1. Introduction
Most idiopathic kidney stones are composed of an organic matrix and crystals of calcium oxalate (CaOx) mixed with small amounts of apatitic calcium phosphate (CaP). Consensus is that these CaOx stones are formed in association with Randall’s plaques (RP) or Randall’s plugs (RPg) on the renal papillae [1, 2, 3]. The plaques are suburothelial deposits of hydroxyapatite and originate deep inside the papillary tissue, suggested to start in the basement membrane of the loops of Henle. The plugs are crystalline deposits in the terminal collecting ducts. This article explores the role of inflammation and injury in the pathogenesis of CaOx kidney stones with emphasis on the development of RPs and production of stone matrix contents.
2. Randall’s plaques
Plaques are suburothelial deposits of biological apatite on renal papillary surface [1, 4, 5, 6]. They start as interstitial deposits of CaP deep in the renal papillary tip and are common in both the stone formers and non-stone formers. All of the plaques, however, are not associated with or support kidney stone formation [7]. It has been proposed that plaque formation starts as concentrically laminated spherulitic CaP crystals in the tubular basement (Figures 1, 2), most likely that of loops of Henle. Such crystals, 0.5–2 μm across, are seen in the tubular basement membrane and renal interstitium. The calcification proceeds from the tubular basement membrane through the interstitium and reaches the base of the papillary surface urothelium. Erosion of the covering urothelium exposes the underlying crystalline plaque to the urine with its various urinary components. Stones form on the exposed plaque by the addition of crystals on the exposed surface through nucleation, growth and aggregation. The interstitial calcifications include highly dense deposits of elongated calcified strands aggregated with spherulitic units of varying diameters. In addition, collagen fibers, unidentified fibrillary material, (Figures 2, 3) membrane-bound vesicles, and cellular degradation products are also detectable [8]. The results of electron diffraction of the calcified deposits demonstrated a range of crystallinity with the center being more crystalline than the periphery. Higher magnification of the plaques as shown in Figures 2 and 3 clearly demonstrate that spherulites and other crystals are actually a complex of crystals and organic matrix, so called biocrystals. Plaques also express osteopontin (OPN), heavy chain of inter-α-trypsin-inhibitor [2, 9], and zinc [10].
TEM of a section of renal papilla from CaOx kidney stone former. There are a number of damaged tubules surrounded by calcium phosphate (CaP) deposits.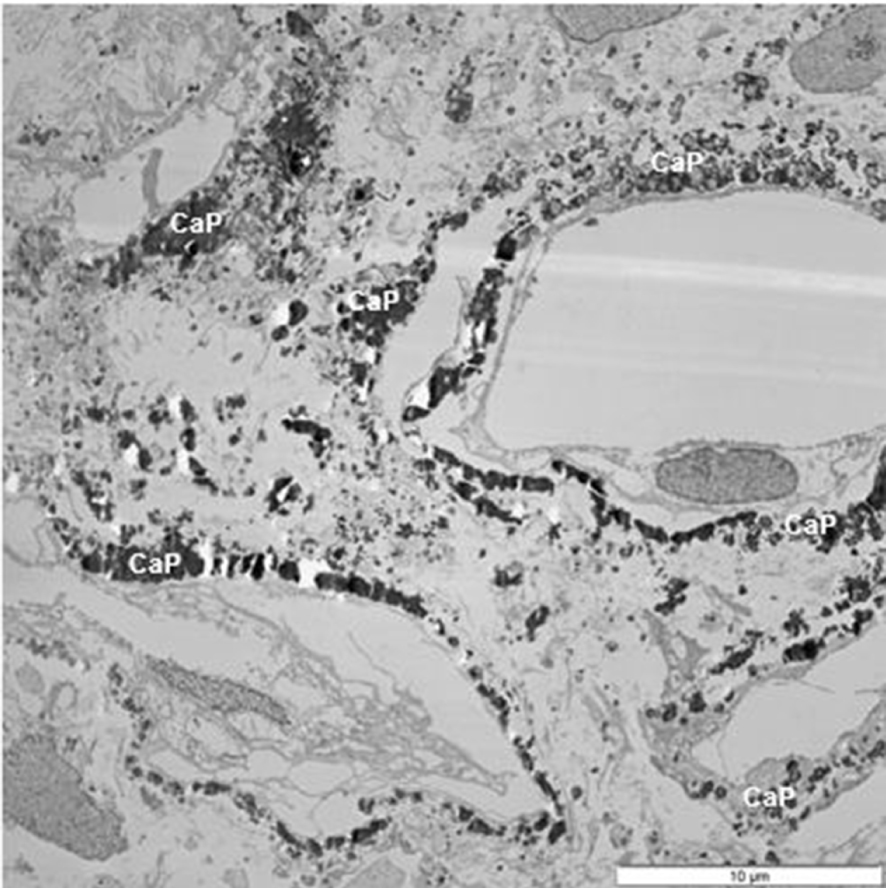
TEM of renal interstitium of a stone former showing aggregated calcium phosphate (CaP) deposits as well as a spherical (CaPs). Interstitium is filled with collagen (C) and other unidentified fibers, and vesicles (V). Part of a normal appearing epithelial cell with nucleus (N) and basement membrane are also seen.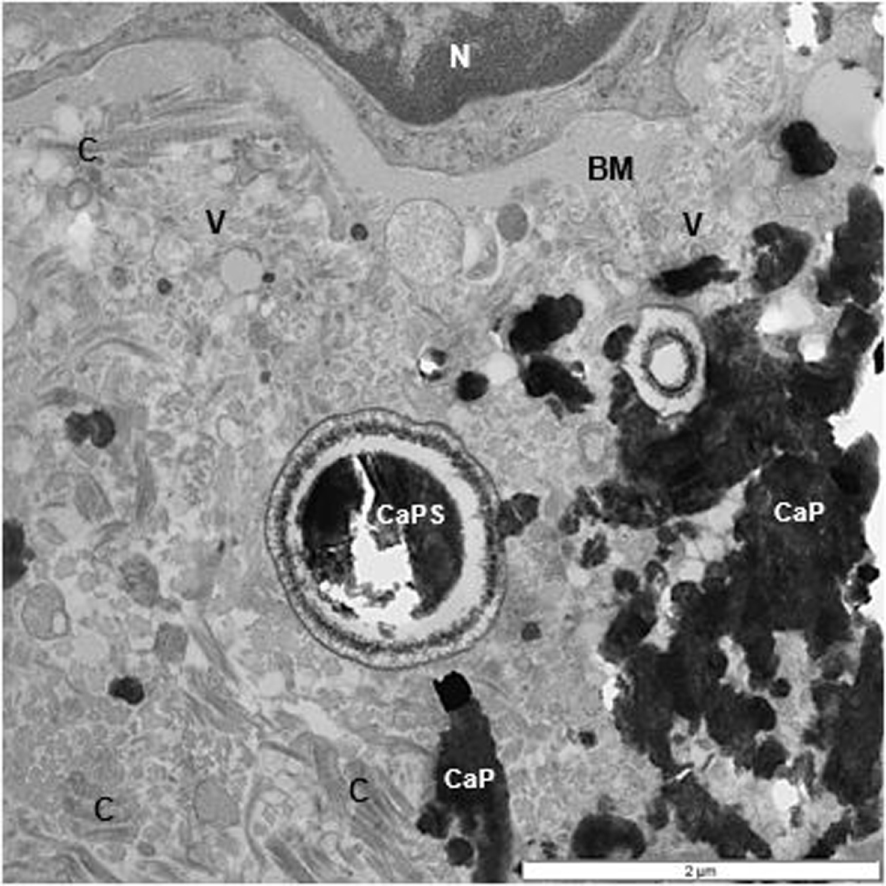
TEM of renal interstitium of a stone former filled with CaP deposits and masses of fibrillary material. Some of them clearly collagenous (C). One of the collagen strands appears to have been calcified (CC).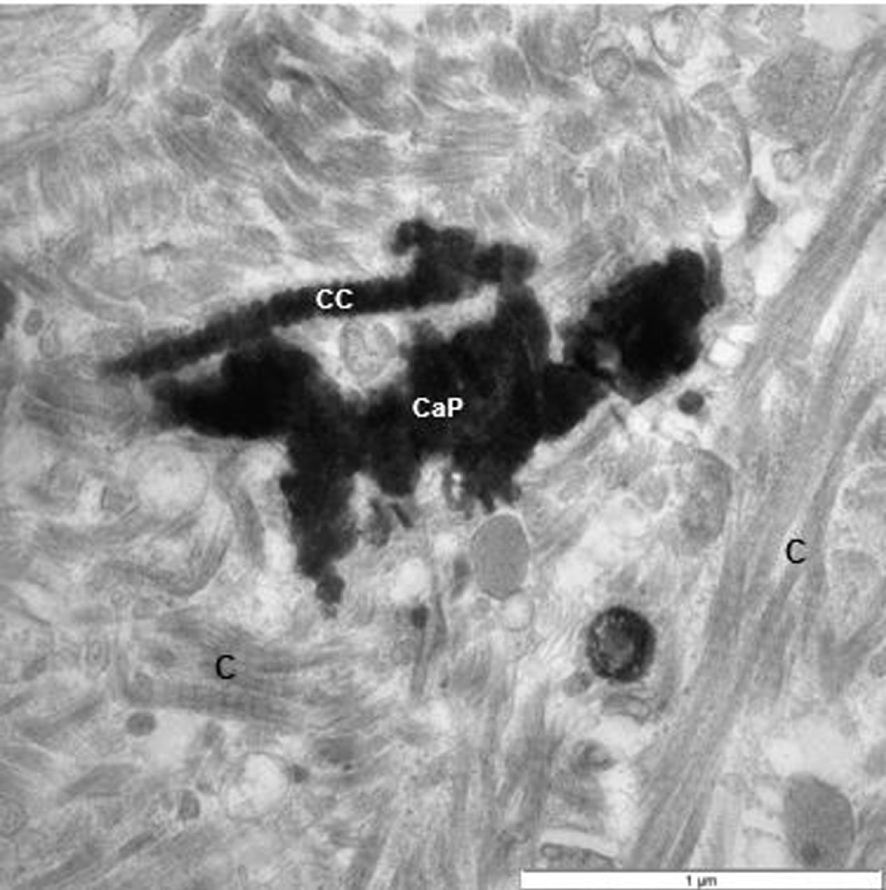
A number of hypotheses have been put forward to explain the pathogenesis of Randall’s plaque. One theory proposes that calcium excretion plays a central role in the development of the plaques [11]. This theory describes plaque and stone formation as basically a supersaturation dependent chemical reaction between various ions. The proposed mechanism involves decreased reabsorption of calcium in the proximal tubule leading to increased calcium in the thick ascending limbs of the loop and reabsorption into the interstitium. This leads to an increased supersaturation for CaP in the interstitium around the limbs of the loops of Henle promoting the formation of CaP crystals [12, 13]. Proponents of the hypercalciuria theory reported the absence of any signs of cell injury, inflammation, interstitial fibrosis or intratubular crystal deposition.
