1. Introduction
Structures of Securidanes A and B (1A). Approaches to the synthesis of triarylmethanes containing biaryl unit(s) (1B–E).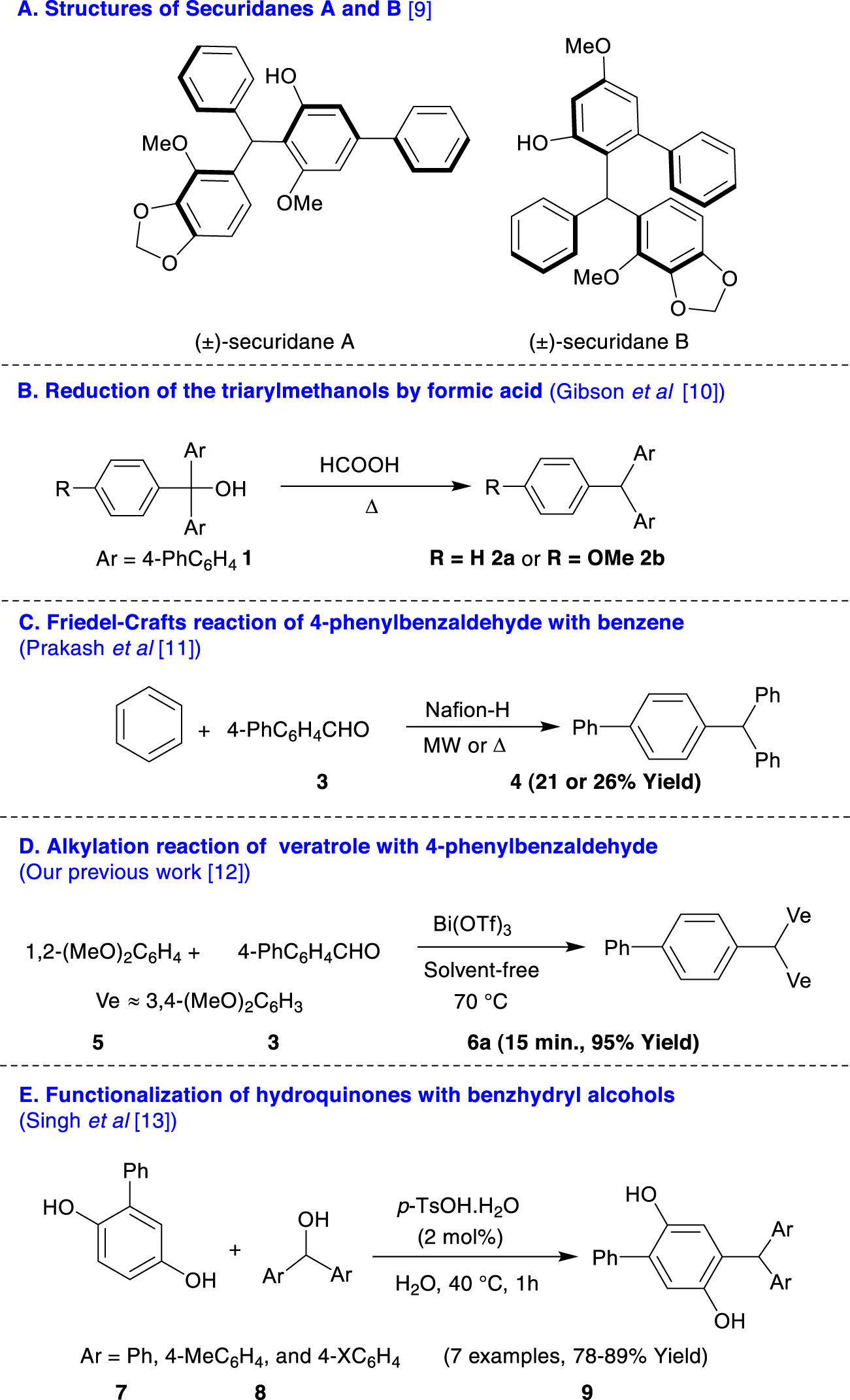
Biaryls are privileged templates that are frequently found in natural products, functional materials, polymers, liquid crystals, chiral reagents, and biologically active compounds. They are also employed as building blocks in organic synthesis and ligands for homogeneous catalysis [1, 2]. The Suzuki–Miyaura coupling reaction of arylboronic acids with aryl and heteroaryl halides/esters has become a powerful tool for the construction of biaryl scaffolds [3, 4, 5, 6, 7]. A new series of diarylmethanes including a biaryl moiety has also been synthesized by Gu et al. through the multi-component reaction of organohalides, tosylhydrazide, and arylboronic acids [8]. Recently, Zhou et al. have reported the isolation of enantiomeric pairs of Securidanes A and B, as two natural triarylmethanes (TRAMs), from Securidaca inappendiculata (Scheme 1A) [9]. To date, to the best of our knowledge, a few TRAMs bearing one or two biphenyl moieties have been synthesized by means of the reduction of triarylmethanols 1 by formic acid (Scheme 1B) [10], Nafion-H-catalyzed microwave or conventionally heated Friedel–Crafts reaction of 4-phenylbenzaldehyde 3 with benzene (Scheme 1C) [11], and the Friedel–Crafts alkylation reaction of veratrole 5 with 3 catalyzed by Bi(OTf)3 under solvent-free conditions (Scheme 1D) [12] as well as p-TsOH-catalyzed functionalization reactions of substituted hydroquinones 7 with benzhydryl alcohols 8 (Scheme 1E) [13]. This limitation on scope originates from three determining factors: (i) Aryl- and heteroarylboronic acids are commercially available with a greater diversity than biarylaldehyde derivatives. (ii) Biarylaldehydes are more expensive than the corresponding arylboronic acids. (iii) The acid-catalyzed reactions of N-containing arylaldehydes with arenes generally fail in acidic media due to the coordination of nitrogen site(s) into H+ or metal cores.
The Mizoroki–Heck-type cross-coupling reaction is a well-known C–C bond forming process, which has been broadly used for vinylation of aryl halides or triflates using Pd(0)-containing catalytic systems [13, 14, 15, 16, 17]. In contrast, vinylation of TRAMs has received little attention from researchers to date. Qian et al. employed the Pd(OAc)2-catalyzed Heck vinylation reactions of fluorophore templates as the key step for the synthesis of fluorescent probes [18]. The Heck coupling reaction of 1-bromo-3-(diphenylmethyl)benzene with styrylboronic acid has also been used for producing the corresponding vinylated TRAM [19]. Additionally, stilbenoid units have been incorporated into a few tetraarylmethanes using Suzuki coupling reactions [20]. As part of an ongoing program on extending the synthetic applications of TRAMs [12, 21, 22, 23], we herein describe our efforts toward the functionalization of TRAMs via the Suzuki–Miyaura and Heck-type reactions of brominated TRAMs with arylboronic acids and olefins.
2. Experimental section
2.1. General considerations
Unless otherwise stated, all chemicals and solvents were obtained from commercial suppliers and were used without further purification. The solvents used as reaction media were anhydrous, and they were purified according to standard procedures. An aqueous solution of K2CO3 (2 M) was freshly prepared in deionized water and was used in reactions without degassing. All the reactions were run in an oven-dried apparatus with dried solvents in an atmosphere of dry nitrogen. Thin layer chromatography (TLC) analyses were performed on pre-coated silica gel F254 plates (commercially available from Merck), and visualized under UV light. Melting points were recorded using a Stuart SMP2 apparatus and were uncorrected. Fourier-transform infrared (FT-IR) spectra were obtained as KBr pellets using a Nicolet Impact 400D spectrophotometer. All 1H and 13C spectra were recorded on a Varian UNITY Inova 500 MHz spectrometer. Elemental analyses were carried out on a LECO CHNS-932 instrument.
