1. Introduction
Names of different products under different reaction conditions
| Reactant A | Reactant B | Reactant C | Ratio (n∕n) | Solvent | Product number |
|---|---|---|---|---|---|
| 1:3:1 | Distilled | MSDS-1 | |||
| SDS | HCHO | C4H11NO2 | |||
| 1:3:2 | Water | MSDS-2 | |||
There are lipophilic and hydrophilic groups in the molecular structure of a surfactant, and the existence of an amphiphilic structure makes adsorption possible on the liquid interface. After the adsorption of a surfactant, the interfacial properties of a solution change significantly [1]. Experimental studies found that the structure of a surfactant has a significant impact on its properties [2, 3] such as washing, wetting, dispersing, emulsifying, solubilizing, foaming, and so on. Surfactants are widely used in the fields of washing products, chemical industry, medicine, textile, remediation of petroleum-contaminated soil [4, 5, 6], and so on. As a result of the continuous development of the economy, the chemical industry in China has grown significantly with the rise in demand and application of surfactants. To effectively promote the application of surfactants in various fields, we need to strengthen the chemical structure of surfactants [7]. For anionic surfactants, the active constituents are anions. In general, the length of carbon chains is between 12 and 18. When they dissolve in water, they produce hydrophilic anionic groups [8]. The catalog of anionic surfactants is very large with a wide variety, which forms a complete system, and it has an extensive range of practical applications [9]. However, it has some shortcomings. For example, it cannot meet the demand of extreme environments such as high methanol content, high salinity, high temperature, and so on. In this work, modified sodium dodecyl sulfate surfactants (MSDS-1 and MSDS-2) were prepared by using methanol and diethanol amine as modifiers to overcome these shortcomings [10].
2. Experimental section
2.1. Materials
Sodium dodecyl sulfate (SDS) was purchased from Xi’an Chemical Reagent Factory. Methanol and formaldehyde were purchased from Tianjin Tianli Chemical Reagent Co., Ltd. Diethanol amine was purchased from Tianjin Kelong Chemical Reagent Factory. All chemicals were used without further purification.
2.2. Synthesis and reaction mechanism of surfactants
Formaldehyde, SDS, and diethanol amine were weighed in proportion (molar ratio as shown in Table 1). Sodium dodecyl sulfate was placed in a 250 mL flask. To this, 200 mL distilled water was added as the solvent. Formaldehyde was added to the flask drop by drop at 70 °C at a rate of 1 drop/s, and the mixture was stirred for 3 h. As shown in Scheme 1, two possible reactions provide two products, which were named as shown in Table 1. They are MS (ESI) of MSDS-1 m∕z: 382; MS (ESI) of MSDS-2 m∕z: 499.
The Mannich reaction of SDS, diethanol amine, and formaldehyde.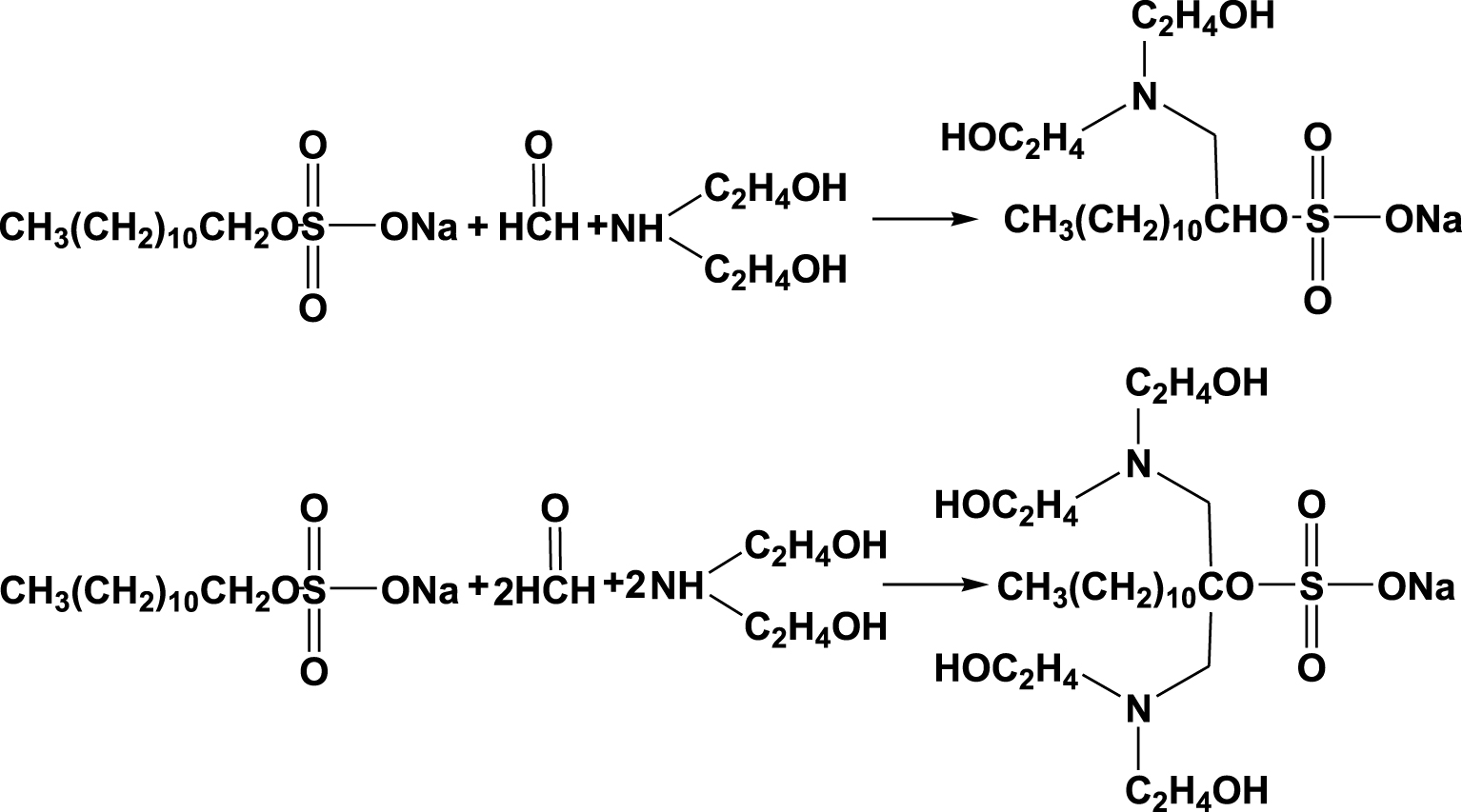
Critical micelle concentration and surface tension for a surfactant concentration of 0.05%
| Surfactant | cmc value (%) | 0.05% surface tension (mN/m) |
|---|---|---|
| SDS | 0.0090 | 33.2 |
| MSDS-1 | 0.0091 | 31.6 |
| MSDS-2 | 0.0094 | 31.8 |
2.3. Surface tension measurements
Distilled water was used to prepare 0.0001%, 0.0005%, 0.001%, 0.003%, 0.005%, 0.008%, 0.01%, 0.02%, 0.03%, 0.04%, and 0.5% (mass fraction) surfactant solutions. The surface tension of each surfactant solution was determined by a tensiometer, and the critical micelle concentration (cmc) was determined [11]. The tensiometer was checked before the surface tension measurement using distilled water to confirm the standard surface tension value of distilled water [12]. Each experiment was repeated thrice.
