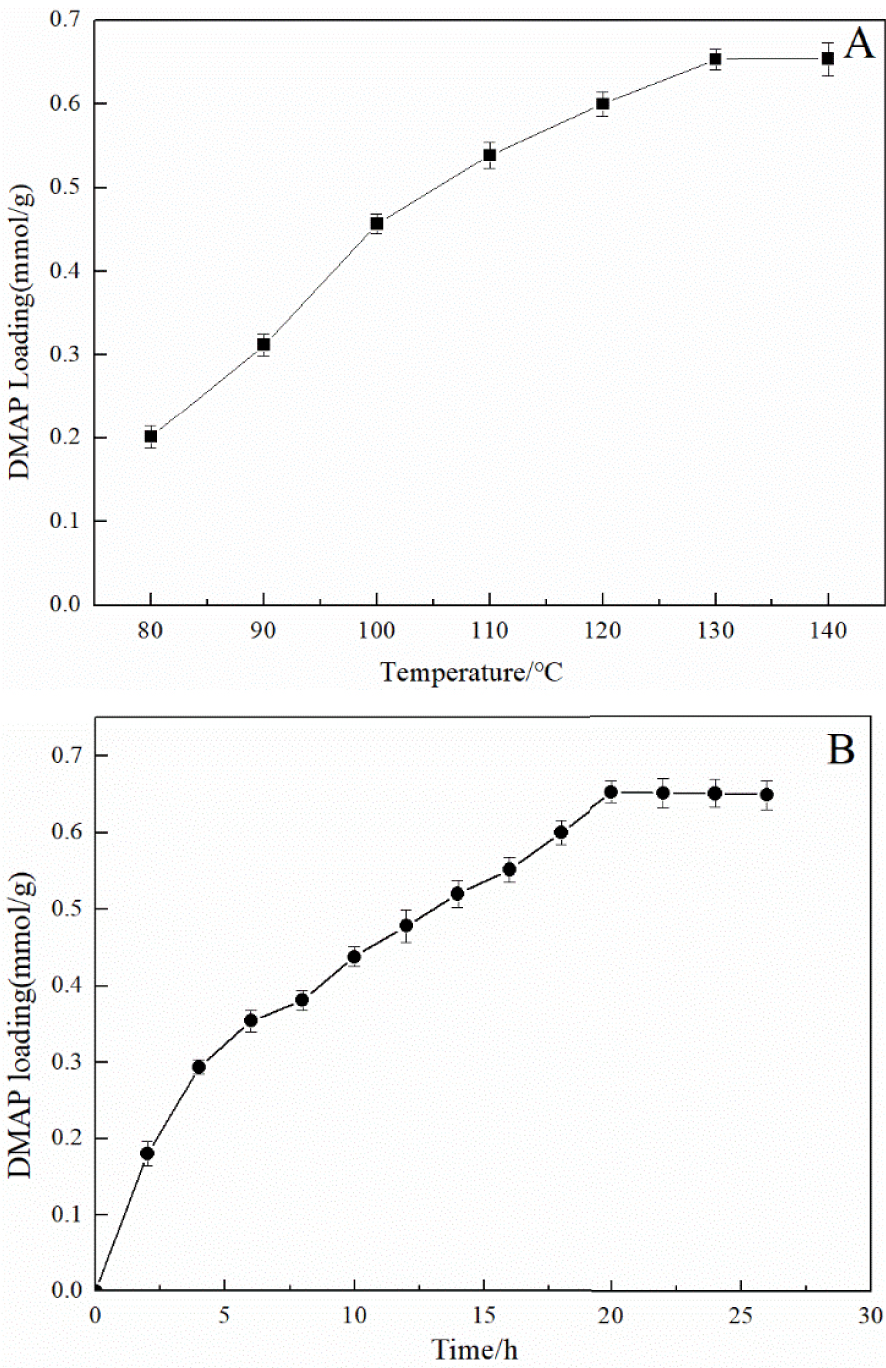
Preparation of immobilized DMAP derivative: the optimum reaction temperature (A) and time (B).
All data were the average values of triple experiments, and error bars represented the standard error of the mean.