Other studies of renal papillary tips from idiopathic stone formers have reported the existence of cellular injury and inflammation in association with the interstitial RPs. Analysis of kidneys obtained from patients undergoing radical nephrectomy showed the presence of mineral deposits in all human renal tissues with as well as without the RPs. Both intratubular and interstitial crystalline deposits were discovered, intratubular in the outer medulla, and interstitial in the papillary tips in close alignment with the vasa recta. A positive correlation was found between proximal intratubular and the distal interstitial crystal deposition. Both types of deposits were made of CaP but intratubular deposits were comprised of large spherical aggregates of several hundred nanometer long plate or needle shaped crystals while interstitial deposits contained small spherical entities united together and embedded in a collagenous matrix. Both types of deposits stained positive for OPN, osteocalcin (OC) and bone specific protein (BSP) [14]. Additionally, vasa recta and renal tubules were also positive for OPN and OC. It was concluded that both vasa recta and renal tubules participate in interstitial calcification and perhaps proximal intratubular CaP deposition stimulates distal interstitial deposition [15]. It is interesting to note that OPN, OC and BSP are osteoblast markers and play a well-established role in vascular calcification. OPN is a known participant in inflammation and a well-known modulator of mineralization. It is ubiquitous in matrices of soft tissue calcifications, RPs and stone and urinary crystal. Osteocalcin is found in matrices of both the bone and stone matrices [16, 17]. Bone specific protein is involved in both physiological and pathological conditions [18, 19, 20].
Our ultrastructural observations of the renal papillae from both the primary hyperoxaluric [21], as well as idiopathic stone formers [8], have shown clear signs of cellular injury and inflammation. Histological examination of paraffin embedded papillectomy specimens from a patient with malabsorption revealed interstitial fibrosis, and focal epithelial hyperplasia in association with PAS (periodic acid schiff) positive calcification [21]. Ultrastructural analysis of the calcified areas disclosed enlarged interstitium filled with myofibroblasts, abundant collagen, unidentified fibrils, and necrotic cellular debris. Tubular epithelial cells appeared necrotic with swollen mitochondria and vesiculating endoplasmic reticulum. Basement membrane of the epithelial cells appeared thickened and multilayered. CaP deposits included 120–150 nm diameter laminated spherules with needle-shaped crystals. In addition, there were large electron dense deposits of CaP which were surrounded on the periphery by vesicular and fibrillary material.
Ultrastructural analysis of renal papillary tissue obtained from 15 idiopathic stone patients through cold cup biopsy revealed pathology somewhat similar to that described above [8]. Two basic configurations of crystals, both identified as basic CaP, were seen in the renal interstitium (Figures 2, 3). Spherical, 0.5–2 μm in diameter, concentrically laminated units were found throughout, within the tubular basement membrane as well as interstitium. Some of them retained concentrically arranged needle-shaped crystallites while others lost them during processing leaving behind the organic matrix as crystal ghosts. Large electron dense calcifications were aggregates of elongated calcified strands mixed with spherulites and were located mainly in the interstitium. They were surrounded by collagen fibers, membrane bound calcified and non-calcified vesicles and a variety of cellular degradation products. Peripheral strands showed a distinct banding pattern, reminiscent of the banding pattern of close by collagen fibers. Center of the large deposits was more compact and appeared more crystalline with radiating needle-shaped crystallites compared to the surface which was less compact and crystalline. Electron diffraction confirmed ultrastructural observations that central hydroxyapatite was more crystalline than the peripheral ones. The epithelial cells with large numbers of spherical CaP crystals in their basement membrane, appeared necrotic. The interstitium was replete with membrane bound vesicles, other forms of cellular degradation products, collagen and other fibriller material. Lumens of some tubules appeared blocked with cellular degradation products and perhaps even CaP crystals.
Nanoscale studies of incipient and fully developed Randall’s plaque using field emission scanning electron microscopy with energy-dispersive X-ray microanalysis, μ-Fourier transform infrared spectroscopy, cryo-transmission electron microscopy coupled with selected area electron diffraction and electron energy loss spectroscopy showed the presence of micro-calcifications around both the loops of Henle and vasa recta [22, 23]. Two types of micro-calcifications were identified including calcified vesicles and mineral granules both containing CaP with carbonate in their cores. Secondary calcifications were found embedded in a fibrillary organic material.
A recent study using a combination of techniques including micro-CT imaging, multiphoton and confocal fluorescence imaging and infrared microscopy found a blue autofluorescence signature unique to RP, suggesting a unique nature of the molecules within [24]. Based upon its intrinsic fluorescence, fibrillar collagen was identified in the plaque.