2.4. Foaming ability measurement
There are many methods for generating foaming, including the sparge tube technique or gas flow and “whipping” [13, 14]. In the present study, the high-speed stirring method was used to evaluate the foaming properties of surfactant solutions. The foaming ability was measured as the volume of the foam produced immediately after the mechanical agitation stopped. The foam volume and foam half-life (time taken to separate 50 mL water) were recorded.
2.5. Microstructure of foams
The microstructure of foams produced by different surfactant solutions and their changes with time were studied using an optical microscope, whose light source is polarized light (DM4500P LFD, Germany).
3. Results and discussion
3.1. Surface tension measurements
Surfactant solutions having mass fractions of 0.0001%, 0.0005%, 0.001%, 0.003%, 0.005%, 0.008%, 0.01%, 0.02%, 0.03%, 0.04%, and 0.5% were prepared by using distilled water. Then, the surface tension of each solution was measured. The results are as follows.
Surface tension patterns of SDS, MSDS-1, and MSDS-2.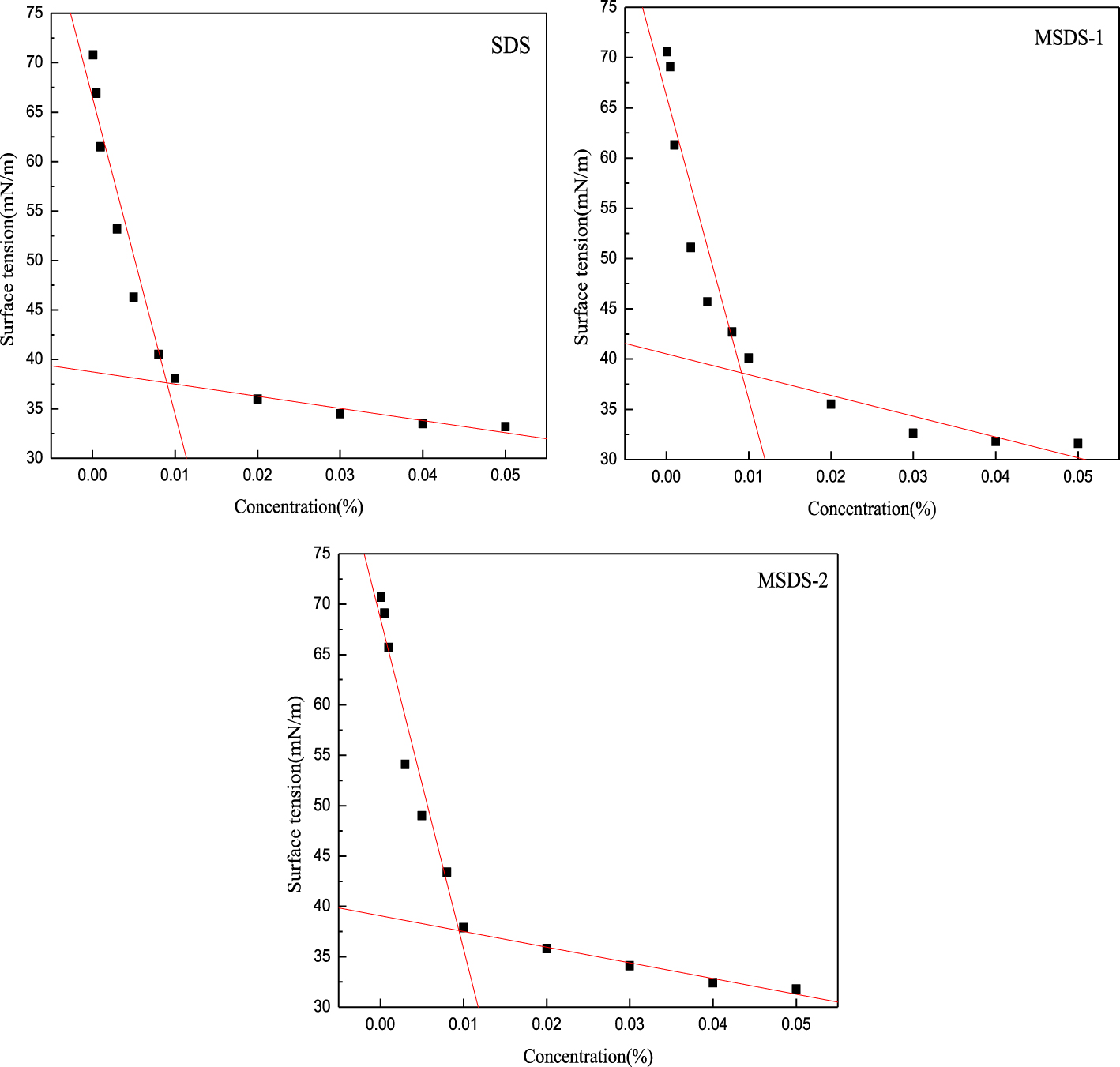
Foaming ability (a) and foam half-life time (b) under different reaction conditions.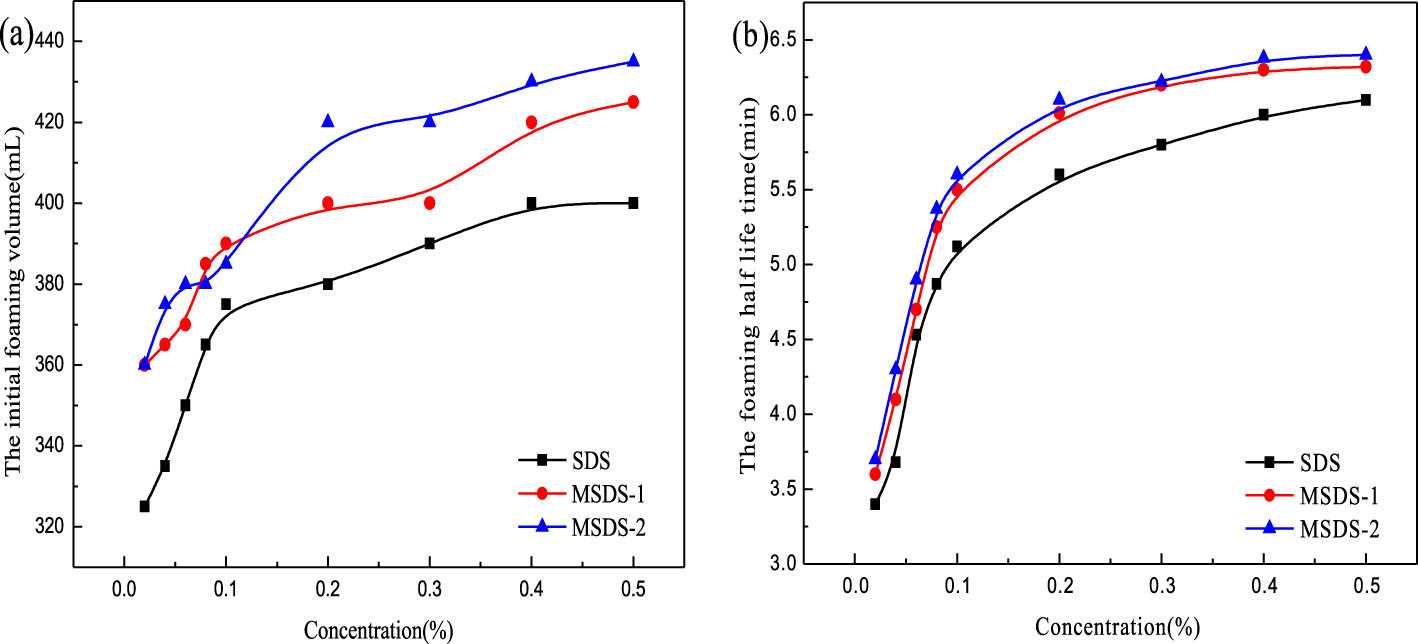
Change in foam volume with temperature for different surfactants (0.5%): (a) SDS, (b) MSDS-1, and (c) MSDS-2.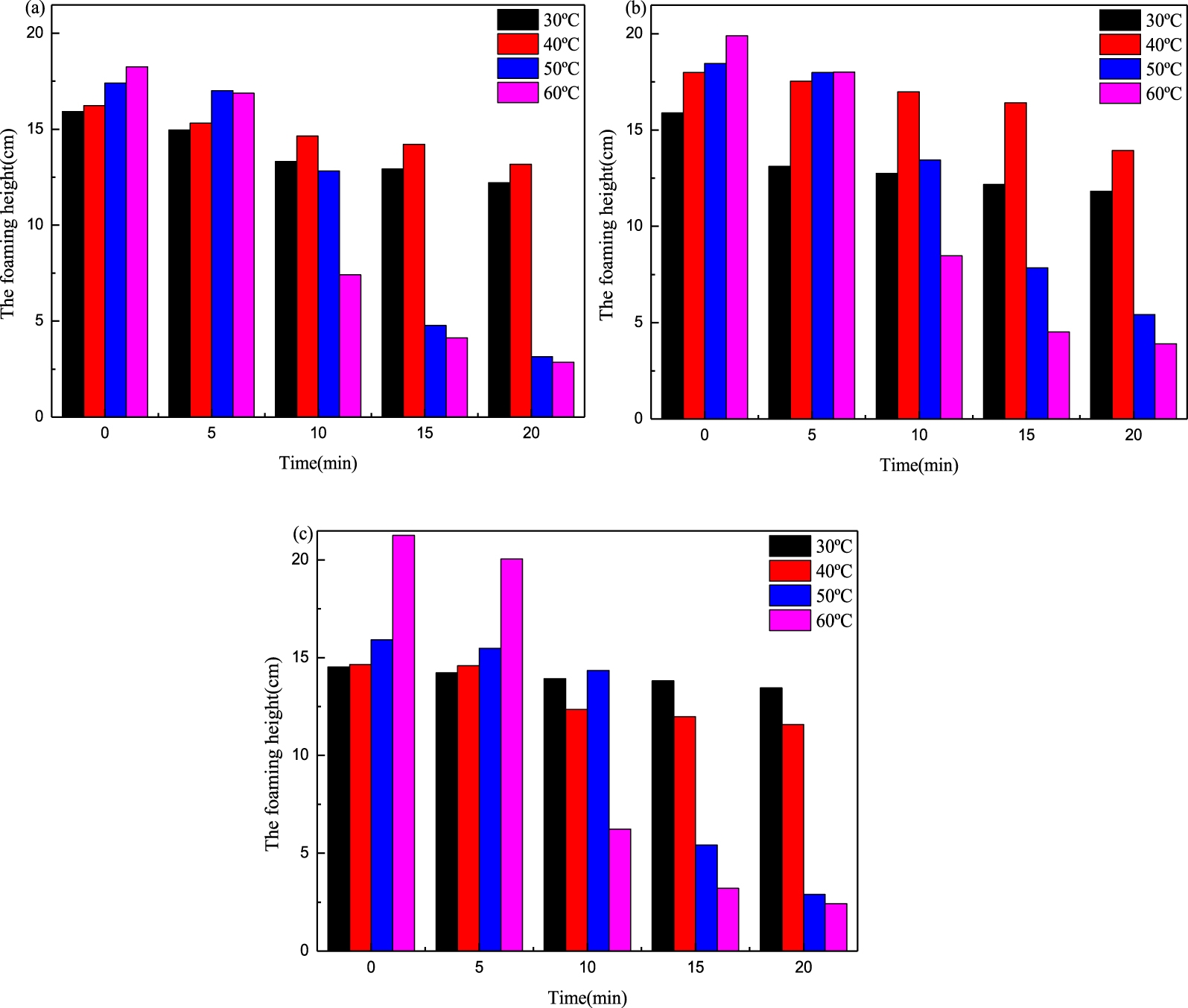
The reason why surfactants produce foam is that the surface-active-agent molecules can be arranged regularly on the interface between the gas phase and the liquid phase in water medium. When the concentration of the surfactant solution is lower than the cmc, the surface tension decreases and it is not easy for the adjacent bubbles to aggregate and merge. The lower the surface tension, the more easily the gas–liquid system is dispersed and the more ideal the foaming effect of the surface-active agent. As can be seen from Figure 1, with increase in concentration, the surface tension of the surfactant solution decreases sharply at first and then tends to stabilize. This is because the surfactants begin to automatically assemble and arrange themselves on the surface, reducing the contact area between water and air, and the surface tension of the solution decreases dramatically [15]. After processing the data in Figure 1, the cmc values of SDS, MSDS-1, and MSDS-2 for different molar ratios are obtained as shown in Table 2. Compared with other surfactants at room temperature, the cmc of SDS is 0.0090%. The minimum surface tension of MSDS-1 is 31.6 mN∕m. There is no significant difference in the cmc values of the products of the three reaction ratios. Therefore, the change in surface tension is not the main factor affecting the foaming performance.