3. Results and discussion
3.1. Catalyst preparation
The preparation of the immobilized DMAP derivative required two steps as shown in Figure 1. The first step was to synthesize the chloropropyl-modified nanoscale silicon dioxide by covalently coupling hydroxyl on the surface of nanoscale silicon dioxide particles using CPTMS. The second step was to prepare the immobilized DMAP derivative by the N-alkylation reaction of MAP and chloropropyl-modified nanoscale silicon dioxide. During immobilization, KI was used as the catalyst and K2CO3 was employed to create an alkaline environment, which enabled N-alkylation to be carried out smoothly. To study the optimum conditions for the synthesis of the immobilized DMAP derivative, the effects of reaction time and temperature were systematically investigated. As shown in Figure 2A, it was observed that for temperatures below 130 °C, the loading amount of DMAP increased with temperature. When the temperature exceeded 130 °C, the loading amount barely changed. As shown in Figure 2B, the loading amount of DMAP increased with time, while the DMAP loading amount did not significantly increase above 20 h. Consequently, the maximum loading amount of the immobilized DMAP derivative, 0.65 mmol/g, was obtained at 130 °C after 20 h.
3.2. Catalyst characterizations
The characterization of the immobilized DMAP derivative is carried out by thermogravimetric analysis/differential thermal gravimetry. As shown in Figure 3, the weight loss of the immobilized DMAP derivative is clearly divided into three stages. Region 1 includes the weight loss from 50 °C to 130 °C. It is observed in all cases and corresponds to the desorption of physically absorbed water. Region 2 includes the weight loss from 350 °C to 600 °C. It is observed in modified silica and the immobilized DMAP derivative and corresponds to the desorption of the coupled CPTMS [26]. Region 3 includes the weight loss from 150 °C to 350 °C. It is attributed to the decomposition of the immobilized DMAP derivative. This result confirms that the DMAP is successfully immobilized on the nanoscale silicon dioxide surface.
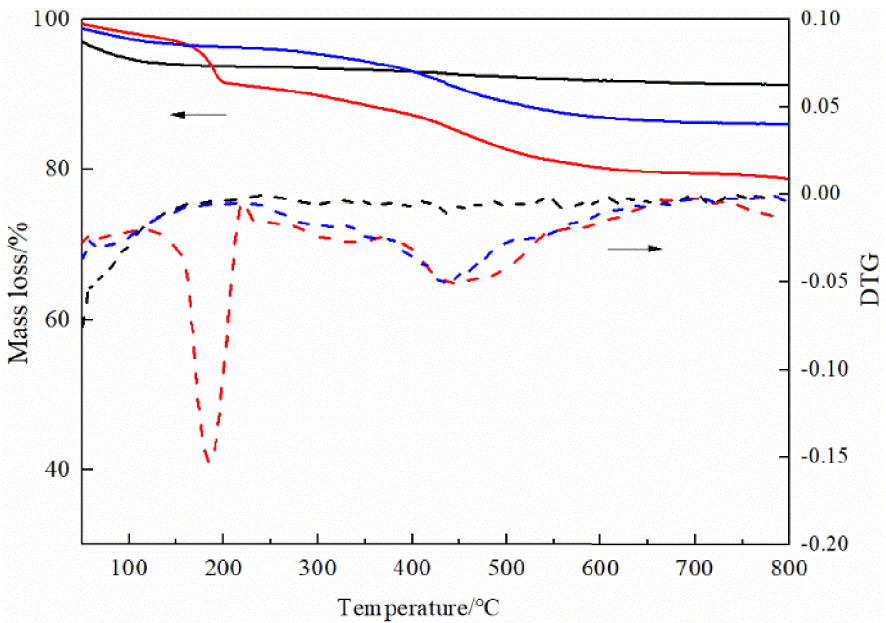
Thermogravimetric analysis (solid line) and differential thermal gravimetry (dashed line): nanoscale silicon dioxide (black line), chloropropyl-modified nanoscale silicon dioxide (blue line), and immobilized DMAP derivative (red line).
The results of FT-IR spectra are shown in Figure 4. In all curves, the peaks at 1100 cm−1 and 796 cm−1 are attributed to Si–O–Si asymmetric and symmetric stretching [27]. After the surface coupling of chloropropyl-modified nanoscale silicon dioxide, a new peak at 695 cm−1 is attributed to the stretching vibration of C–Cl groups; the asymmetric and symmetric stretching peaks of alkyl C–H appear at 2893 cm−1 and 2900 cm−1 [28]. The new characteristic peak at 1654 cm−1 after N-alkylation is attributed to the stretching of aromatic rings [29]. The analysis by FT-IR proved that DMAP was successfully immobilized on the nanoscale silicon dioxide surface.