Another study investigated the presence of crystals and the expression of known kidney stone related genes in kidneys from stone formers and non-stone formers [25]. Calcific crystal deposits were found in both groups. Stone formers kidneys showed significantly more crystals in renal medullas and papillae. More crystals were seen in the papillae of stone formers than those of controls. There were signs of crystal movement from tubular lumen to the interstitium. Osteopontin expression was higher and that of THP lower in stone formers papillae. Expression of superoxide dismutase was also low, indicating signs of oxidative stress. The presence of renal papillary crystals was determined to be significantly related to stone formation (odds ratio 5.55, 95% confidence interval 1.08–37.18, P = 0.0395). Based upon the observations of the presence of intratubular crystals in proximal medullary tubules and interstitial crystals later in the renal papillae, a new theory entitled, “upstream mineral formations initiate downstream Randall’s plaque” has been presented [26].
Investigation of renal papillary tissue using the technique of genome-wide gene expression profiling revealed considerable differences between the tissue from idiopathic CaOx stone formers and controls. Differences were also found between stone formers Randall’s plaque and non-Randall’s plaque tissues. There was an increase in the expression of LCN2, IL11, PTGS1, GPX3 and MMD, and decrease of SLC12A1 and NALCN in the renal papillary tissue associated with Randall’s plaque compared with the normal non-plaque associated papillary tissue [27]. There was also an increase in number of immune cells and apoptotic cells in renal tissue associated with Randall’s plaque compared with non-plaque associated renal papillary tissue. The above mentioned genes play important roles in inflammatory processes. There is also an increase in M1 macrophage related genes and a decrease in M2 macrophage related genes in renal papillary tissue of stone patients [28]. Presence of giant cells in the renal interstitium, where crystals are surrounded by macrophages, is a well-known occurrence [29].
A study of renal papillary tips of kidney stone patients utilizing the technique of quantitative PCR showed a significant increase, compared to controls, in the presence of inflammatory cytokine, CCL2, CCL5, CCL7, CC-chemokine receptor 2 (CCR2), CD40, macrophage colony-stimulating factor 1 receptor (CSF1), CXC-chemokine ligand 9 (CXCL9), CXCL10, Fas ligand (FASLG), receptor interacting serine–threonine kinase 2 (RIPK2), e-selectin (SELE) and Toll-like receptor 3 (TLR3), irrespective of the stone type [30].
2.1. Randall’s plugs
Randall described two types of renal papillary lesions, Type 1 and Type 2, as sites for future stone formation. Plaque 1 is now generally known as Randall’s plaque and Type 2 plaque is mostly referred as ductal plug. Plaques are present on the papillary surface while plugs are crystalline deposits clogging the openings of the terminal collecting ducts [1, 6, 31]. As discussed above, Randall’s plaques are deposits of mostly apatitic CaP crystals and are found in idiopathic CaOx, brushite, primary hyperparathyroid, small bowel resection, ileostomy stone disease [4]. The composition of ductal plugs varies. Plugs can be apatitic in primary hyperparathyroid stone formers, mixed with CaOx in brushite stone formers, obesity bypass patients, patients with small bowel resection, renal tubular acidosis. Ductal plugs can also be apatite mixed with urate in ileostomy stone formers [4]. The Randall’s plugs of stone patients with primary hyperoxaluria are made of CaOx [4, 21]. Plugging is also common in non-stone forming kidneys where it is mainly composed of carbonate apatite and amorphous CaP [32].