Foam volume of 0.5% surfactant solution for different concentrations of methanol: (a) 1%, (b) 2%, (c) 3%, and (d) 4%.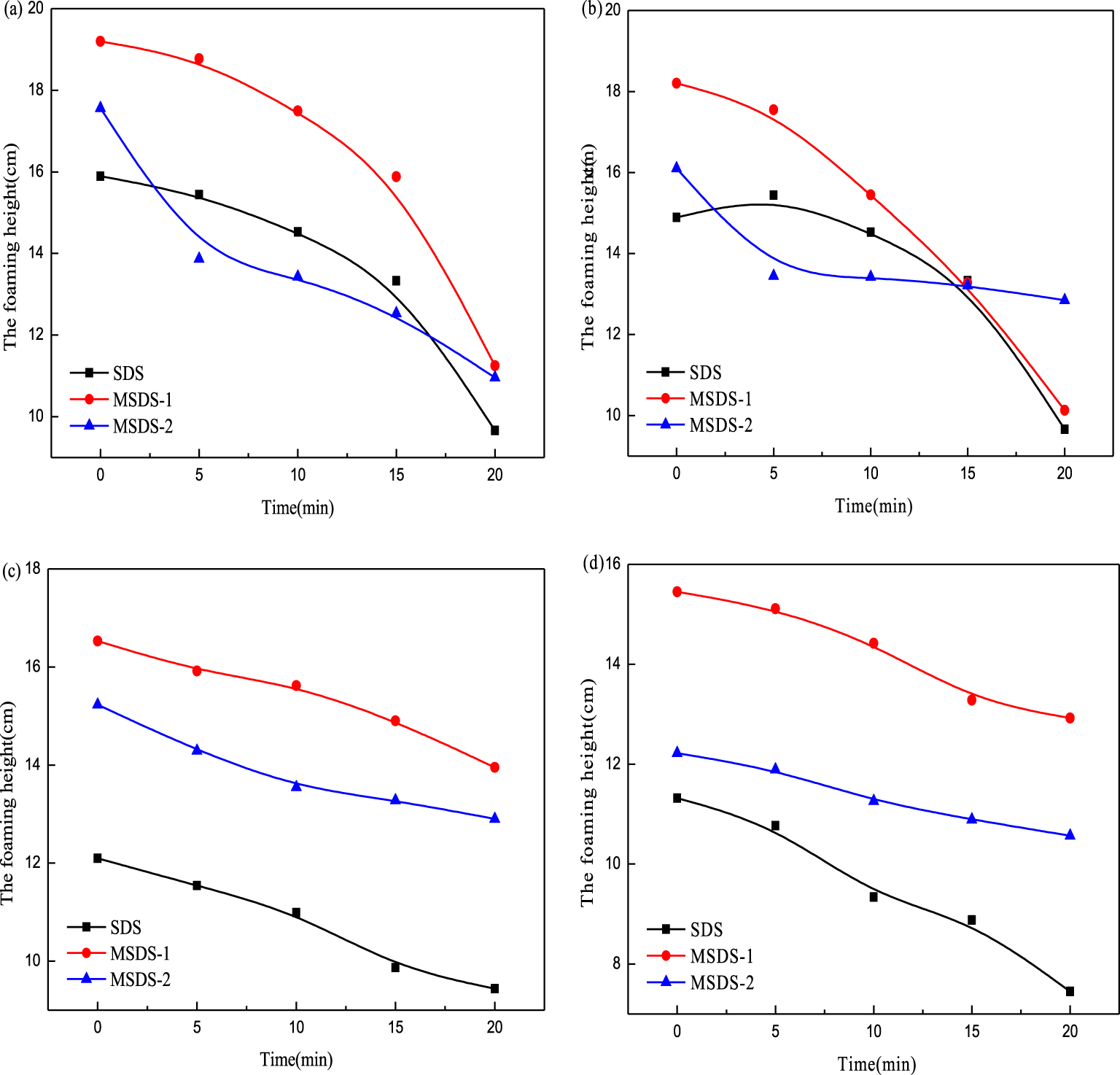
Foaming ability of SDS, MSDS-1, and MSDS-2 for different salinities: (a) NaCl and (b) MgCl2.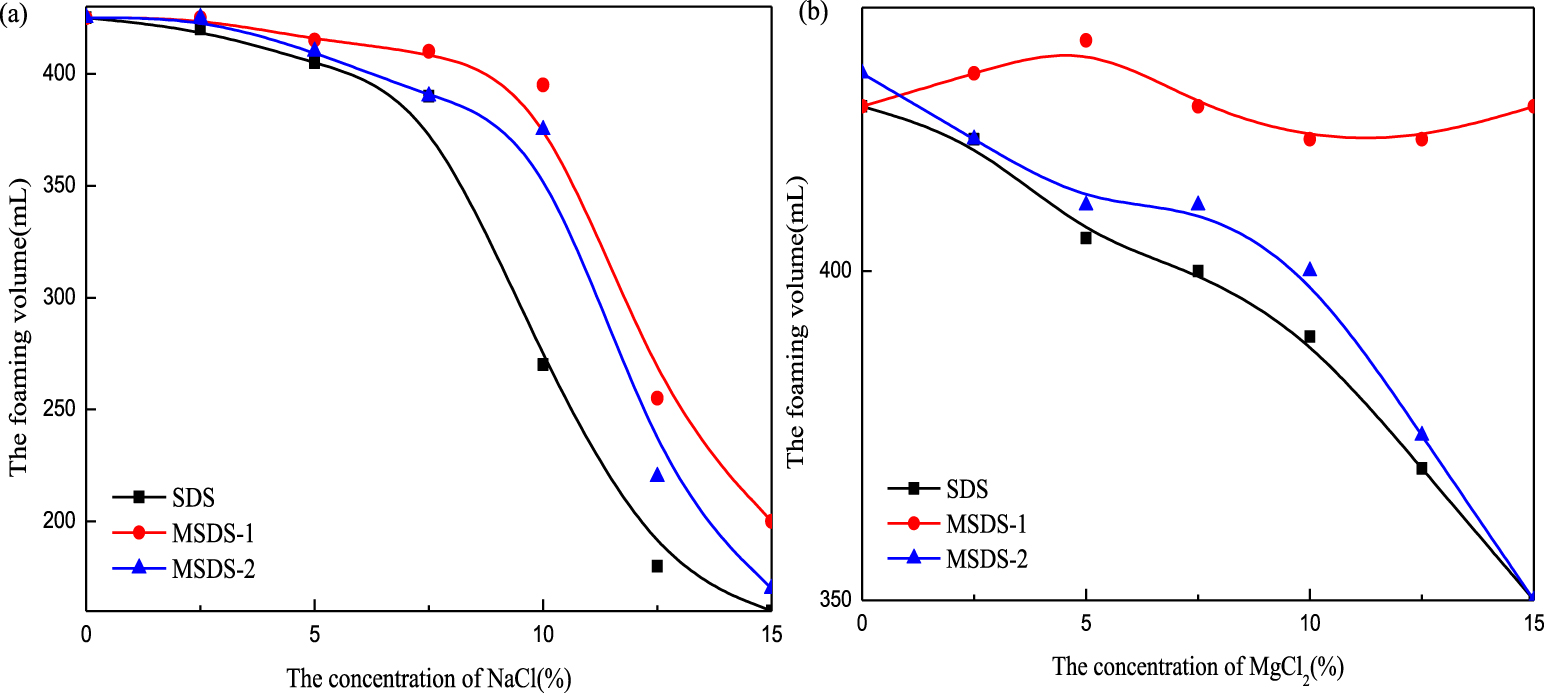
Foaming ability of different surfactants for different concentrations of condensate oil: (a) MSDS-1 and (b) MSDS-2.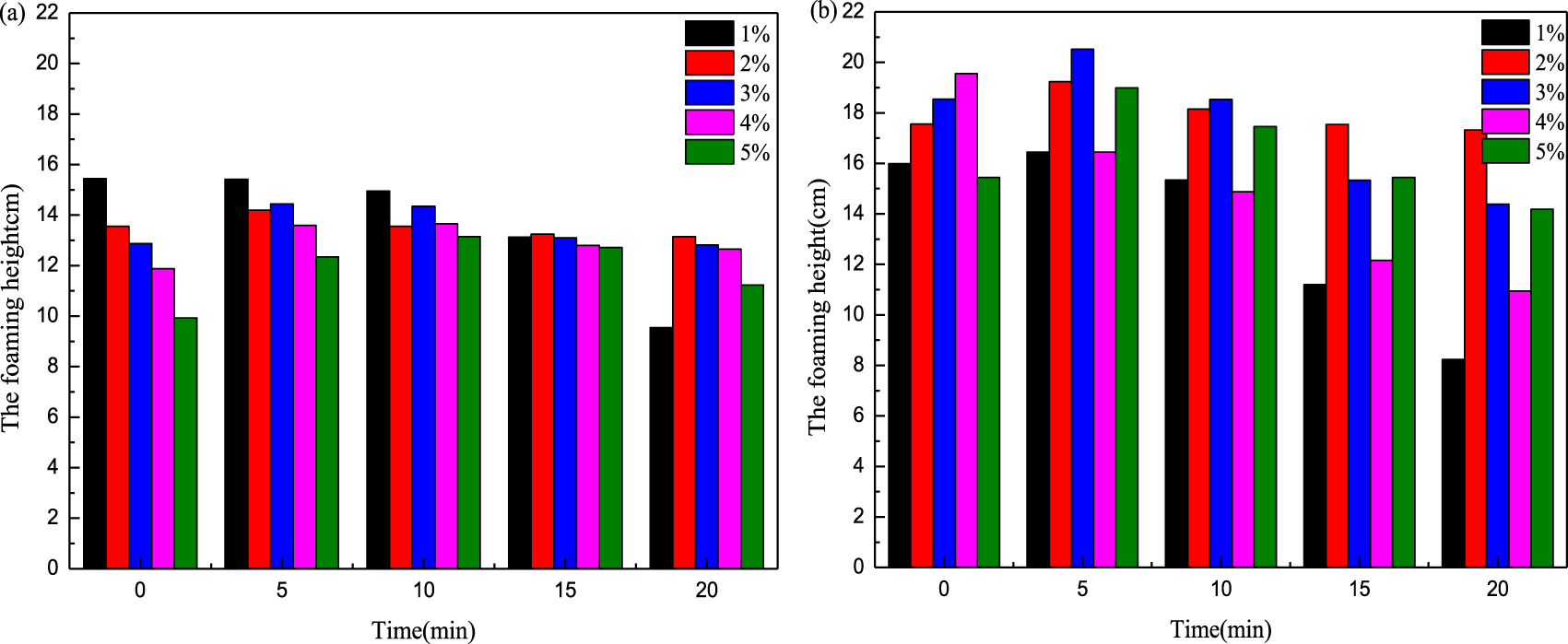
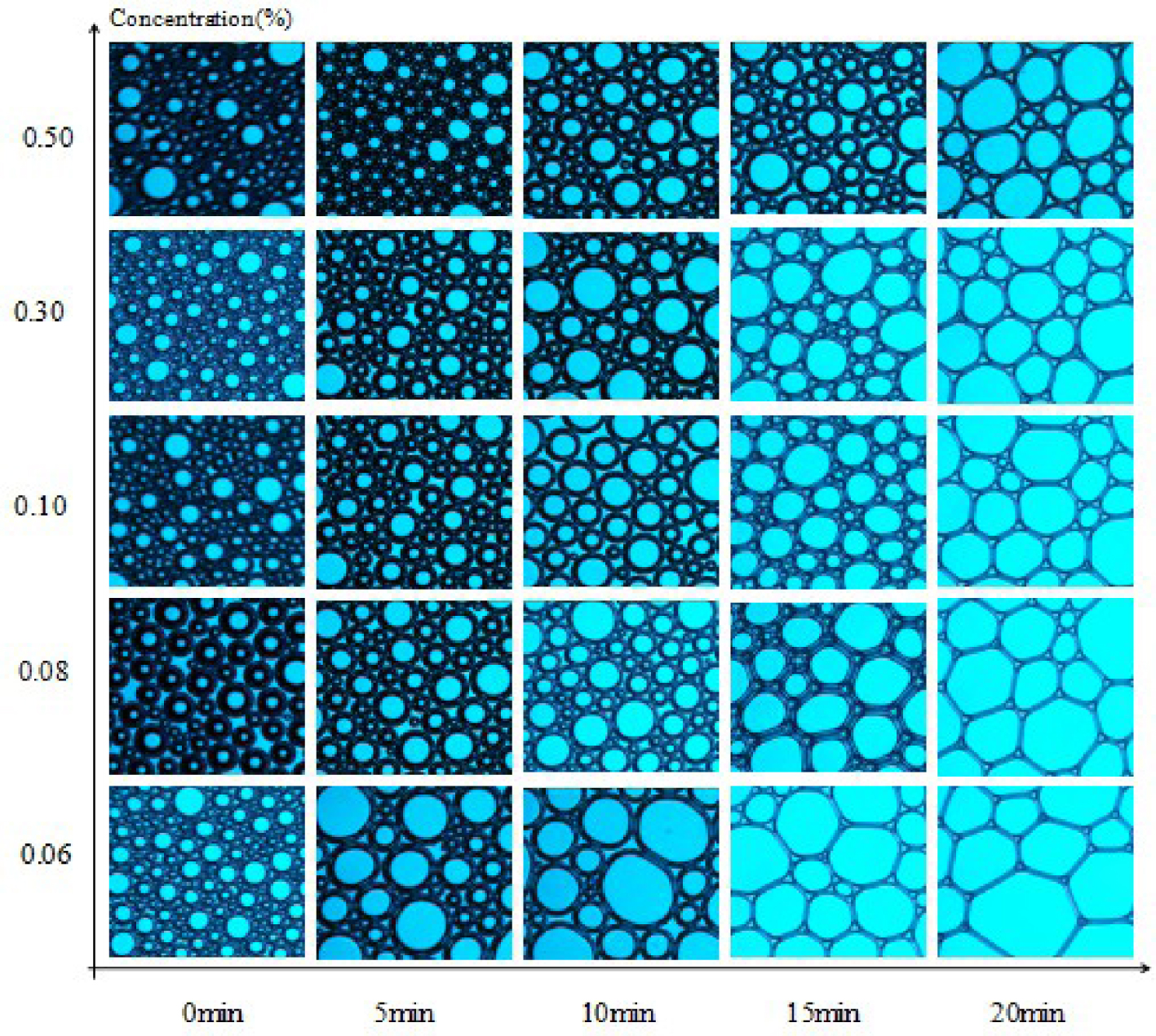