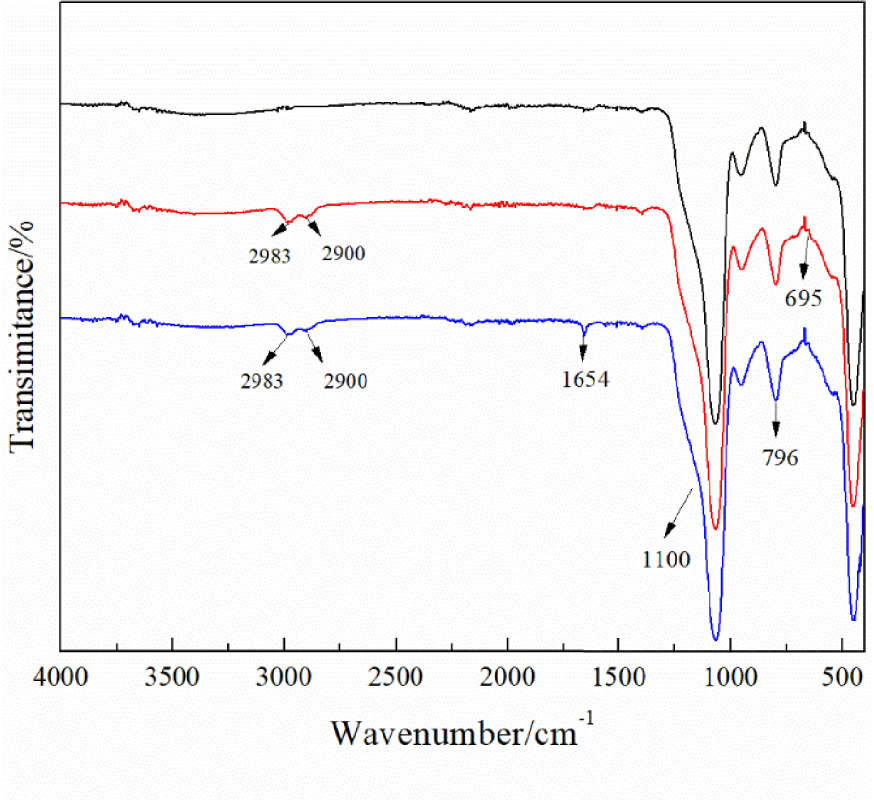
FT-IR spectra of nanoscale silicon dioxide (black line), chloropropyl-modified nanoscale silicon dioxide (blue line), and immobilized DMAP derivative (red line).
3.3. Immobilized DMAP derivatives on different carriers
To study the role of carriers in immobilization, several different carriers such as silica gel, MCM-41, and nanoscale silicon dioxide are used to immobilize DMAP. The loading amount of DMAP and the yield of monomenthyl succinate in three kinds of immobilized DMAP derivatives are systematically assessed in Figure 5.