Obviously, plug formation is a result of crystal retention within the terminal collecting ducts. Results of animal model and tissue culture studies clearly show that crystals can be retained by either attachment to the epithelial surface or aggregation during excessive supersaturation [33, 34, 35]. Crystal nucleation, and aggregation can be supported by the membranous products of renal tubular injury [36, 37, 38]. Massive crystallization is possible in the presence of acute or persistent urinary supersaturation and excretion of poorly soluble salts and cause tubular plugging in any section of the renal tubules [32]. Examples include CaOx ductal plugs in patients with primary hyperoxaluria [21, 39], or cystine crystal plugs in the cystine stone formers [39]. The overgrowth on plugs will form an attached stones. Parts of a stone may break off and act as nidus for an unattached stone [6, 40]. Plugs are exposed to two different environments, tubular and pelvic, with different urinary composition. As a result the final product, the stone, may have one crystalline type on the tubular side and another on the pelvic side. Thus an apatite nucleus may have brushite on the one side and CaOx on the other [41].
3. Organic matrix of idiopathic kidney stones
Calcium oxalate kidneys stones, like other stones, are composed of crystals (Figure 4) mixed with small amounts of a pervasive organic matrix (Figures 5, 6) which contains carbohydrates, proteins and lipids [37, 42, 43]. The matrix composition varies between various stone types. There is more matrix material in the outer layers of the stones than their centers and its make-up may change from the surface to the center. Urinary crystals isolated from the human urine or produced in vitro in human urine also contain an organic matrix. Similarly CaOx and CaP crystals and stones produced experimentally in rats also contain an organic matrix, indicative of universal phenomenon of incorporation of biological substances from the immediate environment into the growing crystals. Both chromatograph, biochemical and microscopic techniques have been used to study various aspects of organic matrix.
Calcium oxalate (CaOx) kidneys stone examined using scanning electron microscopy (SEM). (A) Stone surface showing CaOx dihydrate (COD) crystals sticking out on the surface. (B) Stone surface showing tops of the tabular CaOx monohydrate (COM) crystals. (C) Fractured surface showing COM crystals organized in concentric laminations. (D) Spherical calcium phosphate crystals.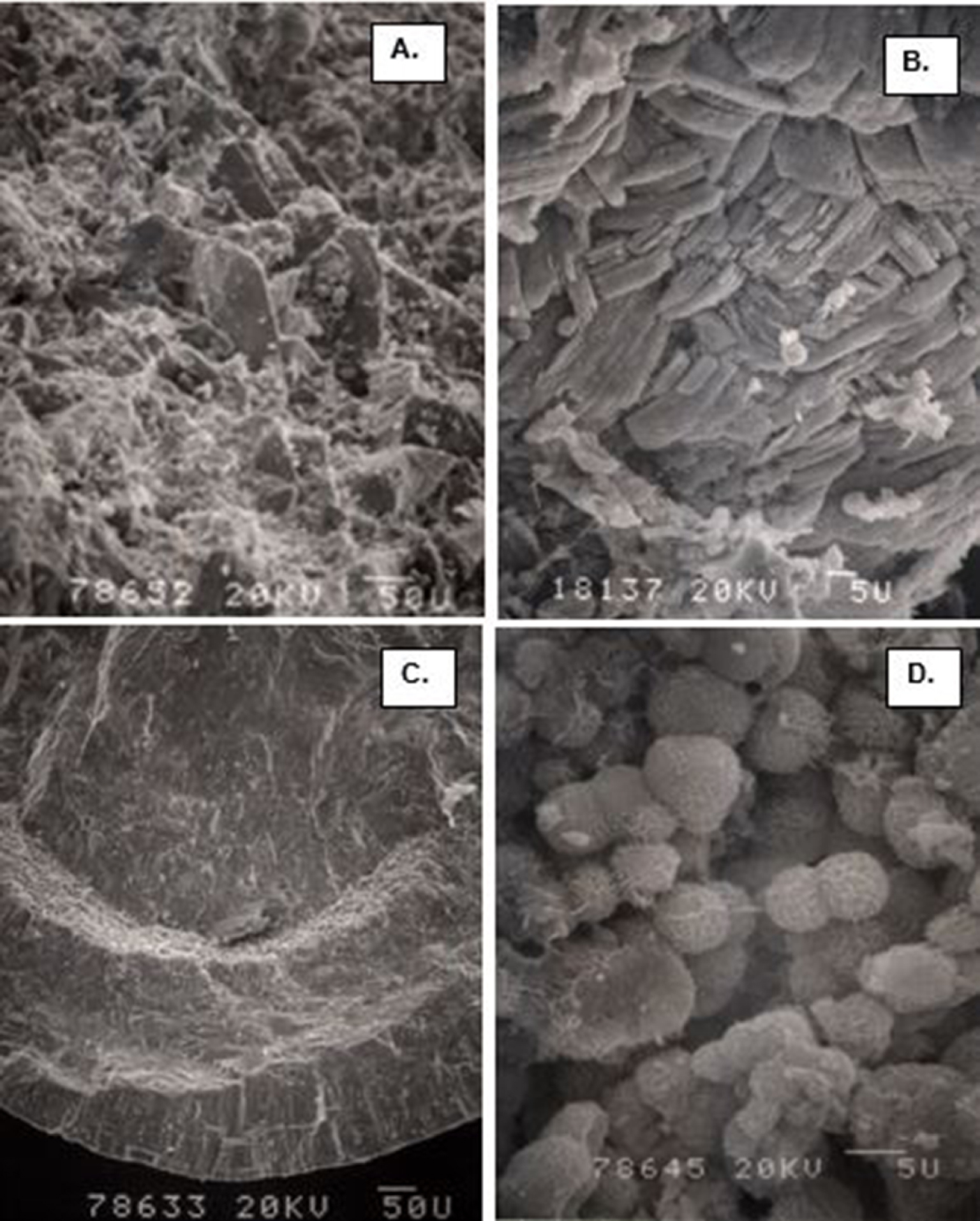
SEM and TEM appearance of demineralized COM stones demonstrating ubiquitous nature of the organic matrix, and its intimate association with the crystalline components of the stone. Stone pieces were demineralized and then processed for electron microscopy. The stone and crystals maintained their architecture even after the removal of the crystalline component. (A) Low magnification SEM appearance of demineralized COM stone, very much similar to the non-demineralized stone shown in Figure 4(C). (B) SEM of the fractured ghosts of tabular COM crystals showing loss of the crystals, leaving behind the organic matrix which allowed the ghost to maintain tabular morphology. COM crystals tightly organized in radial striations and concentric laminations. (C) TEM appearance of a COM stone showing ghosts of both the tabular COM and spherical CaP crysals. The interface is occupied by the organic matrix. Bar = 1 μm. (D) TEM of the cross section of tabular COM crystals demonstrating the inter and intra-crystalline electron dense organic matrix. Bar = 0.2 μm.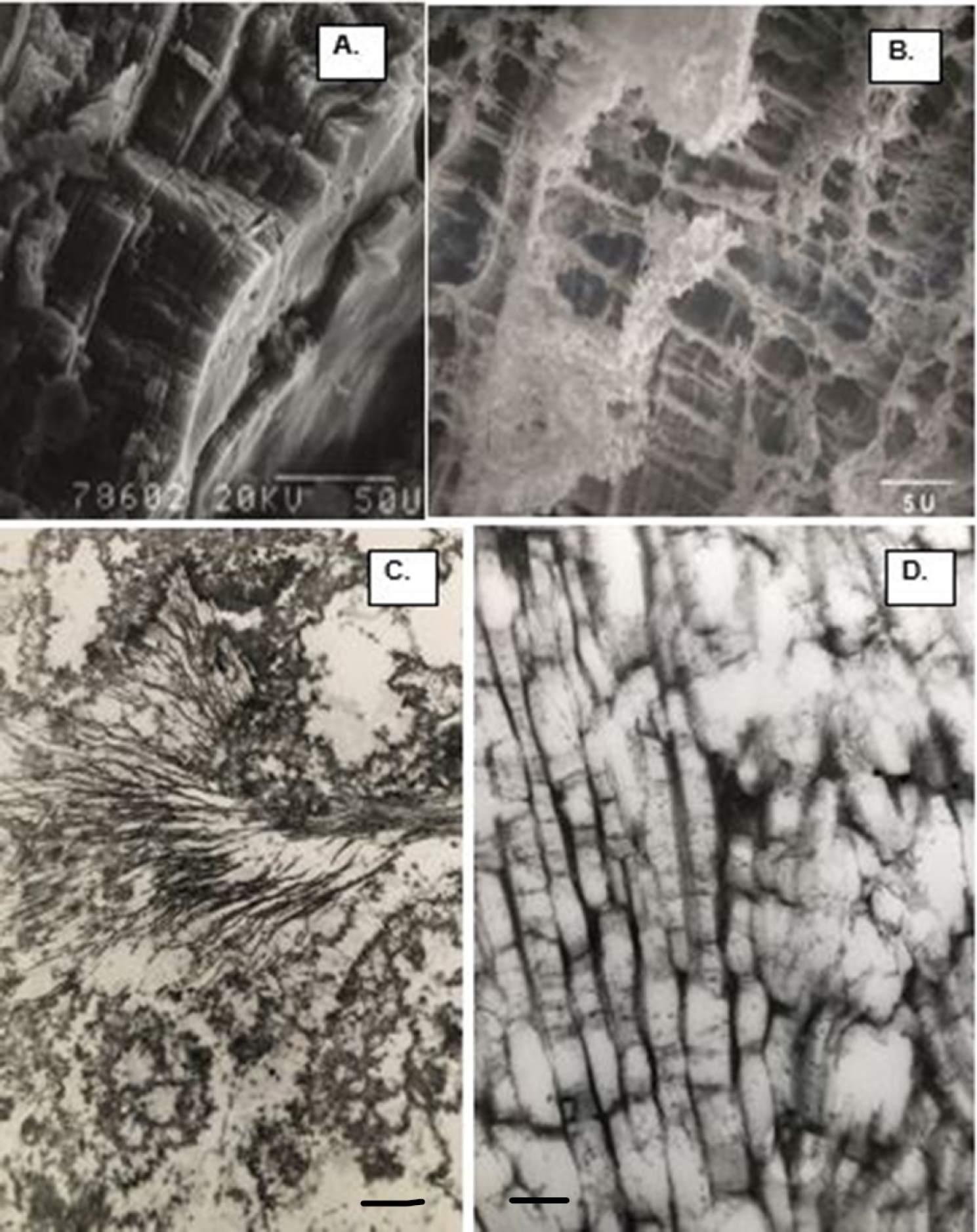
Organic matrix of the CaP crystals in a COM stone. (A) SEM of the fractured demineralized spherical CaP crystals. There is electron dense organic matrix in the center as well as on the surface. Closer look at the surface show needle shaped appearance. (B) TEM of the CAP crystal ghosts showing electron dense material organized on the periphery and also present in the center, similar to what is shown in A. Bar = 1 μm.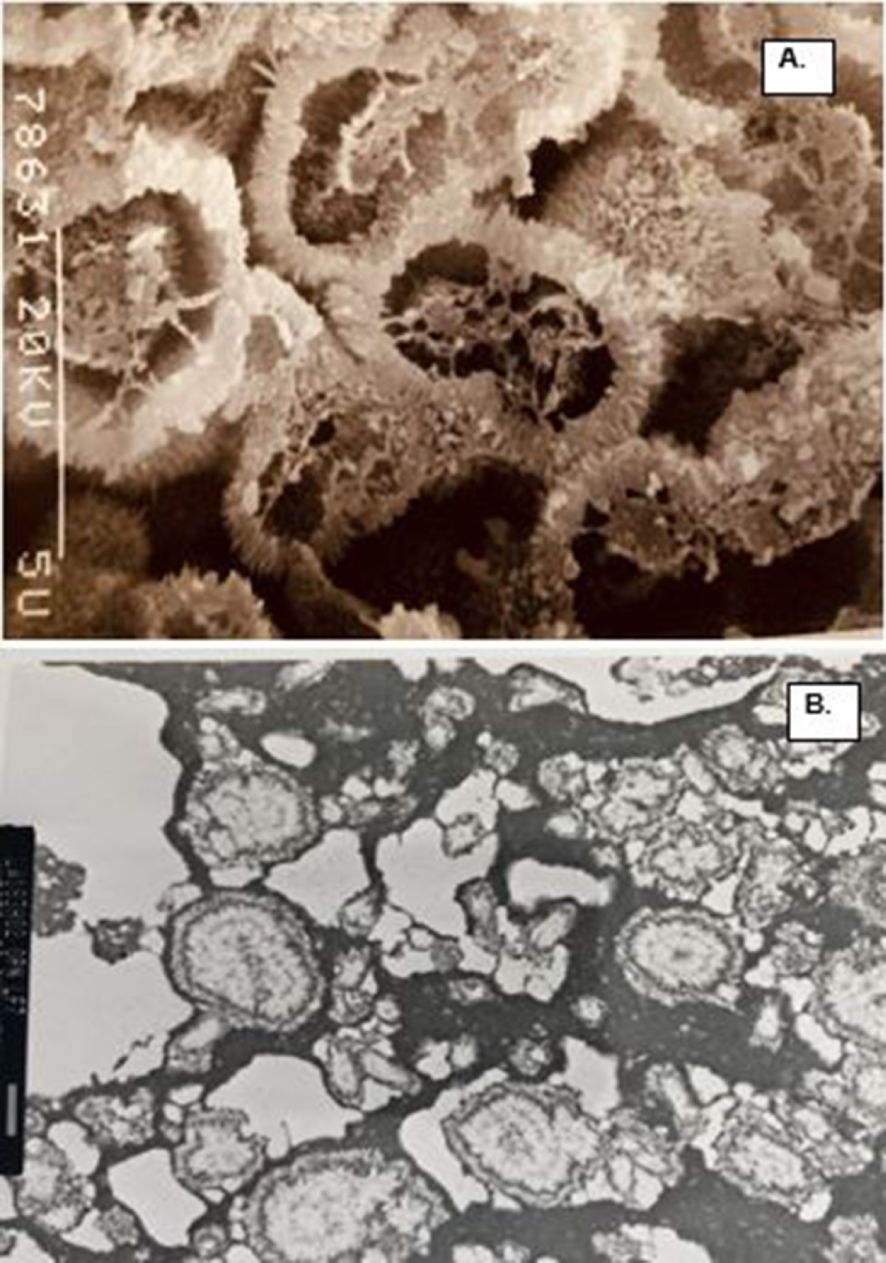
3.1. Results of biochemical, chromatographic, and spectrophotometric analyses
Ever since Boyce described Substance A as the major component of stone matrix, several studies have examined matrix composition using ever more sophisticated techniques with a significant increase in the number of components identified. Recent studies, using a variety of methods, have identified up to 1059 unique proteins in stone matrix [44].