Microscopic morphology of SDS.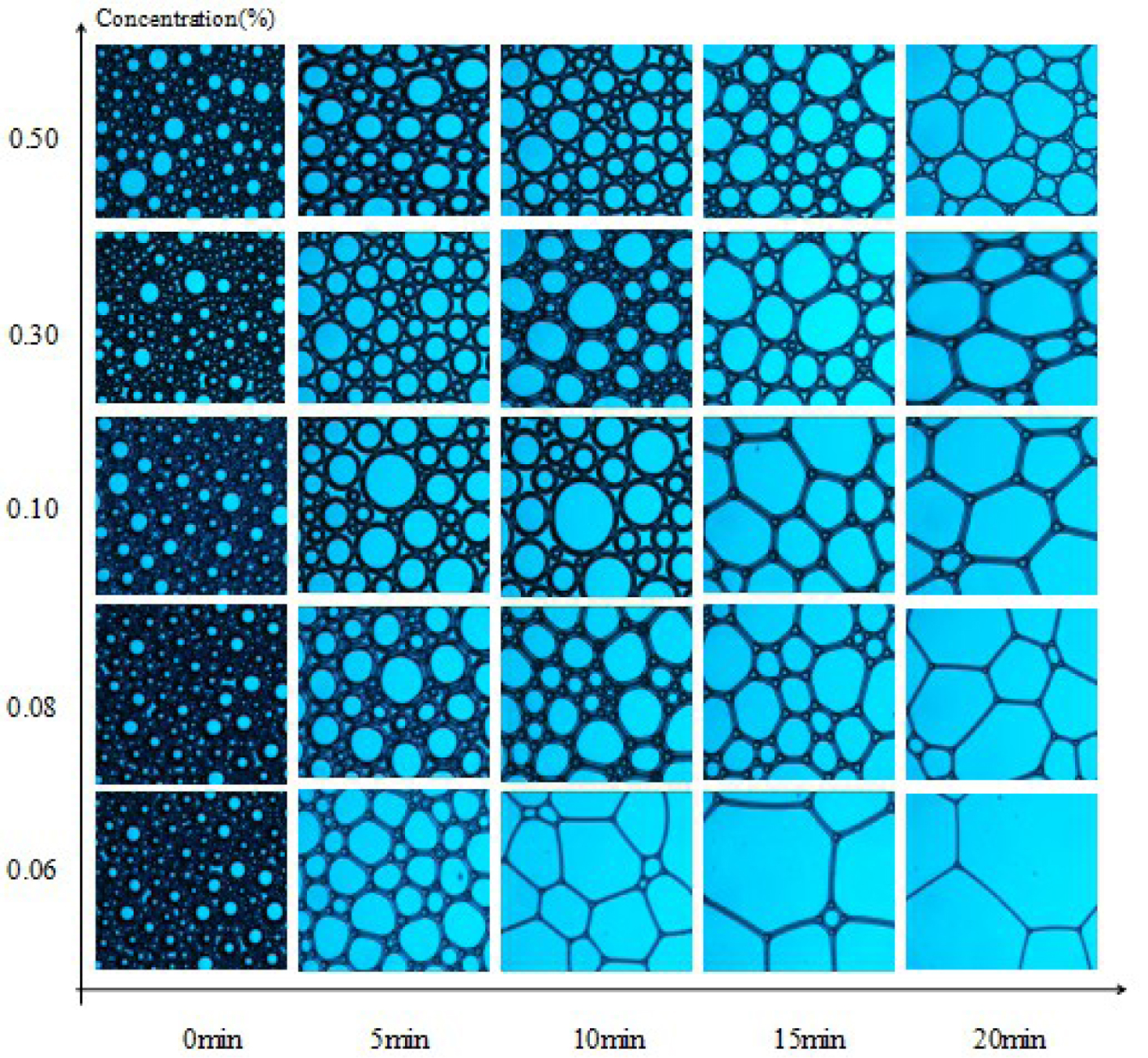
3.2. Foaming ability and foam stability
The foaming ability is the initial height of the foam, which is measured immediately after mechanical stirring is stopped. Figure 2 shows the relation among the foam volume, foam half-life time, and the range of concentration. The foam volume increases significantly with increase in concentration and reaches a maximum foam height at a concentration of 0.5%. To some extent, the foam volume is highly dependent on the concentration of the surfactant. The foam half-life time is found to obviously increase with increase in surfactant concentration. This shows that the stability of foam is related to the concentration of the surfactant. When the solution concentration is the same, the initial foam volumes of all three products are different. When the concentration of the surfactant solution is 0.5%, the initial foam volume of MSDS-2 can reach 435 mL and the half-life time of MSDS-2 can reach 6.4 min.