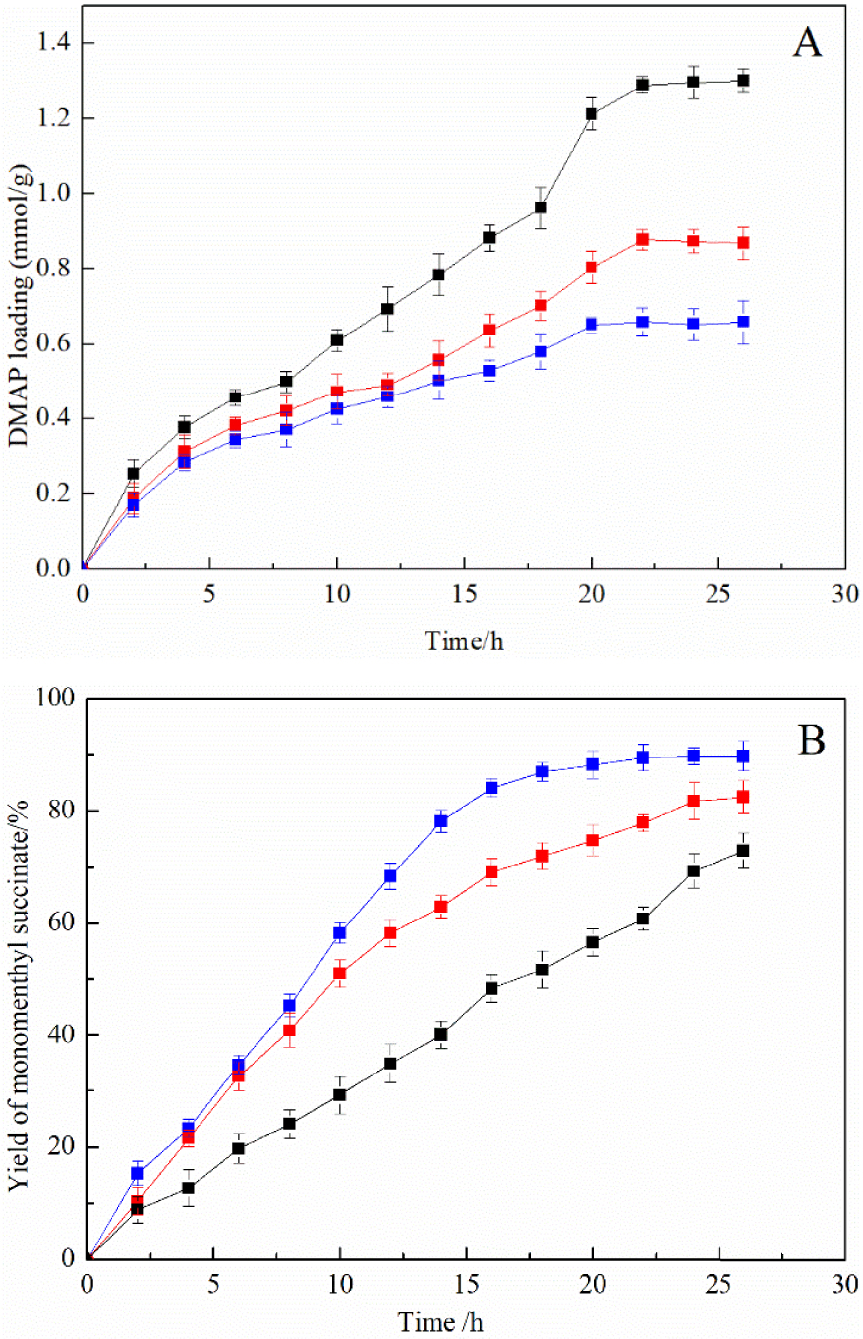
Effect of different carriers for the immobilized DMAP derivative on the DMAP loading amount (A) and the yield of monomenthyl succinate (B). Nanoscale silicon dioxide immobilized DMAP derivative (blue line), MCM-41 immobilized DMAP derivative (black line), and silica gel immobilized DMAP derivative (red line).
The comparison of the results in Figure 5A showed that the immobilized DMAP derivative with MCM-41 had the largest DMAP loading amounts followed by silica gel and nanoscale silicon dioxide had the smallest. This phenomenon could be explained by the carrier’s porous structure, which led to a large specific surface area and more amounts of hydroxyl on the surface. The specific surface areas of MCM-41, silica gel, and nanoscale silicon dioxide are 1500 m2∕g, 386 m2∕g, and 139 m2∕g, respectively [30]. The carriers with large specific surface areas had a better chance of interacting with active intermediates, which greatly increased the efficiency of N-alkylation [31]. Interestingly, it was found that the MCM-41 immobilized DMAP derivative with the highest catalyst loading showed the poorest catalytic performance as illustrated in Figure 5B. It might be explained that the porous structure was easy to generate internal resistance in the process of catalytic reaction. The pore of MCM-41 is an ordered hexagon, and the pore diameter can be regulated between 2 nm and 10 nm. The pore diameter of silica gel is 10.8 nm, and nonporous nanoscale silicon dioxide was used in this research. Therefore, the internal resistance increased with decreasing pore diameter, which significantly reduced the efficiency of alcoholysis.