Dussol et al. analyzed soluble matrix of 5 different types of kidney stones using electrophoretic techniques [45], and identified 13 proteins. Human serum albumin, α1-antitrypsin, α1-microglobulin, were three of the nine proteins common in all types of stones. B2-microglobulin was found only in CaOx stone matrix. THP was not found in matrix of any of the stones.
Canales et al. used mass spectroscopy to analyze matrix of 25 stones including 9 CaOx monohydrate (COM), 4 CaOx dihydrate (COD), 9 apatite and 3 brushite stones [46]. A total of 113 proteins were identified. 42 proteins were unique to CaOx, 36 to CaP and 35 were common in both. Calgranulin A and B, hemoglobin α and β chains, fibrinogen, Tamm–Horsfall protein (THP), α 1 antitrypsin, and vitronectin were found in more than 10 stones. Gene ontology analysis showed that COM stones matrix contained more cell structure, cell membrane, intracellular transport proteins and coagulative proteins while the matric of apatite stones contained more immunity and defense proteins. Proteins involved in inflammatory processes were common in both CaOx and CaP stones.
Marchant et al. analyzed the organic matrix of stones obtained from 5 patients. They used a variety of techniques including spectrophotometry and one-dimensional polyacrylamide electrophoresis [47]. A total of 158 proteins were identified with 28 of them detected in all three LC-MS analyses. Proteins included such commonly known ones as THP, OPN, calgranulins, and albumin. Gene ontology analyses suggested that 61% had extracellular origin, 36% intracellular and 3% intra-/extracellular. Ingenuity pathway analyses identified tumorigenesis, immunological disease and inflammatory disease.
Okumura et al. examined CaOx stones obtained from 9 stone patients and their organic matrix was analyzed using nanoLC-MALDI-tandem mass spectrometry following in-solution protein digestion [48]. A total of 92 proteins were identified and included proteins of kidney, blood and leukocyte origin. Osteopontin, prothrombin, THP, serum albumin, Calgranulins, Fetuin A, Lactotransferin, myeloperoxidase were common to all stones. There was an inverse relationship between Prothrombin and Calgranulins. Some samples had more Prothrombin and OPN while others more Calgranulins. Calgranulins rich stones were also rich in myeloperoxidase and lactotransferin.
Boonla et al. analyzed proteome of stone matrix and urine obtained from same patient to identify the urinary proteins that become part of the stone matrix. Proteins known to be involved in inflammation and fibrosis were detected in both the urine and stone matrix of stone patients [49]. Eighteen proteins with a role in immunity, inflammation, coagulation and fibrosis were common to stone matrix and patient urine and were absent from normal healthy human urine. Calgranulin A, a calcium binding protein with important role in inflammation was suggested to be a urinary marker of nephrolithiasis.
Rodgers group applied solid state nuclear magnetic resonance spectroscopy technique to characterize the biomolecular composition of phosphate stones. They detected varying amounts of proteins, glycosaminoglycans (GAGS) and lipids. Their analysis showed that GAGS and proteins, “composited into or onto the mineral lattice by strong physicochemical interactions” [50]. Interestingly, their results also showed that CaOx and uric acid, when present, existed as separate entities, no direct epitaxial-like relationship.
3.2. Results of microscopic and immune- histochemical analyses
Results of the studies discussed above have provided significant information about the composition of stone matrix and have identified more than a thousand proteins. These studies have provided some indication about the matrix origin as well as clues for stone pathogenesis. The role of matrix is however, still uncertain. To appreciate the role we have to know the exact location of matrix and its components in the stones and their association with the crystals. Boyce and associates realized the significance of such information and performed some of the earliest histological and histochemical examinations of kidney stones [51, 52, 53]. We performed a number of light and electron microscopic studies of decalcified CaOx crystals and stones, both from stone patients and those produced experimentally in rats, to localize various matrix components. Scanning electron microscopy and energy dispersive X-ray microanalysis of CaOx stones after staining with colloidal iron showed the presence of acidic mucosubstances in association with CaOx crystals [54]. But stones, particularly CaOx kidney stones, being highly crystalline, it is difficult to perform critical microscopic studies of their organic matrix. Therefore, we developed a technique involving decalcification of stone sections using 0.25 M ethylenediaminetetracetic acid (EDTA), after embedding them in agar, which allowed full or partial removal of crystals without excessively disturbing the matrix material [55]. Crystals were replaced by crystal ghosts (Figures 5, 6) and could be easily processed for various microscopic and immune-histochemical techniques. Light and electron microscopic examination of decalcified stones, helped localize the organic matrix and its various components within the stones and demonstrated that matrix is ubiquitous within the stones and is present not only on crystals surfaces but also within them [56, 57, 58]. Crystal ghosts generally maintained the original shape of the crystals. Ghosts of CaOx monohydrate crystals appeared as radially arranged tabular units, showing concentric laminations and radial striations (Figure 5). Those of CaOx dihydrate appeared dipyramidal while those of apatite as spherical units. Matrix contains both amorphous and fibrous components and could be stained just like any other tissue components. Staining of demineralized stones with Sudan Black B showed sudanophilic material within the intercrystalline spaces indicating its lipidic nature [59]. The presence of lipids in the intercrystalline spaces was further confirmed through transmission electron microscopy after malachite green staining [60]. Ultrastructural studies of kidney stones, crystal deposits induced in experimental hyperoxaluric rats, as well as those produced in human urine in vitro, showed the presence of proteins, membranes and lipids in their matrices [37]. Crystal ghosts of both CaOx and CaP were made of amorphous material and were associated with fibrillary, amorphous and membranous elements including membrane bound vesicles. Transmission electron microscopy and backscattered electron microscopy of crystal ghosts revealed the presence of organic material on the surface as well as inside the CaOx and CaP crystals and was organized in discrete layers. In addition the interface between CaOx and CaP crystals was occupied by an amorphous organic material [61].