3.3. Temperature resistance
In the exploitation of various oil fields, the underground temperature increases with gradual downward exploitation. The change in temperature has an important effect on the formation and elimination of foam. With increase in temperature, the dissociation speed of the surfactant in the aqueous solution increases, the force between the hydrophilic group with the positive charge and the water medium is enhanced, the fluidity between bubbles is accelerated, and there is loss of liquid in the bubbles. The air bubbles are active between the molecules due to the temperature rise so that the adjacent bubbles are concentrated, the air bubbles with a large area are combined, the free energy can be reduced, and the bubble walls gradually thin until the bubbles are broken [16]. As is shown in Figure 3, when the temperature is low (30 °C, 40 °C), the foam height increases with increase in temperature; at the same time, the speed of decay is also very low. The reason is that the velocity of movement of surfactant molecules increases with temperature and leads to more frequent collisions between molecules; so the gradual increase in the quantity of foam is not surprising [17]. At the same time, because the effect of temperature on foam attenuation is not sufficiently obvious, the rate of foam height attenuation is also very slow. When the temperature is 60 °C, the initial foam height is the highest, but the decline rate is the fastest. At high temperatures, the foam bursts from the top and the foam volume increases and decreases regularly with time. With increase in temperature, the adsorption capacity decreases, the exclusive area of molecules increases, the surface viscosity decreases, the Marangoni effect weakens, and the surface elasticity decreases, which leads to a reduction in foam stability. With increase in temperature, the surface tension of the foam system decreases, which is beneficial to the improvement of foam stability. However, surface tension is not the dominant factor affecting the stability of foam [18, 19].
3.4. Methanol resistance
The addition of methanol serves the following two functions. First, the outdoor temperature is generally lower during winter mining, which may freeze oil and gas; methanol can prevent them from freezing [20]. Second, methanol can prevent or slow down the interaction between gas and bottom-hole accumulation in gas fields to produce hydrates, thereby preventing the blockage and corrosion of pipelines by natural compounds. It is propitious to increase the production from the gas well and reduce the hidden risks of high-temperature and high-pressure vessel. Therefore, it is necessary to study the methanol resistance of surfactants. At 40 °C, the foaming ability of three 0.5% surfactant aqueous solutions with different concentrations of methanol was determined by a Roche foam instrument. As shown in Figure 4, the initial foam height decreases with increase in methanol concentration. When the concentration of methanol is lower than 2%, the stability of the three surfactants is similar; the methanol resistance of MSDS-1 is the best. When the concentration of methanol is higher than 2%, the initial foam height decreases obviously. The reason is that methanol is volatile, and it accelerates the gas diffusion rate in the foam. The higher the methanol concentration, the faster the gas diffusion rate. Bubbles whose decay is dominated by gas diffusion are greatly affected by methanol. However, the resistance of MSDS to methanol is better, and the foam stability is improved. Therefore, MSDS-1 and MSDS-2 exhibit much better methanol resistance than SDS. This may be because methanol, as a defoamer, expands on the foam surface so that the original surfactant molecules are replaced by a new liquid film. The molecules of synthesized surfactants MSDS-1 and MSDS-2 have a larger functional group, which makes the substitution more difficult; so the foam decay rate weakens [21].
3.5. Salinity resistance
In oil and gas field construction sites, it is often necessary to inject a certain amount of surfactants into the wellbore to bring it into contact with formation water so as to further produce a large quantity of water-bearing foam. This is because the formation water contains some mineral ions that accelerate the burst of foam. Different kinds of surfactants have different functions and different physical and chemical effects on inorganic salts. Therefore, the salt resistance of surfactant solutions is also worth studying. It can be seen from Figure 5(a) that when the concentration of NaCl is less than 7.5%, the initial foam heights of the three surfactants synthesized at different ratios do not change much, but when the concentration of NaCl is greater than 7.5%, the foam heights decrease sharply. This is because the solubility of different surfactants decreases with increase in the quality of inorganic salts in the solution so that their foaming properties are reduced. It can be seen from Figure 5(b) that with increase in MgCl2 concentration, the initial foam height of MSDS-1 does not change much, the salt resistance of MSDS-1 is the best, MSDS-2 is second, and SDS is the worst. Compared with SDS, the salt resistance of the modified products is significantly enhanced.