2.2. General procedures
2.2.1. General procedure for the preparation of halogenated TRAMs (Scheme 2schemesc2)
Halogenated TRAMs 10a–h were prepared following our previously reported method [24]. A mixture of arene (3 mmol), corresponding aldehyde (1 mmol), and silica sulfuric acid (SSA, 200 mg) was stirred at 65 °C for 25–30 min. After completion of the reaction as indicated by TLC (eluent: n-hexane/EtOAc 10:4), the reaction mixture was cooled to room temperature and washed twice with absolute EtOH (5 mL). The catalyst was separated by simple filtration, and the crude product was purified by recrystallization from EtOH. The products 10a [22, 24, 26], 10b [12], 10c [26], 10e [12], and 10h [21, 25, 27] have already been described and matched with bibliographic data.
Structures of prepared TRAMs. Reaction conditions: arene (3 mmol), aldehyde (1 mmol), and SSA (200 mg) at 65 °C. aReaction time. bIsolated yield.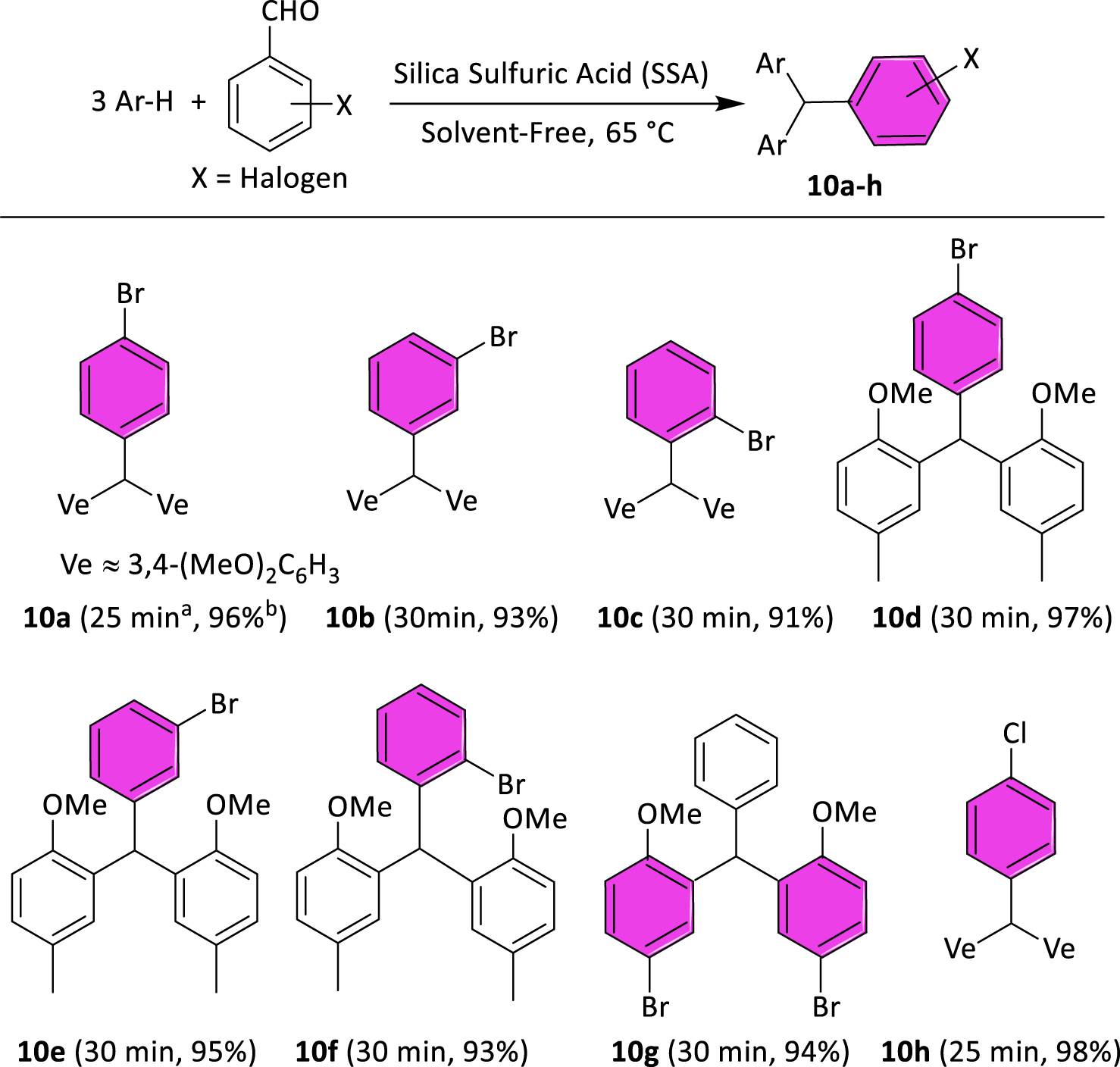
2,2′-((4-Bromophenyl)methylene)bis(1-methoxy-4-methylbenzene) (10d)
White powder; 398 mg (97%); Mp 153–155 °C; FT-IR (KBr): ν (cm−1) 2998, 2934, 1513, 1463, 1245, 1262, 1139, 1027, 474; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.35 (d, J = 8.4 Hz, 2H), 7.01 (dd, J1 = 8.3 Hz, J2 = 2.4 Hz, 2H), 6.93 (d, J = 8.5 Hz, 2H), 6.77 (d, J = 8.2 Hz, 2H), 6.58 (d, J = 2.5 Hz, 2H), 6.09 (s, 1H, Ar3CH), 3.67 (s, 6H, OMe), 2.21 (s, 6H, Me); 13C NMR (125 MHz, CDCl3): δ (ppm) 155.17, 143.38, 131.76, 131.05, 130.90, 130.61, 129.27, 127.73, 119.42, 110.93, 55.91, 42.70, 20.74; Anal. Calcd for C23H23BrO2: C, 67.16; H, 5.64. Found: C, 67.10; H, 5.59.
2,2′-((2-Bromophenyl)methylene)bis(1-methoxy-4-methylbenzene) (10f)
White powder; 382 mg (93%); Mp 133–135 °C; FT-IR (KBr): ν (cm−1) 2987, 2938, 1613, 1526, 1464, 1127, 1087, 1035, 809, 456; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.43 (d, J = 7.9 Hz, 1H), 7.09 (t, J = 7.5 Hz, 1H), 6.96–7.00 (m, 1H), 6.92 (dd, J1 = 8.2 Hz, J2 = 2.0 Hz, 2H), 6.69 (d, J = 8.3 Hz, 1H), 6.39 (d, J = 1.75 Hz, 2H), 6.25 (s, 1H, Ar3CH), 3.57 (s, 6H, OMe), 2.09 (s, 6H, Me); 13C NMR (125 MHz, CDCl3): δ (ppm) 155.21, 143.52, 132.71, 130.80, 130.55, 130.33, 128.97, 127.61, 126.86, 125.34, 111.11, 55.99, 43.19, 20.83; Anal. Calcd for C23H23BrO2: C, 67.16; H, 5.64. Found: C, 67.11; H, 5.61.
2,2′-(Phenylmethylene)bis(4-bromo-1-methoxybenzene) (10g)
White powder; 427 mg (92%); Mp 148–150 °C; FT-IR (KBr): ν (cm−1) 2935, 1484, 1461, 1244, 1115, 913, 743, 450; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.32 (dd, J1 = 8.5 Hz, J2 = 2.5 Hz, 2H), 7.27–7.30 (m, 2H), 7.20–7.26 (m, 1H), 7.04 (d, J = 7.6 Hz, 2H), 6.87 (d, J = 2.5 Hz, 2H), 6.74 (d, J = 8.7 Hz, 2H), 6.06 (s, 1H, Ar3CH), 3.68 (s, 6H, OMe); 13C NMR (125 MHz, CDCl3): δ (ppm) 156.27, 141.90, 134.29, 132.43, 130.35, 129.18, 128.26, 126.36, 112.77, 112.54, 55.86, 43.43; Anal. Calcd for C21H18Br2O2: C, 54.57; H, 3.93. Found: C, 54.49; H, 3.90.