Silicon dioxide is usually chosen as a carrier because of its low density and good dispersibility [32]. In this work, using a nonporous nanoscale silicon dioxide as a supporter to immobilize DMAP was an efficient way to solve the problem of internal diffusion. According to the Stokes–Einstein and collision theory, nanometric scale silicon dioxide with a small size can also increase the collision between reaction substrates and catalysts, which greatly improves the catalytic activity of catalysts [33]. The maximum yield of monomenthyl succinate reached 91.11% using a nanoscale silicon dioxide immobilized DMAP derivative as a catalyst. According to parallel experiment results, the maximum yield of monomenthyl succinate catalyzed by free DMAP reached 96.32%, and its catalytic efficiency was close to the immobilized DMAP derivative.
3.4. Influence of organic solvents on the yield of monomenthyl succinate
The solvent system was a significantly important factor that influenced the catalytic performance in the reaction. Therefore, finding the right solvent for a catalytic reaction, or determining how the solvent affected the reaction, was important [34]. The performance of six different solvents, cyclohexane, hexane, cyclohexanone, acetone, acetonitrile, and DMSO, was compared in the enzymatic synthesis of monomenthyl succinate. As seen in Table 1, the higher yield of monomenthyl succinate was synthesized efficiently in the less-polar solvent. This phenomenon could be explained by the proposed mechanism for the synthesis of monomenthyl succinate as described in Figure 6. DMAP is a powerful nucleophile catalyst that attacks the carbonyl carbon atom of succinic anhydride to form intermediate A.

Proposed mechanism for the synthesis of monomenthyl succinate.
Effect of different organic solvents on the yield of monomenthyl succinate
| Organic solvents | Solvent polarity | IDLH (mg∕m3) | Yield |
|---|---|---|---|
| Cyclohexane | 0.06 | 35000 | 58.3 |
| Hexane | 0.1 | 18000 | 34.5 |
| Cyclohexanone | 5.3 | 20000 | 35.6 |
| Acetone | 5.4 | 48000 | 35.3 |
| Acetonitrile | 6.2 | 6800 | 25.3 |
| DMSO | 7.2 | — | 10.2 |
During the nucleophilic addition of alcohols to intermediate A, the pyridine-linked acyl carbon atom of intermediate A underwent a nucleophilic attack by the hydroxyl of menthol to form an unstable intermediate B. The latter released DMAP to form monomenthyl succinate, which is also the rate-limiting step [35].
The amine atom of pyridine in DMAP was easily polarized and generated a negative charge. It might interact with the high-polar solvent containing some electrophilic group such as carboxyl, carbonyl, and so on. Therefore, a high-polar solvent might weaken the nucleophilic offensive capability of DMAP, compete with succinic anhydride, and then hinder the formation of intermediate A. On the other hand, the electrophilic group in high-polar solvents (such as keto-carbonyl) might interact with menthol, hindering the formation of intermediate B. Theoretically, low-polarity solvents were better for this catalytic reaction [36]. However, the use of these solvents with lower polarity was limited by their very poor solubilization of the substrate succinic anhydride. Herein, the mixture solvent system that significantly improved the efficiency of the catalytic reaction was introduced to synthesize monomenthyl succinate. The mixture consisted of acetone of high polarity and cyclohexane of low polarity.
As seen in Table 1, the larger numerical value of Immediately Dangerous to Life or Health concentration (IDLH) represents higher safety of the solvent. According to the GHS classification standard for acute toxicity of chemicals, both acetone and cyclohexane have the lowest grades with the highest safety [37]. In this mixture solvent system, acetone and cyclohexane were promising candidates for environment-friendly solvents due to their low toxicity and solubility in water to assist biodegradation. Phase resistance was avoided and the activity of the whole system was enhanced because of the good miscible property of these two solvents to maximize the reaction efficiency. As shown in Figure 7, the optimal volume ratio of the cyclohexane/acetone mixed solvent system is confirmed as 3/2.