TEM of the CaP and COM crystal ghosts stained for osteopontin (black dots) demonstrating its substantial presence in the matrix. (A) Spherical CaP ghosts. OPN is specifically present in the electron dense organic matrix in the center and on the periphery of the ghosts. Electron dense amorphous material outside the spherical units is not stained. (B) Tabular COM ghosts. OPN is specifically associated with the inter and intra-crystalline organic matrix of the ghosts. Electron dense materials outside the ghosts is negative for OPN. Bar = 200 nm.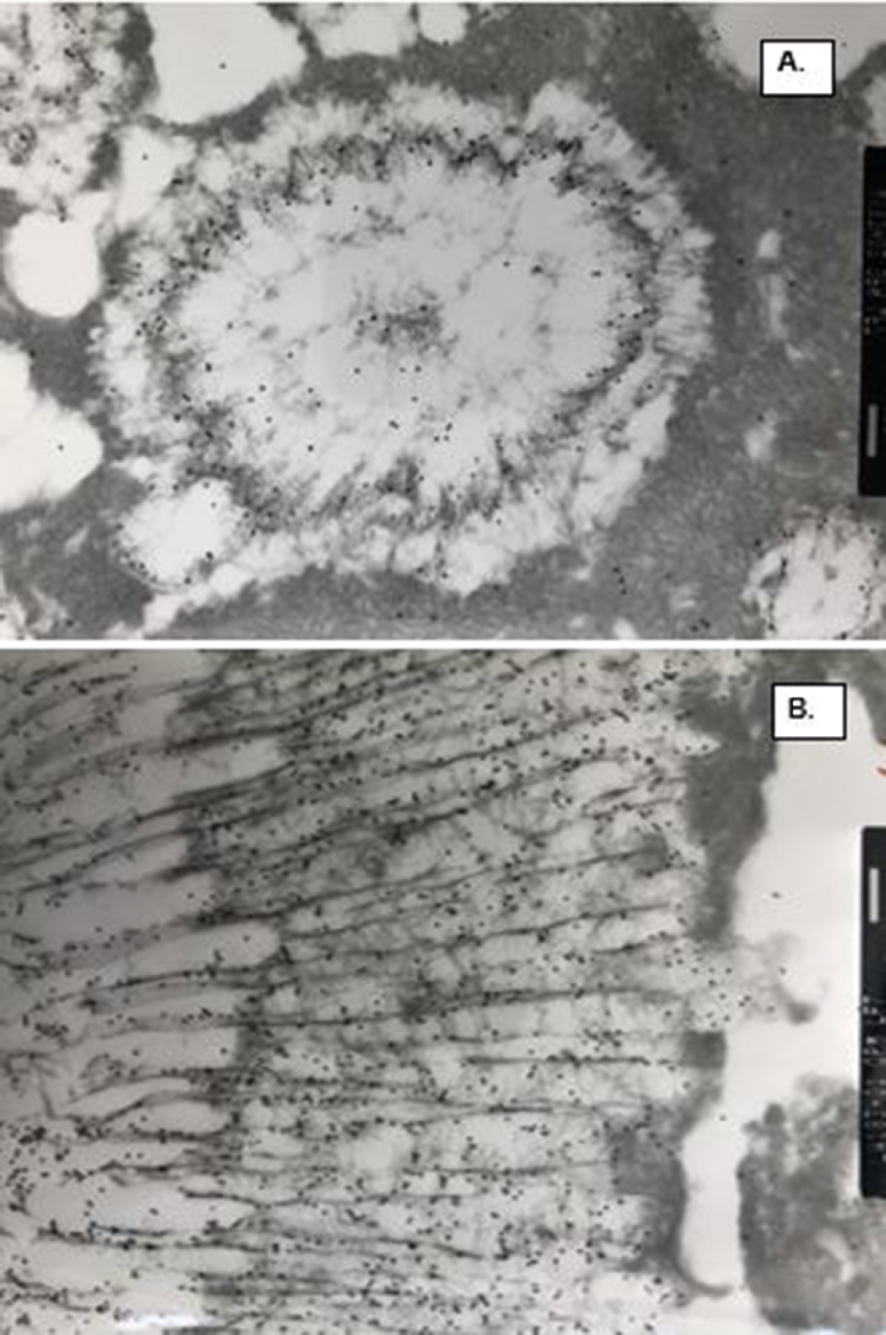
We also performed ultrastructural immuno-detection of OPN, THP and albumin, the three most common organic matrix proteins, in the CaOx crystals of the human stone and those produced in a rat model [16]. Osteopontin was a prominent constituent of CaOx crystals and stones (Figures 7, 8). Intense labelling for OPN was seen in the nucleus, laminas and striations of crystal ghost from rat urine. Similarly, concentric laminas and radial striations of human CaOx kidney stones were also intensely positive for OPN. CaOx crystal ghosts were negative for THP and albumin. Labelling for THP was mostly seen on the stone surface. Similarly, albumin was seen on stone surface and between crystals but not in direct contact with the crystals. Organic material between the Cox and CaP crystals stained positive for OPN.
OPN staining in demineralized COM stones. (A) Interface between ghosts of CaP and COM is occupied by OPN positive electron dense organic matrix. (B) OPN positive vesicles in between the ghosts of tabular COM crystals.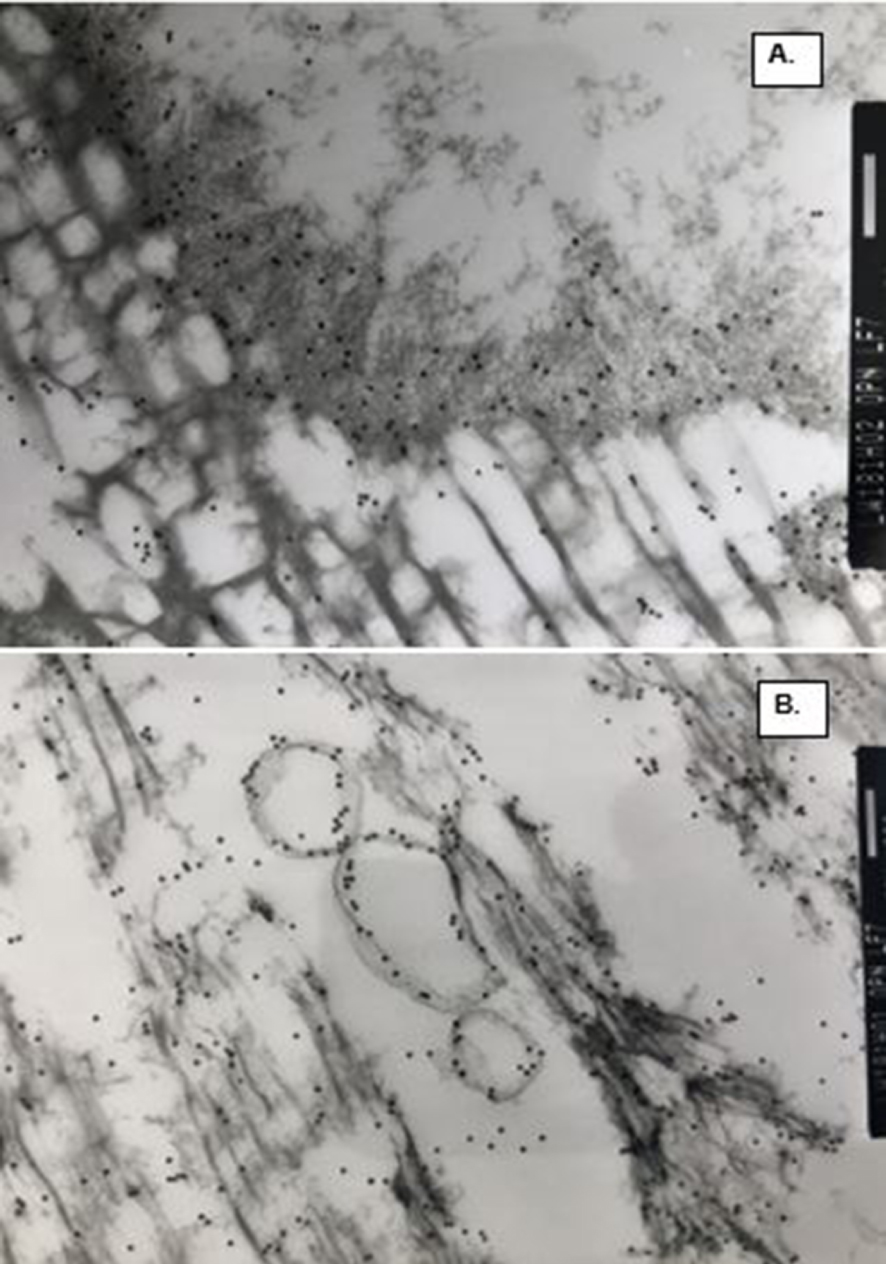
4. Discussion
Results of most current studies indicate that, (1) renal papillae of idiopathic CaOx kidney stone formers show signs of injury and inflammation; and (2) organic matrix of calcific kidney stones contains biomolecules known to be involved in immune response and inflammation (Table 1). It is however unclear whether injury and inflammation play a causative role in the development of RP and stones. It has been suggested that Randall’s plaque development is dependent upon an increase in urinary calcium [11]. Calcium reabsorption is decreased in the proximal tubules (PT), hence more calcium reaches the thick ascending limbs of the loop. Calcium reabsorption into the interstitium is increased, increasing the supersaturation thus promoting the formation of interstitial plaques [12, 13, 62]. Surprisingly they did not find any sign of injury or inflammation associated with the plaque as well as crystal deposits in the nearby tubules. Surprising, because results of earlier studies including those of Randall implied calcification in association with injury. Randall suggested “that calcium plaques appear to be a natural reparative process to some form of tubule damage, the occurrence of which is in much higher incidence than actual frequency of renal stone” [63]. Electron microscopic study of necropsy material by Haggit and Pitcock described calcification as spherical laminated electron-dense bodies in the papillary interstitium and basement membrane of collecting ducts [7]. They showed excessive deposition of collagen in the papillary interstitium, tubular epithelial necrosis and thickening of the tubular epithelial basement membrane. We have always found signs of tubular and interstitial injury in association with Randall’s plaque in renal papillae from idiopathic kidney stone formers [6]. Additionally, in every animal model study and every tissue culture study where renal epithelial cells become exposed to crystals or even high oxalate, there are signs of oxidative stress which eventually leads to cellular injury [33, 34, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74]. Some studies of both CaOx and CaP nephrocalcinosis have also shown signs of osteogenic transformation of renal tubular epithelial cells [75, 76, 77]. In addition, hypercalciuria, genetic or induced by other means, by itself leads only to tubular deposition of crystals [69]. Interstitial crystals have so far only been seen in animal models of nephrocalcinosis with a deficiency of crystal inhibitors such as pyrophosphate, OPN and THP [78, 79, 80, 81, 82].
Biomolecules involved in both, the inflammation and idiopathic CaOx stone disease
| Molecule | Description | Immune function | Findings in Nephrolithiasis |
|---|---|---|---|