2.2.2. General procedure for the Suzuki–Miyaura coupling reactions of TRAMs with arylboronic acid derivatives 6a–l
An oven-dried 25 mL two-neck flask equipped with a condenser and a magnetic stir bar was charged with Pd(PPh3)4 (5 mol%), TRAM 10 (1 mmol), and arylboronic acid 11 (1.2 mmol). After performing two cycles of vacuum N2, degassed tetrahydrofuran (THF, 5 mL) and K2CO3 (1 mL of 2 M non-degassed solution) were added using a disposal syringe. The resulting mixture was refluxed for the allotted time, cooled to room temperature, and filtered through a plug of Celite; the volatiles were then removed in vacuo. The crude product was purified by flash chromatography on silica gel (eluent: petroleum ether/methanol 10:1) to obtain the pure product 6a–j. The products 6k and 6l were synthesized using one-pot, double Suzuki coupling reactions of TRAM 10g with two-fold amounts of 11a and 11c under similar conditions.
4-(Bis(3,4-dimethoxyphenyl)methyl)-1,1′-biphenyl (6a)
White powder; 383 mg (87%); Mp 126–128 °C; FT-IR (KBr): ν (cm−1) 3004, 2933, 1591, 1513, 1462, 1266, 1240, 1138, 1027, 753; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.60 (dd, J1 = 8.1 Hz, J2 = 1.3 Hz, 2H), 7.54 (d, J = 8.3 Hz, 2H), 7.43 (t, J = 7.6 Hz, 2H), 7.33 (t, J = 7.4 Hz, 1H), 7.20 (d, J = 8.1 Hz, 2H), 6.82 (d, J = 8.4 Hz, 2H), 6.73 (d, J = 2.1 Hz, 2H), 6.66 (dd, J1 = 8.2 Hz, J2 = 2.1 Hz, 2H), 5.49 (s, 1H, Ar3CH), 3.88 (s, 6H, OMe), 3.79 (s, 6H, Me); 13C NMR (125 MHz, CDCl3): δ (ppm) 148.86, 147.59, 143.47, 140.81, 139.08, 136.67, 129.66, 128.72, 127.13, 126.95, 126.92, 121.45, 112.88, 111.00, 55.88, 55.87, 55.65; Anal. Calcd for C29H28O4: C, 79.07; H, 6.41. Found: C, 79.13; H, 6.40.
3-(Bis(3,4-dimethoxyphenyl)methyl)-1,1′-biphenyl (6b)
Pale yellow oil; 357 mg (81%); FT-IR (KBr): ν (cm−1) 3018, 2933, 1595, 1512, 1462, 1262, 1184, 1139, 1028, 751, 701; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.55 (d, J = 8.3 Hz, 2H), 7.48 (d, J = 7.2 Hz, 1H), 7.38–7.44 (m, 4H), 7.32–7.35 (m, 1H), 7.14 (d, J = 7.7 Hz, 1H), 6.82 (d, J = 8.3 Hz, 2H), 6.76 (d, J = 1.7 Hz, 2H), 6.66 (dd, J1 = 8.3 Hz, J2 = 1.7 Hz, 2H), 5.55 (s, 1H, Ar3CH), 3.88 (s, 6H, OMe), 3.80 (s, 6H, OMe); 13C NMR (125 MHz, CDCl3): δ (ppm) 148.86, 147.59, 144.93, 144.90, 141.11, 136.69, 128.71, 128.68, 128.27, 128.11, 127.24, 127.11, 127.08, 125.12, 121.54, 112.97, 111.11, 58.13, 56.05, 55.85; Anal. Calcd for C29H28O4: C, 79.07; H, 6.41. Found: C, 79.36; H, 6.41.
4-(Bis(2-methoxy-5-methylphenyl)methyl)-1,1′-biphenyl (6c)
White powder; 367 mg (90%); Mp 169–171 °C; FT-IR (KBr): ν (cm−1) 3025, 2923, 1497, 1241, 1077, 1035, 759; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.62 (d, J = 7.4 Hz, 2H), 7.50 (d, J = 8.2 Hz, 2H), 7.43 (t, J = 7.6 Hz, 2H), 7.32 (t, J = 7.4 Hz, 1H), 7.14 (d, J = 8.1 Hz, 2H), 7.02 (dd, J1 = 8.1 Hz, J2 = 1.5 Hz, 2H), 6.80 (d, J = 8.3 Hz, 2H), 6.78 (d, J = 1.6 Hz, 2H), 6.21 (s, 1H, Ar3CH), 3.70 (s, 6H, OMe), 2.22 (s, 6H, Me); 13C NMR (125 MHz, CDCl3): δ (ppm) 155.30, 143.31, 141.12, 138.26, 132.41, 130.76, 129.71, 129.24, 128.64, 127.55, 126.90, 126.87, 126.54, 111.03, 56.05, 42.74, 20.79; Anal. Calcd for C29H28O2: C, 85.26; H, 6.91. Found: C, 85.20; H, 6.88.
3-(Bis(2-methoxy-5-methylphenyl)methyl)-1,1′-biphenyl (6d)
White powder; 347 mg (85%); Mp 173–175 °C; FT-IR (KBr): ν (cm−1) 2943, 2883, 1591, 1498, 1470, 1242, 1153, 1035, 806; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.53–7.56 (m, 2H), 7.4–7.45 (m, 3H), 7.29–7.35 (m, 3H), 6.98–7.10 (m, 3H), 6.79 (d, J = 8.2 Hz, 2H), 6.67 (d, J = 2.3 Hz, 2H), 6.23 (s, 1H, Ar3CH), 3.69 (s, 6H, OMe), 2.21 (s, 6H, Me); 13C NMR (125 MHz, CDCl3): δ (ppm) 155.27, 144.62, 141.51, 140.56, 132.34, 130.77, 129.20, 128.57, 128.33, 128.27, 128.19, 127.55, 127.15, 126.94, 124.54, 110.96, 56.02, 43.17, 20.81; Anal. Calcd for C29H28O2: C, 85.26; H, 6.91. Found: C, 85.18; H, 6.86.
1-(4-(Bis(2-methoxy-5-methylphenyl)methyl)phenyl)naphthalen (6e)
White powder; 339 mg (74%); Mp 184–186 °C; FT-IR (KBr): ν (cm−1) 3007, 2952, 1608, 1498, 1439, 1242, 1153, 1072, 1035, 808, 755; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.06 (s, 1H), 7.85–7.91 (m, 3H), 7.77 (d, J = 8.3 Hz, 1H), 7.62 (d, J = 8.2 Hz, 2H), 7.45–7.52 (m, 2H), 7.18 (d, J = 8.1 Hz), 7.03 (d, J = 8.0 Hz, 2H), 6.81 (d, J = 8.2 Hz, 2H), 6.70 (s, 1H, Ar3CH), 3.71 (s, 6H, OMe), 2.23 (s, 6H, Me); 13C NMR (125 MHz, CDCl3): δ (ppm) 155.30, 143.46, 138.17, 133.72, 132.48, 132.39, 131.05, 130.91, 130.77, 130.61, 129.82, 129.25, 128.25, 128.12, 127.73, 127.57, 126.83, 126.12, 125.66, 125.54, 125.40, 111.03, 55.91, 42.78, 20.75; Anal. Calcd for C33H30O2: C, 86.43; H, 6.59. Found: C, 86.40; H, 6.61.