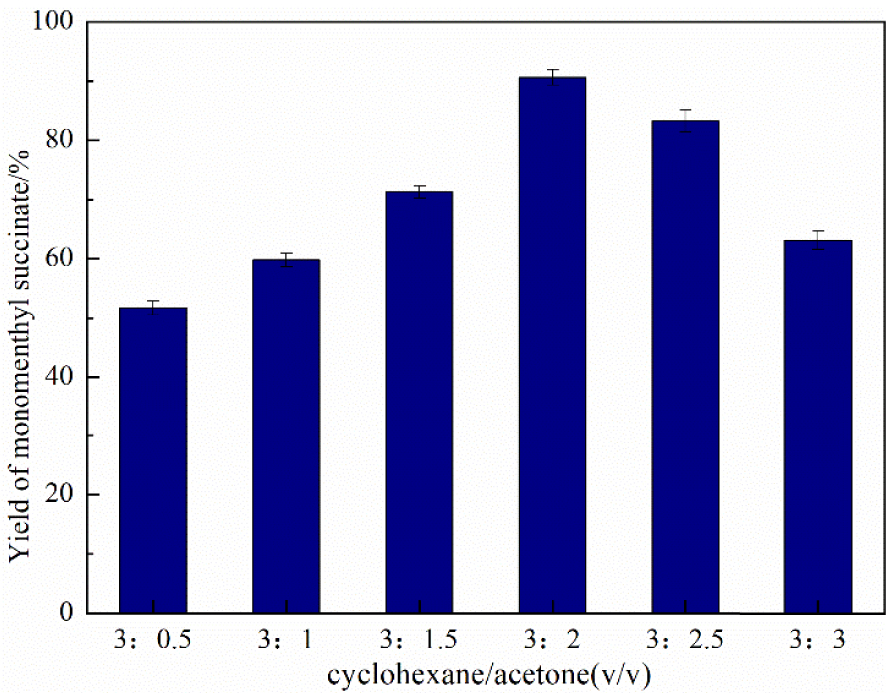
Effect of different cyclohexane/acetone volume ratios using immobilized DMAP derivative as the catalyst. Time—20 h, amount of catalyst—20 g/L, temperature—50 °C, and stirring speed—500 rpm.
3.5. Effect of catalyst dosage on the yield of monomenthyl succinate
The efficiency of the catalytic reaction was also influenced by the catalyst dosage, which directly affected the production cost. Accordingly, it was crucial to determine the optimum conditions for the amount of catalyst. Figure 8 shows that the yield of monomenthyl succinate significantly improved from 71.4% to 91.52% due to the increase in the amount of catalyst from 5 g/L to 20 g/L. However, the yield of monomenthyl succinate did not significantly change when the amount of catalyst was higher than 20 g/L; the excessive catalyst increases production costs. Thus, the optimal dosage of the catalyst was confirmed as 20 g/L.
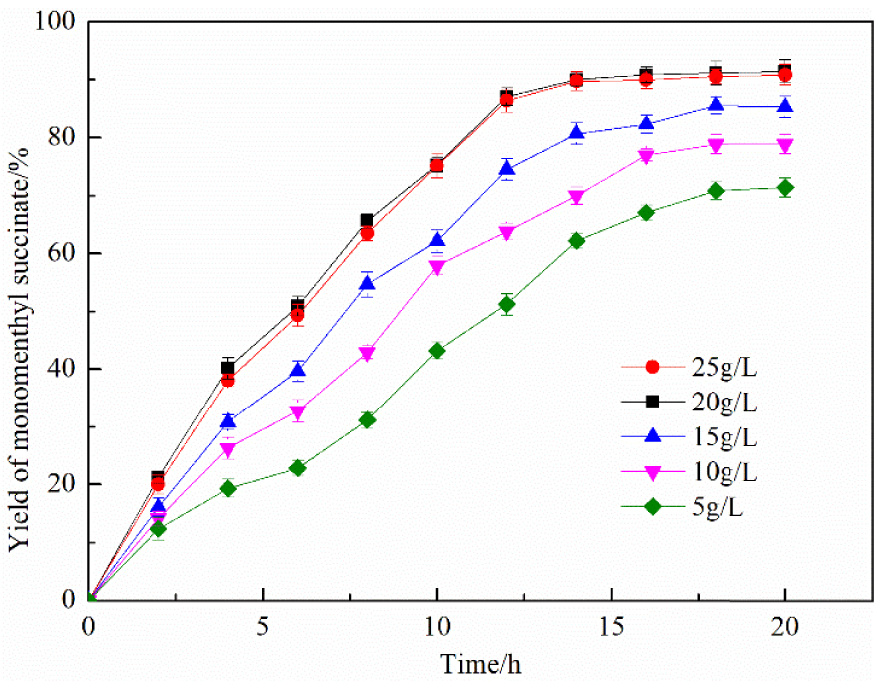
Effect of different catalyst dosages. Time—20 h, stirring speed—500 rpm, and temperature—50 °C.