| α-1 microglobulin (A1M) | A reductase that degrades heme and removes free radicals and oxidizing agents from tissue. It is induced by reactive oxygen species and broken down in the kidney | Urinary marker for renal inflammation & hypertension | CaOx crystallization inhibitor, present in KS matrix |
| Albumin (ALB) | Globular protein commonly found in blood and has a good binding capacity for water, Ca(2+), Na(+), K(+), fatty acids, and hormones | Decrease in response to inflammation. Hypoalbuminemia is a predictor of mortality in patients with chronic renal failure | Promotes crystallization and crystal adhesion, present in KS matrix |
| Bikunin | A single glycosaminoglycan chain, is a plasma serine protease inhibitor | Anti-inflammatory | CaOx crystallization inhibitor, present in KS matrix |
| Calgranulin (S100) | S100 calcium-binding protein family (A8, A9, A12) that is expressed in multiple cell types, including renal epithelial cells and neutrophils | Inflammatory, act as damage-associated molecular pattern (DAMP) proteins, signaling through the receptor for advanced glycation end products (RAGE), and acting as endogenous toll-like receptor 4 (TLR4) ligands | Inhibitor of CaOx crystallization in urine, present in KS matrix |
| Fetuin-A | Also known as alpha-2-HS-glycoprotein (AHSG), is a human plasma glycoprotein belonging to the cystatin proteases inhibitors family that has a calcium phosphate-binding site and TGF (transforming growth factor)-β cytokine-binding motif | Anti-inflammatory (stimulates anti-inflammatory cationic polyamines and directly inhibits PAMP-induced HMGB1 release by innate immune cells) | Binds to CaP to form nanoparticles and promotes crystal aggregation, present in KS matrix |
| Fibronectin | Glycoprotein of the extracellular matrix that binds to integrins, collagen, fibrin, and heparan sulfate proteoglycans | Modulator of inflammation | Inhibitor of CaOx crystallization and adhesion, present in KS matrix |
| Heparin sulfate (HS) | A linear polysaccharide that occurs as a proteoglycan which is capable of binding Wnt | Pro-inflammatory by assisting macrophage attachment and chemokine binding | Inhibitor of CaOx crystal growth and adhesion, present in KS matrix |
| Inter-α-inhibitor (IαI) | A plasma proteins consisting of three of four heavy chains selected from the group ITIH1, ITIH2, ITIH3, ITIH4 and one light chain selected from the group AMBP which function as protease inhibitors. IαI form complexes with HA | Anti-inflammatory | Inhibitor of CaOx crystallization and adhesion, present in KS matrix |
| Matrix-gla-protein (MGP) | A member of a family of vitamin-K2 dependent, Gla-containing proteins that binds to calcium. It acts as an inhibitor of vascular mineralization and plays a role in bone organization | Anti-inflammatory | Inhibitor of mineralization, present in KS matrix |
| Osteonectin | Also known as secreted protein acidic and cysteine rich (SPARC), is a calcium binding glycoprotein that belongs to the matricellular protein family and is expressed in remodeling tissues, such as bone, mucosa of the gut, and healing wounds | Anti-inflammatory (may regulate macrophage viability and chronic immune response) | Inhibitor of CaOx crystal growth and adhesion |
| Osteopontin (OPN) | A phosphorylated glycoprotein that binds integrins and functions as a mediator of cell adhesion, migration, immune responses, and tissue repair | Pro-inflammatory cytokine | Both a promoter and inhibitor calcification, crystal aggregation and attachment; present in KS matrix |
| Phospholipids | Components of cellular membranes | Membrane exposure to reactive oxygen species creates oxidized phospholipids which are potent anti-inflammatory mediators | Membrane lipids provide site for face selective crystal nucleation, aggregation and retention within the kidneys |
| Prothrombin fragment 1 | Peptide formed during conversion of prothrombin to thrombin; excreted in urine | Binds calcium and is involved in coagulation, as well as in the recruitment and activation of immune cells | Inhibitor of crystal growth and aggregation, present in KS matrix |
| Tamm–Horsfall protein (THP) | Also known as uromodulin, is a large glycoprotein in the ascending limb of the loop of Henle extending into the early portion of the distal tubule and is the most abundant protein excreted in normal urine | Modulator of inflammation | Important modulator of CaOx crystallization and aggregation in urine as well as CaOx adhesion to uroepithelium, present in KS matrix |
Modified from Khan et al. [69].