3-(4-(Bis(2-methoxy-5-methylphenyl)methyl)phenyl)pyridine (6f)
White powder; 307 mg (75%); Mp 181–183 °C; FT-IR (KBr): ν (cm−1) 2968, 2922, 1608, 1497, 1464, 1241, 1219, 1108, 1035, 772; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.88 (d, J = 2.0, 1H), 8.56 (dd, J1 = 4.9 Hz, J2 = 1.9 Hz, 1H), 7.90 (td, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H), 7.47 (d, J = 8.5 Hz, 2H), 7.36 (dd, J1 = 7.8 Hz, J2 = 4.3 Hz, 1H), 7.17 (d, J = 7.8 Hz, 2H), 7.02 (dd, J1 = 8.5 Hz, J2 = 2.1 Hz, 2H), 6.79 (d, J = 8.4 Hz, 2H), 6.65 (d, J = 2.4 Hz, 2H), 6.21 (s, 1H, Ar3CH), 3.69 (s, 6H, OMe), 2.21 (s, 6H, Me); 13C NMR (125 MHz, CDCl3): δ (ppm) 155.26, 148.24, 148.09, 144.41, 136.56, 134.95, 134.07, 132.10, 130.70, 130.01, 129.27, 127.66, 126.66, 126.56, 123.42, 111.01, 60.40, 42.78, 29.68, 20.77; Anal. Calcd for C28H27NO2: C, 82.12; H, 6.65; N, 3.42. Found: C, 82.05; H, 6.60; N, 3.36.
4′-(Bis(2-methoxy-5-methylphenyl)methyl)-[1,1′-biphenyl]-4-carbaldehyde (6g)
White powder; 318 mg (73%); Mp 166–168 °C; FT-IR (KBr): ν (cm−1) 2952, 1883, 1699, 1603, 1557, 1497, 1462, 1242, 1169, 1034, 825, 733; 1H NMR (500 MHz, CDCl3): δ (ppm) 10.05 (s, 1H, CHO), 7.94 (d, J = 8.5 Hz, 2H), 7.77 (d, J = 8.4 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 7.03 (dd, J1 = 7.3 Hz, J2 = 1.7 Hz, 2H), 6.80 (d, J = 8.3 Hz, 2H), 6.63 (d, J = 2.2 Hz, 2H), 6.22 (s, 1H, Ar3CH), 3.70 (s, 6H, OMe), 2.22 (s, 6H, Me); 13C NMR (125 MHz, CDCl3): δ (ppm) 201.05, 155.24, 147.18, 144.97, 136.76, 134.90, 132.01, 130.70, 130.23, 129.97, 129.27, 127.71, 127.39, 126.85, 110.98, 55.97, 42.85, 20.80; Anal. Calcd for C30H28O3: C, 82.54; H, 6.47. Found: C, 82.47; H, 6.41.
2-(Bis(3,4-dimethoxyphenyl)methyl)-1,1′-biphenyl (6h)
White powder; 299 mg (68%); Mp 117–120 °C; FT-IR (KBr): ν (cm−1) 3055, 2999, 2930, 2831, 1590, 1514, 1463, 1250, 1181, 1183, 1025, 798; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.23–7.33 (m, 6H), 7.10–7.14 (m, 3H), 6.74 (d, J = 8.3 Hz, 2H), 6.52 (dd, J1 = 8.2 Hz, J2 = 2.1 Hz, 2H), 6.48 (d, J = 2.2 Hz, 2H), 5.46 (s, 1H, Ar3CH), 3.85 (s, 6H, OMe), 3.71 (s, 6H, OMe); 13C NMR (125 MHz, CDCl3): δ (ppm) 148.61, 147.27, 142.32, 141.90, 141.63, 136.98, 134.58, 130.06, 129.68, 129.28, 128.24, 127.82, 127.37, 127.25, 126.92, 126.86, 124.04, 121.53, 121.84, 110.73, 55.81, 55.76, 52.21; Anal. Calcd for C29H28O4: C, 79.07; H, 6.41. Found: C, 79.00; H, 6.35.
1-(2-(Bis(3,4-dimethoxyphenyl)methyl)phenyl)naphthalen (6i)
Pale yellow oil; 260 mg (53%); FT-IR (KBr): ν (cm−1) 3054, 3002, 2934, 2834, 1591, 1512, 1462, 1414, 1263, 1242, 1184, 1139, 1028, 912, 742; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.87–7.90 (m, 1H), 7.81 (d, J = 8.2 Hz, 1H), 7.70–7.73 (m, 2H), 7.30–7.38 (m, 4H), 7.20–7.24 (m, 1H), 6.78 (d, J = 8.3 Hz, 2H), 6.59 (dd, J1 = 8.2 Hz, J2 = 2.1 Hz, 2H), 6.50 (d, J = 2.2 Hz, 2H), 5.51 (s, 1H, Ar3CH), 3.87 (s, 6H, OMe), 3.67 (s, 6H, OMe); 13C NMR (125 MHz, CDCl3): δ (ppm) 174.38, 169.72, 148.69, 147.34, 142.18, 142.16, 139.07, 137.04, 132.89, 132.33, 130.32, 129.85, 128.20, 128.18, 128.02, 127.82, 127.79, 127.64, 127.41, 127.36, 126.21, 125.95, 121.60, 112.89, 110.81, 55.88, 55.83, 55.74, 55.70, 52.54; Anal. Calcd for C33H30O4: C, 80.79; H, 6.16. Found: C, 80.72; H, 6.18.
3-(3-((5-Bromo-2-methoxyphenyl)(phenyl)methyl)-4-methoxyphenyl)pyridine (6j)
White powder; 381 mg (83%); Mp 136–138 °C; FT- IR (KBr): ν (cm−1) 3007, 2936, 2839, 1607, 1486, 1459, 1438, 1289, 1241, 1113, 1021, 806, 708; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.68 (dd, J1 = 2.4 Hz, J2 = 0.9 Hz, 1H), 8.50 (dd, J1 = 4.8 Hz, J2 = 1.7 Hz, 1H), 7.68 (qd, J1 = 8.0 Hz, J2 = 2.4 Hz, J3 = 1.7 Hz, 1H), 7.33 (dd, J1 = 8.7 Hz, J2 = 2.6 Hz, 1H), 7.26–7.31 (m, 3H), 7.20–7.24 (m, 1H), 7.05–7.10 (m, 2H), 7.06 (d, J = 1.7 Hz, 1H), 6.98 (d, J = 8.5 Hz, 1H), 6.93 (dd, J1 = 2.6 Hz, J2 = 0.6 Hz, 1H), 6.76 (d, J = 8.8 Hz, 1H), 6.17 (s, 1H, Ar3H), 3.76 (s, 3H, OMe), 3.69 (s, 3H, OMe); 13C NMR (126 MHz, CDCl3): δ (ppm) 157.36, 156.37, 148.01, 147.71, 142.39, 136.33, 134.64, 133.78, 132.67, 132.45, 130.28, 129.61, 129.21, 128.61, 128.22, 126.30, 123.42, 112.74, 112.52, 111.28, 55.93, 55.82, 43.53; Anal. Calcd for C26H22BrNO2: C, 67.83; H, 4.82; N, 3.04. Found: C, 67.77; H, 4.75; N, 2.98.
3,3′′-(Phenylmethylene)bis(4-methoxy-1,1′-biphenyl) (6k)
White powder; 356 mg (78%); Mp 65–67 °C; FT-IR (KBr): ν (cm−1) 3028, 3000, 2933, 1605, 1483, 1462, 1242, 1110, 1075, 1025, 760, 700; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.56–7.61 (m, 6H), 7.45–7.49 (m, 4H), 7.40–7.44 (m, 2H), 7.30–7.40 (m, 7H), 7.06 (d, J = 8.4 Hz, 2H), 6.49 (s, 1H, Ar3CH), 3.84 (s, 6H, OMe); 13C NMR (125 MHz, CDCl3): δ (ppm) 157.14, 143.60, 141.28, 133.15, 132.96, 129.52, 129.00, 128.78, 128.20, 126.81, 126.58, 126.15, 126.11, 111.29, 55.95, 43.79; Anal. Calcd for C33H28O2: C, 86.81; H, 6.18. Found: C, 86.75; H, 6.15.