3.6. Resistance of condensate oil
The condensate oil affects the physical chemistry of the surfactant solution system and further hinders the generation of the foam. The hydrocarbon penetrates the surface-active-agent liquid film, which is surrounded by air bubbles, and is then expanded into a monomolecular film on the inner wall of the foam. The surfactant fraction adsorbed on the foam interface is extruded, and a new surface film is generated. However, the surface film has poor strength and is not stable so that the foam is easily damaged [22]. The anti-condensate oil properties of MSDS-1 and MSDS-2 are measured by a Roche foam instrument at 40 °C. The results in Figure 6 show that the two surfactants have good anti-condensate oil properties. The foam height increases with time because the volatilization of condensate oil leads to an increase in foam height. The initial foam height of MSDS-1 is lower than that of MSDS-2 at different concentrations, and the foam height continues to increase from the original value with time. It can be seen that the anti-condensation oil performance of MSDS-2 is better than that of MSDS-1. This may be because the molecules on the surface of the foam liquid film produced by the foam are more difficult to be replaced by hydrocarbon molecules. The foam does not burst; so the foam height increases faster than the foam burst rate.
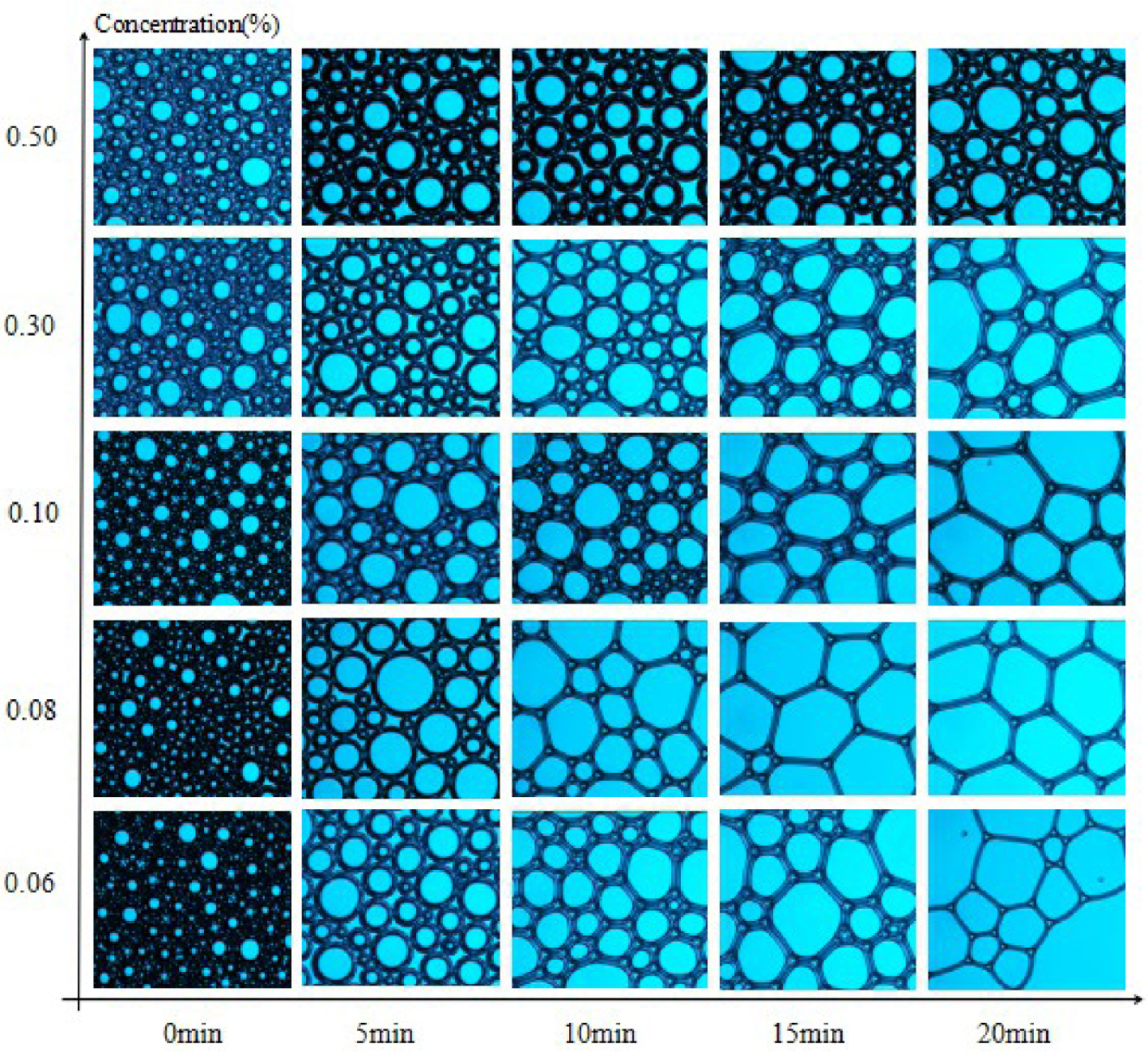
3.7. Microstructure of foams
Microstructures of SDS, MSDS-1, and MSDS-2 were observed with an optical microscope. Polarized light was chosen as the light source. The results are shown in Figures 7–9. The stability of foam is also related to the properties of the solution itself such as the surface tension and viscosity of the solution. In addition, it is also related to the thickness of the foam wall. Generally speaking, the thicker the foam wall, the higher the mechanical strength and the better the stability. The thickness of the bubble wall can be easily observed with a microscope. The microstructure of the initial foam is generally round, and the bubble membrane wall is thicker. However, with time, the bubble wall gradually becomes polygonal and thins and eventually breaks. From Figures 7–9, at 0 min, the microstructures of the three synthesized surfactants are basically the same at different concentrations. From Figures 7–9, as the concentration of surfactants increases, the foam stability is improved. When the concentration is 0.5% and the time is 20 min, the foam wall of MSDS-2 is the thickest, indicating that this foam wall is the most stable and that of MSDS-1 is second, which corresponds to the previous foam stability test results.
4. Conclusion
In this work, modified sodium dodecyl sulfates (MSDS-1 and MSDS-2) are prepared by the Mannich reaction of SDS, formaldehyde, and diethanol amine. After modification, the surface tension is basically unchanged, and the foaming performance and foam stability are improved. At the concentration of 0.5%, the initial foam volumes of MSDS-1 and MSDS-2 can reach 425 mL and 435 mL, respectively, which are much higher than that of SDS. Compared with SDS, the temperature resistance, methanol resistance, salt resistance, and condensate resistance of MSDS-1 and MSDS-2 are improved. Among them, the temperature resistance, salt resistance, and methanol resistance of the MSDS-1 solution are the best. The MSDS-2 solution has the best anti-condensate performance. In addition, the foam size becomes smaller, the foam wall thickens, and the foam stability increases.



 CC-BY 4.0
CC-BY 4.0