3.6. Effect of stirring rate on the yield of monomenthyl succinate
In this heterogeneous system, the internal diffusion resistance was eliminated due to the use of nonporous nanoscale silicon dioxide for the immobilization of DMAP. Moreover, external diffusion resistance could be reduced by stirring. As seen in Figure 9, the yield of monomenthyl succinate gradually increased with increase in the speed of stirring. However, the yield hardly changed when the stirring speed exceeded 500 rpm. A reasonable explanation for this phenomenon was that the high speed of stirring increased the collisions of the reactant particles and the catalyst, which reduced the resistance of external diffusion and increased the conversion rate of the reaction. On the contrary, an excessive stirring rate could break the connection between the catalyst and the carriers, which not only increases the difficulty in catalyst separation but also leads to more energy consumption. Therefore, the immobilized DMAP derivative catalyzed the synthesis of monomenthyl succinate at the optimal stirring rate of 500 rpm, and the yield reached 91.55%.

Effect of different stirring rates. Time—20 h, amount of catalyst—20 g/L, and temperature—50 °C.
3.7. Stability of catalyst
With the growing call for sustainable development and the emergence of the concept of green chemistry, the longevity and reusability of catalysts have become important indicators for evaluation. To investigate the recyclability and the stability of the immobilized DMAP derivative, the catalysts were reused 10 times under optimal reaction conditions. As seen in Figure 10, the catalyst activity suffered no obvious loss after being reused for 10 consecutive cycles. This result clearly proved that the immobilized DMAP derivative had remarkable longevity and reusability. It also proved the applicability of using this method for the large-scale production of monomenthyl succinate. The immobilized DMAP derivative could be easily extracted from the mixture solvent to investigate its stability by HPLC after the reaction [38]. The result showed that the immobilized DMAP derivative had good stability, which prevented catalyst contamination and increased safety in production.
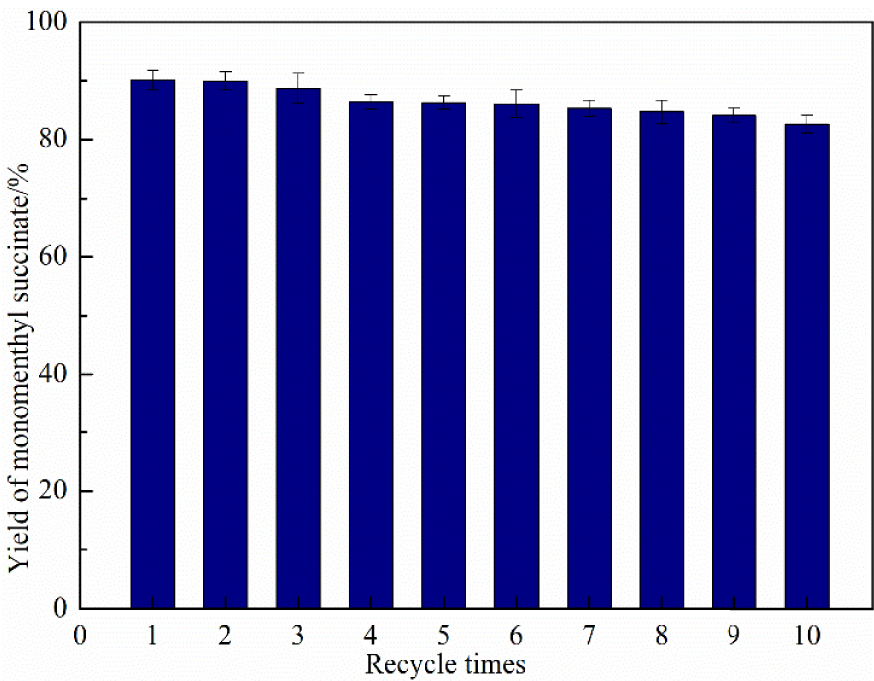
The recyclability and stability of nanoscale silicon dioxide immobilized DMAP derivative.