3,3′-((Phenylmethylene)bis(4-methoxy-3,1-phenylene))dipyridine (6l)
White powder; 248 mg (54%); Mp 190–192 °C; FT-IR (KBr): ν (cm−1) 3027, 2931, 2836, 1607, 1503, 1474, 1288, 1249, 1113, 1065, 1022, 801, 751, 712; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.66 (d, J = 2.3 Hz, 2H), 8.47 (dd, J1 = 4.8 Hz, J2 = 1.6 Hz, 2H), 7.66–7.69 (m, 2H), 7.46 (dd, J1 = 8.4 Hz, J2 = 2.4 Hz, 2H), 7.20–7.31 (d, J = 2.3 Hz, 2H), 6.99 (d, J = 8.5 Hz, 2H), 6.26 (s, 1H, Ar3CH), 3.77 (s, 6H, OMe); 13C NMR (125 MHz, CDCl3): δ (ppm) 157.47, 149.35, 148.23, 147.98, 147.65, 142.84, 136.39, 134.42, 133.76, 133.02, 129.54, 129.23, 128.59, 128.18, 126.22, 123.78, 123.41, 111.27, 55.87, 43.71; Anal. Calcd for C31H26N2O2: C, 81.20; H, 5.72; N, 6.11. Found: C, 81.15; H, 5.70; N, 6.06.
2.2.3. General procedure for the Mizoroki–Heck coupling reactions of TRAMs with olefins 13a–j.
An oven-dried 25 mL two-neck flask equipped with a condenser and a stir bar was charged with PdCl2 (0.05 mmol), PPh3 (0.1 mmol), and LiBr (0.1 mmol); two cycles of vacuum N2 were performed. Anhydrous dimethylformamide (DMF, 2 mL) was added, and the resulting suspension was heated at 140 °C for 30 min. After cooling the yellowish mixture to room temperature, Et3N (2 mmol) was added. This was followed by the addition of a solution of TRAM 10 (1 mmol) and olefin 12 (2 mmol) in anhydrous DMF (3 mL). The reaction mixture was heated at 100 °C for 36 h, allowed to reach room temperature, and filtered through a plug of Celite. After the removal of volatiles in vacuo, the crude product was purified by flash chromatography on silica gel using n-hexane/EtOAc (5:1) as an eluent to obtain the corresponding pure product 13a–j.
Methyl (E)-3-(4-(bis(2-methoxy-5-methylphenyl)methyl)phenyl)acrylate (13a)
White powder; 371 mg (89%); Mp 160–162 °C; FT-IR (KBr): ν (cm−1) 3022, 2948, 2834, 1719, 1634, 1605, 1498, 1463, 1242, 1168, 1035, 858, 757; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.69 (d, J = 16.0 Hz, 1H), 7.42 (d, J = 7.2 Hz, 2H), 7.09 (d, J = 8.1 Hz, 2H), 7.02 (d, J = 7.1 Hz, 2H), 7.78 (d, J = 8.3 Hz, 2H), 6.61 (s, 2H), 6.41 (d, J = 16.0 Hz, 1H), 6.16 (s, 1H), 3.80 (s, 3H), 3.67 (s, 6H), 2.21 (s, 6H); 13C NMR (125 MHz, CDCl3): δ (ppm) 167.64, 155.20, 147.28, 145.09, 131.82, 131.72, 130.67, 129.80, 129.27, 127.82, 127.75, 116.66, 110.93, 55.93, 51.60, 43.11, 20.76; Anal. Calcd for C27H28O4: C, 77.86; H, 6.78. Found: C, 77.80; H, 6.72.
Tert-butyl (E)-3-(4-(bis(2-methoxy-5-methylphenyl)methyl)phenyl)acrylate (13b)
White powder; 389 mg (85%); Mp 147–149 °C; FT-IR (KBr): ν (cm−1) 3023, 2956, 2871, 1711, 1635, 1606, 1498, 1462, 1243, 1169, 1071, 1035, 807, 734; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.73 (d, J = 16.0 Hz, 1H), 7.41 (d, J = 8.3 Hz, 2H), 7.08 (d, J = 8.3 Hz, 2H), 7.01 (dd, J1 = 8.2 Hz, J2 = 2.7 Hz, 1H), 6.78 (d, J = 8.3 Hz, 2H), 6.60 (d, J = 2.3 Hz, 2H), 6.40 (d, J = 16.0 Hz, 1H), 6.16 (s, 6H, Ar3CH), 3.67 (s, 3H, 6H, OMe), 2.20 (s, 6H, Me), 1.54 (s, 9H, But); 13C NMR (125 MHz, CDCl3): δ (ppm) 167.62, 155.21, 147.27, 145.07, 131.82, 131.73, 130.67, 129.78, 129.28, 127.79, 127.74, 116.64, 110.96, 55.95, 55.91, 51.54, 28.36, 20.74; Anal. Calcd for C30H34O4: C, 78.57; H, 7.47. Found: C, 78.51; H, 7.40.
(E)-2,2′-((4-Styrylphenyl)methylene)bis(1-methoxy-4-methylbenzene) (13c)
White powder; 308 mg (71%); Mp 105–107 °C; FT-IR (KBr): ν (cm−1) 3003, 2938, 2836, 1837, 1558, 1533, 1458, 1416, 1342, 1258, 1233, 1165, 1028, 876, 763; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.88 (d, J = 8.2 Hz, 2H), 7.67 (d, J = 8.2 Hz, 2H), 7.56 (d, J = 7.5 Hz, 2H), 7.32–7.43 (m, 3H), 7.16 (d, J = 16.4 Hz, 1H), 7.00 (dd, J1 = 8.2 Hz, J2 = 1.7 Hz, 2H), 6.92 (d, J = 8.4 Hz, 2H), 6.77 (d, J = 8.3 Hz, 2H), 6.57 (d, J = 1.7 Hz, 2H), 6.08 (s, 1H), 3.66 (s, 6H), 2.20 (s, 6H); 13C NMR (125 MHz, CDCl3): δ (ppm) 155.27, 131.75, 131.06, 130.91, 130.72, 130.62, 129.64, 129.26, 129.21, 128.61, 127.73, 127.56, 126.38, 126.51, 110.96, 110.96, 55.91, 42.69, 20.75; Anal. Calcd for C31H30O2: C, 85.68; H, 6.96. Found: C, 85.60; H, 6.93.
Methyl (E)-3-(4-(bis(3,4-dimethoxyphenyl)methyl)phenyl)acrylate (13d)
Pale yellow oil; 390 mg (87%); FT-IR (KBr): ν (cm−1) 2999, 2951, 2835, 1716, 1635, 1604, 1512, 1462, 1415, 1324, 1264, 1169, 1140, 1028, 731; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.67 (d, J = 16.15 Hz, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.14 (d, J = 8.2 Hz, 2H), 6.79 (d, J = 8.3 Hz, 2H), 6.65 (d, J = 2.2 Hz, 2H), 6.58 (dd, J1 = 8.6 Hz, J2 = 1.8 Hz, 2H), 6.41 (d, J = 16.0 Hz, 1H), 5.45 (s, 1H, Ar3CH), 3.85 (s, 6H, OMe), 3.79 (s, 3H, CO2Me), 3.76 (s, 6H, OMe); 13C NMR (125 MHz, CDCl3): δ (ppm) 160.78, 148.84, 147.62, 147.01, 144.56, 136.04, 132.46, 129.80, 128.07, 121.34, 117.32, 112.64, 110.91, 55.87, 55.84, 55.81, 51.68; Anal. Calcd for C27H28O6: C, 72.30; H, 6.29. Found: C, 72.25; H, 6.25.
Methyl (E)-3-(3-(bis(3,4-dimethoxyphenyl)methyl)phenyl)acrylate (13e)
Pale yellow oil; 351 mg (78%); FT-IR (KBr): ν (cm−1) 3003, 2932, 2835, 1604, 1512, 1463, 1415, 1262, 1139, 1062, 754; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.04 (d, J = 15.8 Hz, 1H), 7.55 (dd, J1 = 7.6 Hz, J2 = 1.1 Hz,1H), 7.23–7.30 (m, 2H), 6.93 (d, J = 7.6 Hz, 1H), 6.77 (d, J = 8.3 Hz, 2H), 6.64 (d, J = 1.4 Hz, 2H), 6.53 (dd, J1 = 8.2 Hz, J2 = 1.7 Hz, 2H), 6.27 (d, J = 15.8 Hz, 1H), 3.86 (s, 6H), 3.77 (s, 6H), 3.77 (s, 3H); 13C NMR (125 MHz, CDCl3): δ (ppm) 167.13, 148.89, 147.59, 143.58, 142.60, 135.67, 133.70, 129.80, 129.72, 126.88, 126.80, 121.64, 119.71, 112.85, 110.95, 55.82, 55.80, 52.28, 51.59; Anal. Calcd for C27H28O6: C, 72.30; H, 6.29. Found: C, 72.25; H, 6.23.
Methyl (E)-3-(2-(bis(3,4-dimethoxyphenyl)methyl)phenyl)acrylate (13f)
White powder; 287 mg (64%); Mp 115–117 °C; FT-IR (KBr): ν (cm−1) 3000, 2950, 2834, 1716, 1631, 1512, 1462, 1316, 1263, 1243, 1139, 1027, 757; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.04 (d, J = 15.8 Hz, 1H), 7.55 (dd, J1 = 7.4 Hz, J2 = 1.8 Hz, 1H), 7.23–7.32 (m, 3H), 6.93 (dd, J1 = 7.5 Hz, J2 = 1.7 Hz, 1H), 6.78 (d, J = 8.3 Hz, 2H), 6.64 (d, J = 2.1 Hz, 2H), 6.53 (dd, J1 = 8.3 Hz, J2 = 2.1 Hz, 2H), 6.27 (d, J = 15.8 Hz, 1H), 5.76 (s, 6H), 3.86 (s, 6H), 3.77 (s, 6H), 3.75 (s, 3H); 13C NMR (125 MHz, CDCl3): δ (ppm) 167.16, 148.86, 147.56, 143.60, 142.60, 135.66, 133.69, 129.82, 129.76, 126.89, 126.82, 121.63, 119.71, 112.78, 110.89, 55.82, 55.80, 52.28, 51.16; Anal. Calcd for C27H28O6: C, 72.30; H, 6.29. Found: C, 72.32; H, 6.25.
(E)-4,4′-((3-Styrylphenyl)methylene)bis(1,2-dimethoxybenzene) (13g)
White powder; 317 mg (68%); Mp 97–99 °C; FT-IR (KBr): ν (cm−1) 2998, 2936, 1840, 1560, 1513, 1449, 1412, 1340, 1265, 1249, 1135, 1052, 995, 854, 761; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 7.57 (d, J = 7.7 Hz, 2H), 7.48 (d, J = 7.8 Hz, 1H), 7.17–7.36 (m, 7H), 6.98 (d, J = 7.7 Hz, 1H), 6.87 (d, J = 8.3 Hz, 2H), 6.67 (d, J = 2.1 Hz, 2H), 6.58 (dd, J1 = 8.3 Hz, J2 = 2.1 Hz, 2H), 5.48 (s, 1H), 3.70 (s, 6H), 3.63 (s, 6H, 6H); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 148.99, 147.73, 145.34, 137.41, 137.40, 136.85, 129.11, 129.04, 128.95, 128.45, 128.68, 128.06, 128.01, 126.93, 124.42, 121.49, 113.53, 112.15, 55.94, 55.42; Anal. Calcd for C31H30O4: C, 79.80; H, 6.48. Found: C, 79.74; H, 6.42.
(E)-4,4′-((2-Styrylphenyl)methylene)bis(1,2-dimethoxybenzene) (13h)
White powder; 294 mg (63%); Mp 101–103 °C; FT-IR (KBr): ν (cm−1) 2995, 2935, 1835, 1589, 1512, 1449, 1340, 1263, 1248, 1184, 1136, 1025, 761; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.61 (d, J = 7.5 Hz, 1H), 7.33–7.37 (m, 3H), 7.17–7.28 (m, 3H), 6.93 (d, J = 16.1 Hz, 1H), 6.89 (dd, J1 = 7.6 Hz, J2 = 1.4 Hz, 1H), 6.80 (d, J = 8.3 Hz, 2H), 6.69 (d, J = 2.1 Hz, 2H), 6.60 (dd, J1 = 10.7 Hz, J2 = 2.1 Hz, 2H), 5.74 (s, 1H), 3.87 (s, 6H), 3.77 (s, 6H); 13C NMR (125 MHz, CDCl3): δ (ppm) 148.93, 147.56, 141.98, 137.58, 136.76, 136.19, 130.76, 129.54, 128.62, 127.59, 127.39, 126.87, 126.67, 126.47, 126.20, 121.68, 112.98, 111.04, 55.84, 52.78; Anal. Calcd for C31H30O4: C, 79.80; H, 6.48. Found: C, 79.75; H, 6.43.
(E)-4,4′-((2-(4-Chlorostyryl)phenyl)methylene)bis(1,2-dimethoxybenzene) (13i)
White powder; 302 mg (60%); Mp 131–133 °C; FT-IR (KBr): ν (cm−1) 3020, 2933, 2834, 1590, 1512, 1462, 1414, 1263, 1139, 1028, 812, 754; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 7.68 (dd, J1 = 7.6 Hz, J2 = 1.6 Hz, 1H), 7.51 (d, J = 16.2 Hz, 1H), 7.47 (d, J = 7.1 Hz, 2H), 7.32 (t, J = 7.7 Hz, 2H), 7.17–7.25 (m, 3H), 7.01 (d, J = 16.2 Hz, 1H), 6.86 (d, J = 8.2 Hz, 2H), 6.81 (d, J = 2.1 Hz, 2H), 6.52 (dd, J1 = 8.2 Hz, J2 = 2.0 Hz, 2H), 5.51 (s, 1H, Ar3CH), 3.69 (s, 6H, OMe), 3.63 (s, 6H, OMe); 13C NMR (126 MHz, DMSO-d6): δ (ppm) 149.02, 147.62, 142.70, 137.75, 136.52, 136.14, 129.97, 129.41, 129.06, 129.03, 126.89, 126.85, 126.59, 126.53, 126.05, 121.74, 113.68, 112.06, 55.92, 55.89, 55.85, 55.82, 51.87; Anal. Calcd for C31H29ClO4: C, 74.32; H, 5.83. Found: C, 74.28; H, 5.80.