3.8. Continuous operation for monomenthyl succinate production
From the perspective of industrial production, continuous production was a more efficient strategy to maximize throughput [39]. A process flow for the continuous production of monomenthyl succinate was systematically investigated. This approach was based on a continuous flow of the reaction solution in a fluidic device, which served as a practical platform for the large-scale and cost-effective production of monomenthyl succinate. Figure 11 shows that the maximum yield of monomenthyl succinate, 90.13%, was achieved after 60 cycles and the reaction time was approximately 100 min. The space–time yield is an essential factor in industrial production, which directly decides the production efficiency. The space–time yield of continuous production was 4.14 × 10−3 mg∕min⋅mgcatalyst, which was almost four times that of batch production (1.14 × 10−3 mg∕min⋅mgcatalyst).
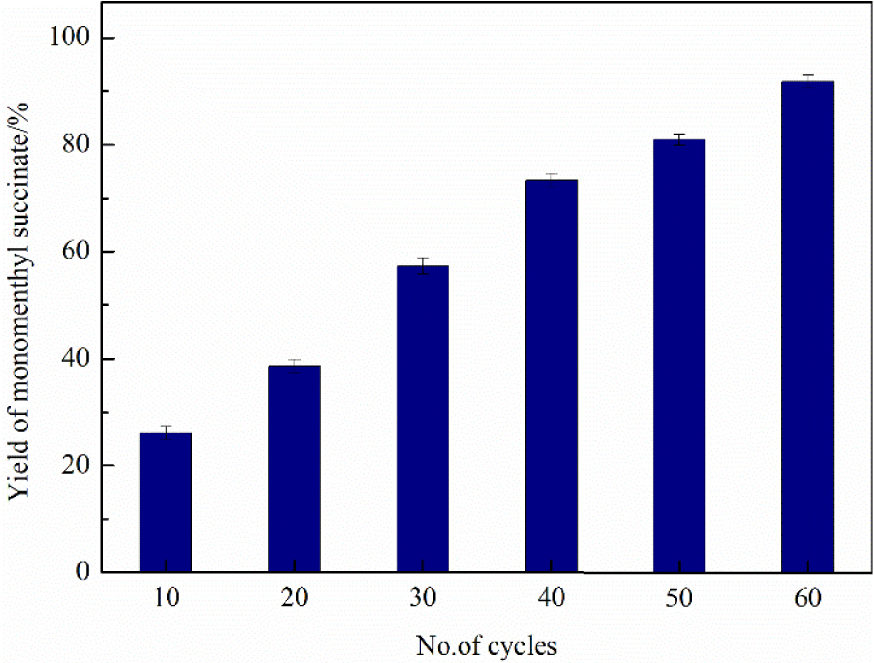
Effect of the number of cycles on the yield of monomenthyl succinate in continuous production.
Compared with batch technology, continuous production, where the catalyst is fixed in the packed-bed reactor, has the advantages of shortening the reaction time, simplifying the separation process, and reducing the catalyst loss. On the other hand, the continuous production process simplified the washing steps and reduced the use of organic solvents, which conforms to the concept of green chemistry. These excellent results made this continuous system more promising for the industrial production of monomenthyl succinate.
4. Conclusion
As a heterogeneous catalyst, an immobilized DMAP derivative was successfully prepared via the N-alkylation reaction of MAP with chloro- propyl-modified nanoscale silicon dioxide under environment-friendly alkaline conditions. The immobilized DMAP derivative was successfully employed to synthesize monomenthyl succinate with satisfactory yields. This permitted easy recycling of DMAP and prevented the catalyst contamination of the product. Besides, a cyclohexane/acetone mixed solvent system was used to improve both the substrate solubility and the activity of the catalyst. As far as we know, this is the first method to synthesize monomenthyl succinate by using a nanoscale silicon dioxide immobilized DMAP derivative in a cyclohexane/acetone mixed solvent system. The maximum yield of monomenthyl succinate reached 91.78%. Continuous production with a simpler operation and higher production efficiency was successfully carried out for the synthesis of monomenthyl succinate so as to assess its potential for industrial applications.



 CC-BY 4.0
CC-BY 4.0
Vous devez vous connecter pour continuer.
S'authentifier