3-(4-(Bis(3,4-dimethoxyphenyl)methyl)phenyl)acrylonitrile (13j)
White powder; 266 mg (64%) (E:Z = 60:40 based on 1H NMR spectrum); Mp 98–101 °C; FT-IR (KBr): ν (cm−1) 3003, 2932, 2835, 2215, 1604, 1512, 1463, 1415, 1262, 1244, 1139, 1026, 966, 800, 754; 1H NMR of (E)-isomer (500 MHz, CDCl3): δ (ppm) 7.60 (d, J = 16.8 Hz, 1H), 7.56 (d, J = 8.4 Hz, 2H), 7.16 (d, J = 8.3 Hz, 2H), 6.86 (d, J = 8.3 Hz, 2H), 6.72 (d, J = 2.0 Hz, 2H), 6.55 (dd, J1 = 8.2 Hz, J2 = 2.0 Hz, 2H), 6.38 (d, J = 16.8 Hz, 1H), 5.49 (s, 1H), 3.70 (s, 6H), 3.63 (s, 6H); 1H NMR of (Z)-isomer (500 MHz, CDCl3): δ (ppm) 7.73 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 14.0 Hz, 1H), 7.23 (d, J = 9.2 Hz, 2H), 6.87 (d, J = 8.4 Hz, 2H), 6.74 (d, J = 2.0 Hz, 2H), 6.57 (dd, J1 = 8.1 Hz, J2 = 2.0 Hz, 2H), 5.81 (d, J = 12.1 Hz, 1H), 5.51 (s, 1H), 3.70 (s, 6H), 3.63 (s, 6H); 13C NMR (125 MHz, CDCl3): δ (ppm) 150.74, 149.02, 148.27, 148.05, 136.46, 132.22, 129.96, 129.11, 128.20, 121.44, 113.43, 112.18, 110.03, 55.94, 55.33; Anal. Calcd for C26H25NO4: C, 75.16; H, 6.07; N, 3.37. Found: C, 75.10; H, 6.00; N, 3.30.
Synthesis of TRAMs containing biaryl unit(s). Reaction conditions: TRAMs 10 (1 mmol), 11 (1.2 mmol), Pd(PPh3)4 (5 mol%), K2CO3 (1 mL, 2 M), and THF (5 mL) in N2 atmosphere and under reflux conditions. aReaction time. bIsolated yield.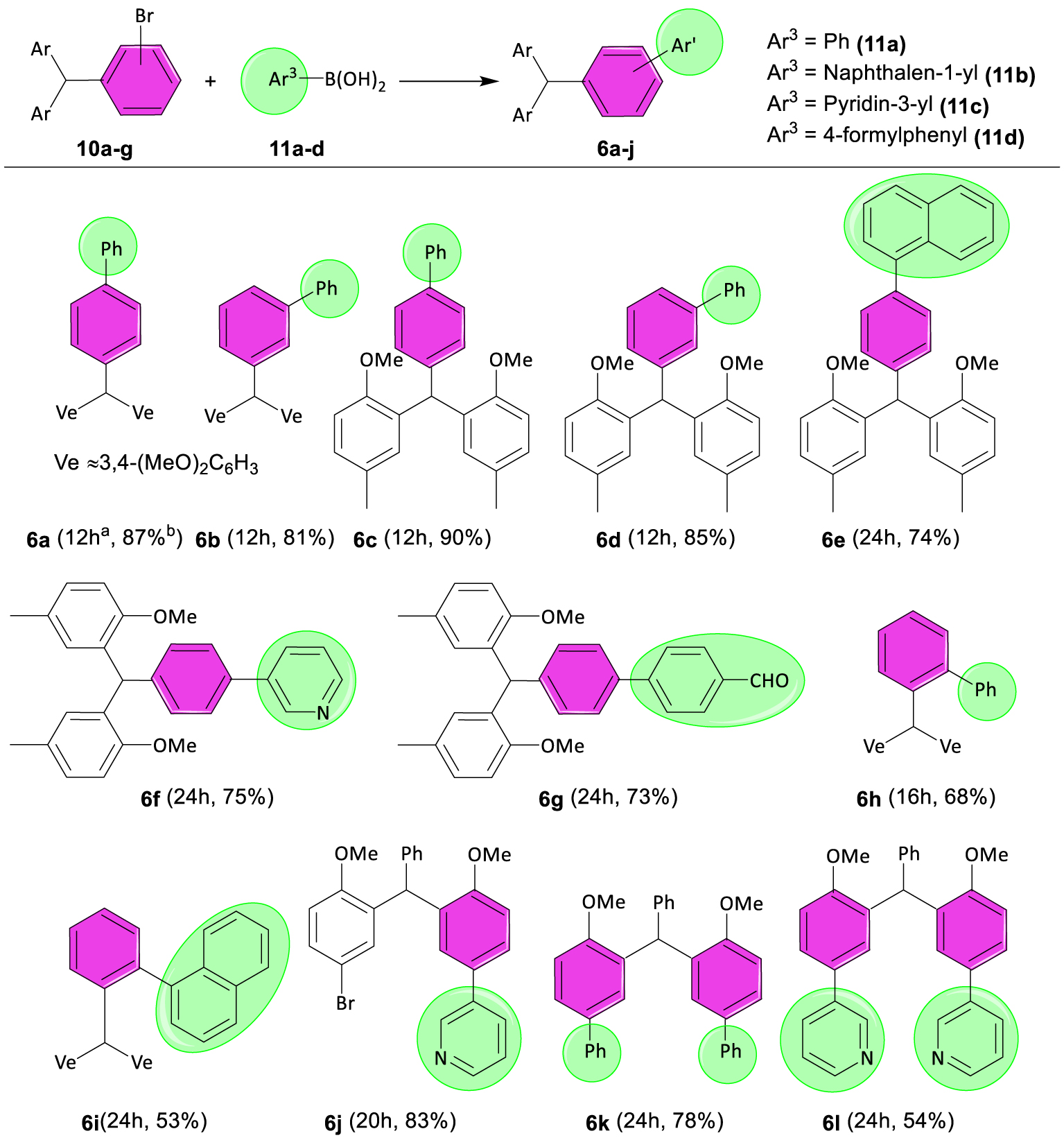
3. Results and discussion
3.1. Synthesis of new TRAMs bearing one or two biaryl moieties via [Pd]-catalyzed Suzuki–Miyaura cross-coupling reactions
Tetrakis(triphenylphosphine)palladium(0) is a commercially available product that has been traditionally used as a convenient catalyst in coupling reactions [7, 28, 29, 30]. We therefore decided to investigate the coupling reaction of brominated TRAMs 10a–g with arylboronic acids 11a–d in the presence of Pd(PPh3)4 for building TRAMs containing biaryl unit(s). Accordingly, the treatment of TRAM 10a with phenylboronic acid 11a in the presence of 5 mol% Pd(PPh3)4 under optimal reaction conditions (see Supporting information) resulted in the formation of coupling product 6a in 87% yield (Scheme 3).
Likewise, brominated TRAMs 10a–h were subjected to the Suzuki coupling reaction with arylboronic acid derivatives 11a–d to produce the corresponding coupling products, and the results are shown in Scheme 3. The reaction of brominated TRAMs 10b, 10d, and 10e with phenylboronic acid 11a proceeded efficiently, and the desired coupling products 6b–d were obtained in yields ranging from 81% to 90%. We next used naphthalen-1-ylboronic acid 11b, pyridin-3-ylboronic acid 11c, and (4-formylphenyl)boronic acid 11d as coupling partners in the reaction with TRAM 10d under optimal conditions, and products 6e–g were acquired in 74%, 75%, and 73% yields, respectively. Then we examined the reactivity of TRAM 10c in the coupling reaction with arylboronic acids. Although the reaction of TRAM 10c with phenylboronic acid 11a generated product 6h in 68% yield after 16 h, product 6i was isolated in moderate yield via the coupling reaction of 10c with naphthalen-1-ylboronic acid 11b after 24 h. Accordingly, ortho-brominated TRAM 10c displayed significant resistance toward the coupling reaction with arylboronic acids probably due to the steric effects of the diveratrylmethyl group (Ve2CH–). In addition, the reaction of chlorinated TRAM 10h with phenylboronic acids 11a did not proceed efficiently even after 24 h due to the strength of the C–Cl bond. This result proved that brominated TRAMs have been selected properly as substrates for this procedure. On the other hand, we found this protocol suitable for the indirect synthesis of unsymmetrical TRAMs. For example, when we treated TRAM 10g with pyridin-3-ylboronic acid 11c under optimal conditions, unsymmetrical TRAM 6j was obtained selectively in 83% yield after 20 h as depicted in Scheme 3. To the best of our knowledge, there exist a few examples of TRAM derivatives containing two biaryl moieties. Moreover, this kind of TRAM could not be synthesized by Friedel–Crafts alkylation reactions of aldehydes. Double Suzuki–Miyaura coupling reactions using palladium catalysts have been repeatedly reported in organic synthesis [31, 32, 33, 34, 35]. Surprisingly, one-pot, double Suzuki coupling reactions of TRAM 10g with two-fold amounts of 11a and 11c under optimal reaction conditions resulted in products 6k and 6l in 78% and 54% yields, respectively. Furthermore, this protocol may offer an efficient approach for the induction of chirality into TRAM molecules by constructing a biaryl or aryl(heteroaryl) moiety on them.
3.2. Synthesis of vinylated TRAMs through Mizoroki–Heck cross-coupling reaction of brominated TRAMs with olefins
Prompted by the results obtained in the Suzuki coupling reactions, we became interested in investigating the Pd-catalyzed Heck coupling reactions of brominated TRAMs with olefins. There are various reports in the literature on the use of PdCl2(PPh3)2 in coupling reactions [4, 36, 37, 38, 39]. We initially set up a one-pot, two-step protocol to react TRAM 10d with methyl acrylate 12a in the presence of in situ generated PdCl2(PPh3)2 as a pre-catalyst. As depicted in Scheme 4, the expected coupling product 10a was isolated in 89% yield under optimal reaction conditions (see Supporting information).
Structures of vinylated TRAMs produced through PdCl2(PPh3)2-catalyzed Mizoroki–Heck-type reaction of brominated TRAMs with olefins.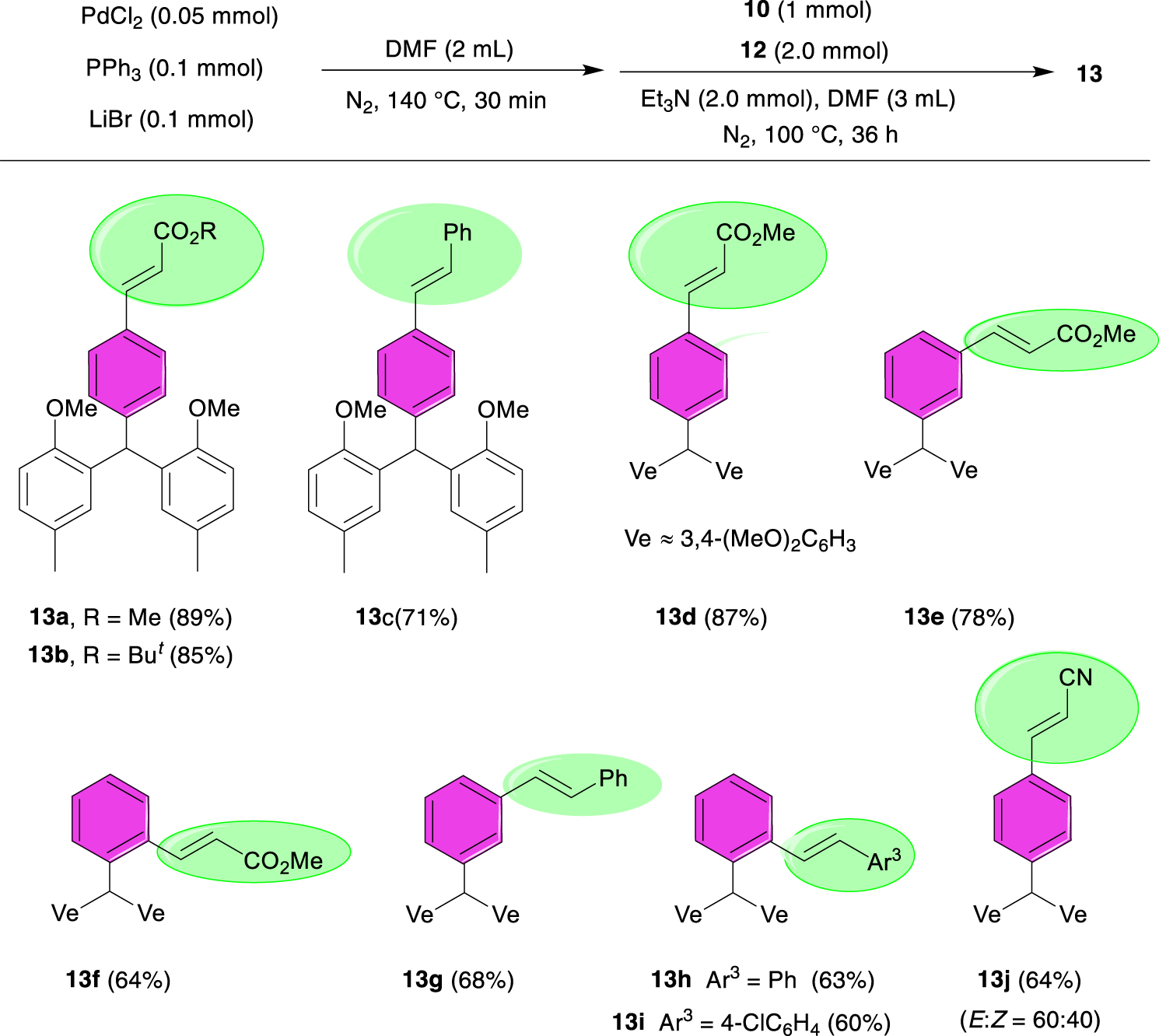
We decided to also examine the generality of this protocol, and the results are illustrated in Scheme 4. The Heck coupling reaction of TRAM 10d with tert-butyl acrylate 12b produced the coupling product 13b in 85% yield. When styrene 12c was used as the coupling partner in the reaction with TRAM 10d, the respective (E)-stilbene product 13c was isolated in 71% yield. However, triarylmethane 10d failed to react with acrylamide 12d under optimal conditions. We then turned our attention to the vinylation–functionalization of TRAMs 10a–c containing two veratryl moieties. Under optimal conditions, these precursors underwent vinylation with 12a to produce the corresponding aryl acrylates 13d–f in good to high yields. Substrate 10b also reacted smoothly with 12c to produce the olefination product 13g in 68% yield. The reaction of TRAM 10c with 12c and 4-chlorostyrene 12e proceeded well to generate the expected (E)-stilbene derivatives 13h and 13i in slightly lower yields. These results indicate that the substrates containing a sterically hindered diveratrylmethyl substituent (Ve2CH–) at the ortho-position of the C–Br bond are less reactive in olefination reactions. Moreover, acrylonitrile 12f reacted with TRAM 10d producing a non-separable mixture of E- and Z-isomers of aryl acrylonitrile 13j in 64% yield in an approximate ratio of 60:40, respectively (based on 1H NMR of the mixture; see Supporting information). Compounds 13a–j produced via this procedure could not be synthesized by means of the common synthetic methods for TRAMs. Furthermore, our initial evaluation showed that these compounds can be promising building blocks in organic synthesis. This study is currently ongoing in our laboratory.
4. Conclusion
In summary, symmetrical and unsymmetrical TRAMs bearing one or two biaryl units can be synthesized in good to high yields from the Suzuki–Miyaura cross-coupling reaction of brominated TRAMs with arylboronic acids in the presence of Pd(PPh3)4, which is a cheap and readily available catalyst. We have also developed a one-pot, two-step Heck-type coupling procedure for the efficient vinylation of brominated TRAMs. This protocol provides an exclusive and efficient method for the synthesis of functionalized TRAMs, which cannot be produced by conventional methods. The obtained products can be used as building blocks in organic transformations or as ligands for catalysis objectives.




 CC-BY 4.0
CC-BY 4.0