It has been proposed that Randall’s plaque and stone formation [6, 38, 83], are mechanistically a form of ectopic calcification, pathological deposition of mostly CaP in the soft tissue, analogous to vascular/valvular calcification (VC) during atherosclerosis (Table 2) [1, 69, 83, 84]. Reactive oxygen species play a crucial role in both VC and stone formation [66, 68, 85, 86]. Vascular calcification involves osteogenic transformation of vascular smooth muscle cells (VSMC) [87, 88, 89, 90], in the presence of high calcium and phosphate. Reactive oxygen species play crucial role [66, 68, 85, 86]. The transcription factors, Runt-related transcription factor 2 (RUNX2), osterix and msh homeobox 2 (MSX-2) are upregulated. Bone morphogenetic proteins and Wnt signaling pathways are activated [84, 91]. Calcification starts in membrane bound matrix vesicles or exosomes [92], produced by the transformed VSMC cells. Apoptotic bodies produced by the deaths of many other cells including lymphocytes and macrophages can also behave as matrix vesicles [90, 93, 94, 95, 96]. The matrix vesicles contain certain proteins and lipids that help crystal nucleation. Once formed, needle shaped CaP crystals pierce through the limiting membrane of the vesicles inducing mineralization of the surrounding extracellular matrix such as collagen, and support onward movement of the mineralization front [90, 97]. RPs contain both empty and calcified vesicles [8, 23], normal and calcified collagen fibers [8, 24], membranous cellular degradation products, and express various mineralization modulators such as OPN [27, 98]. Progression of mineralization is accomplished by calcification of apoptotic bodies and collagen fibers in the extracellular matrix. These features are similar to what happens during vascular calcification as briefly reviewed above. Osteogenic changes and expression of transcription factors in association with RPs has been suggested but not been confirmed [99, 100]. The appearance of transcription factors may be transient or below the level of detection by immunohistochemical means. It has however, been reported in rat models of CaOx and CaP nephrolithiasis [76, 77]. Macrophages play a significant role in both vascular and renal papillary calcification. Pro-inflammatory macrophages are implicated in vascular plaque calcification while anti-inflammatory ones with plaque regression [101, 102, 103]. Macrophages are common around renal interstitial crystals and pro-inflammatory macrophage genes are increased while anti-inflammatory macrophage genes are decreased in the kidneys of patients with kidney stones [28, 104, 105, 106].
Features common to vascular and renal calcification
| Vascular calcification | Renal calcification | |
|---|---|---|
| Reactive oxygen species involvement | ROS activate many steps in calcification. Both Mitochondria and NADPH are involved in ROS production | Crystals and high oxalate induce ROS production by renal epithelium. Mitochondria and NADPH oxidase are involved in ROS production |
| Inflammation | Activation of NLRP 3 inflammasome is required for VSMC calcification | Activation of NLRP3 Inflammasome |
| Osteogenic transformation (OT) | OT of VSMC | OT of renal tubular epithelial cells |
| Transcription factors involved | RUNX2 and Osterix | RUNX1 and 2, Osterix |
| Bone specific gene and protein expression | BMP, OPN, OCN, MGP | BMP, OPG, OPN, MGP, Osteocalcin, Osteonectin, Fibronectin |
| Mineralization modulators | OPN, OCN, MGP, Fetuin, Lipids | OPN, MGP, Fetuin, Osteocalcin, Lipids, THP |
| Monocyte/ macrophages | Macrophages play critical role in plaque calcification. M1 macrophages associated with plaque calcification and M2 with plaque regression | Macrophages are common around interstitial crystals. M1 related genes are upregulated and M2 related genes are downregulated in kidneys of stone patients |
| Matrix vesicles/ exosomes/apoptotic bodies | VC starts in membrane bound vesicles produced by transformed VSMC and apoptotic bodies produced by death of lymphocytes and macrophages | Membrane bound vesicles are associated with both intratubular and interstitial calcifications and are likely starting point for interstitial calcification |
| Mineralization progress | Mineralization continues by calcification of apoptotic bodies and collagen fibers in extracellular matrix | Mineralization continues by calcification of apoptotic bodies and collagen fibers in extracellular matrix |
Results based on animal model, cell culture as well as limited number of clinical studies. (From Khan et al. [69].)
Almost all the recent studies of organic matrix of idiopathic CaOx kidney stones, have concluded that injury and inflammation are significant contributors to the formation of matrix constituents. Over a thousand proteins have so far been identified therein. Many of them participate in immune response and are also well-known modulators of crystallization in the urine (Table 1) [1, 69, 107, 108, 109, 110, 111, 112]. Osteopontin, a phosphorylated glycoprotein, the most common matrix constituent, is a pro-inflammatory cytokine and can act as both a promoter and inhibitor of crystal formation, aggregation and attachment to epithelial surfaces. Urinary prothrombin fragment 1 is formed during conversion of prothrombin to thrombin and is excreted in urine. It binds calcium and plays a role in coagulation, and recruitment and activation of immune cells. It is an inhibitor of crystal growth and aggregation. Of the many proteins present in the urine, CaOx crystals selectively incorporate OPN and UPFT-1 [16, 113, 114, 115]. Albumin is common in blood, urine and stone matrix. It promotes crystal formation and adhesion. Albumin production is reduced in response to inflammation and hypoalbuminaemia is considered a mortality predictor in patients with renal failure. Calgranulins, a family of S100 calcium binding proteins function as damage associated molecular pattern signaling through the receptor for advanced glycation products and as endogenous ligands for tool-like receptor. They are common in stone matrix and have been shown to inhibit CaOx crystallization in urine. Tamm–Horsfall Protein or uromodulin, a large glycoprotein expressed mainly in ascending limb of the loop of Henle and proximal part of the distal tubule, is excreted in the urine in large amounts. It modulates certain steps in inflammation as well as CaOx crystallization and their retention within the kidneys. Inter alpha trypsin inhibitor is a plasma protein complex of a light chain covalently bound to heavy chains 1 and 2. Both light and heavy chains are excreted in the urine and have been shown to inhibit CaOx crystallization and adhesion to tubular epithelium. Matrix-GLA protein contains glutamate residues requiring vitamin K for activation, and functions primarily as an inhibitor of vascular calcification. Fetuin A is plasma glycoprotein, has CaP and TGFB cytokine binding sites and has anti-inflammatory properties.
Macromolecules inhibitors of crystallization present in the kidneys and urine, such as MGP, OPN and Fetuin A, are also inhibitors of mineralization of vascular smooth muscle [84]. MGP inhibits vascular calcification [116] and regulates activities of bone morphogenetic protein 2 (BMP2) [117]. Even though it was first isolated from bone [118], the expression of MGP mRNA is fivefold higher in rat kidneys than in bone [119]. There is widespread arterial calcification in Mgp−∕− mice, which starts as amorphous CaP, and eventually transforms into hydroxyapatite and carbonate apatite [120]. MGP may also play a role in kidney stone formation. MGP polymorphisms might be involved in the development of CaOx kidney stone disease in Japanese [121] and Chinese patients [122].
Fetuin A, which is encoded by AHSG, has high affinity for CaP and is highly enriched in both mineralized tissues [123] and pathological calcifications [124]. Knocking out AHSG gene in mice causes extensive soft tissue calcification [123]. In humans, cardiovascular calcification and aortic stiffness in patients with kidney failure is associated with serum Fetuin A deficit [125, 126]. The urine, kidneys and serum of kidney stone patients have lower Fetuin A levels than healthy controls [127].
Osteopontin is highly phosphorylated with multiple functions and plays a role in both calcification and inflammation [128, 129, 130]. It is modulator of crystallization of CaOx as well as CaP and is prominent in both mineralized tissue and soft tissue calcifications. Knockout of OPN leads to deposition of CaOx in hyperoxaluric mice [111]. It is a chemoattractant for macrophages and T-cells and involved in secretion of IL-3, IL-10, IL-12, interferon-γ, integrin αvB3, NFκβ. SPP1 gene that encodes for OPN, is upregulated in renal tissues of kidney stone patients [28].
Lipids and membranes are present in both the kidney stone matrix as well as vascular calcification. Oxidative stress and inflammation are considered two major pathological mechanisms for endothelial dysfunction. Membrane bound extracellular vesicles derived from smooth muscle cells, valvular interstitial cells and macrophages act similar to matrix vesicles and act as mediators of calcification [92, 131]. We have found that lipid contents of the kidney stone matrix [132], as well as tubular epithelial brush border membrane vesicles can act as mediator of both CaP and CaOx crystallization in vitro [36, 133]. Stone formers urine contains more acidic phospholipids, considered to be involved in promoting calcification, than the urine of non-stone formers [134]. The ultrastructure of human calcified aortic valve [92], appears to be analogous to the ultrastructure of Randall’s plaque seen in the kidneys of patients with idiopathic CaOx kidney stones [1, 8, 69]. Both show membrane bound vesicles with and without needle shaped crystals associated with collagen fibers.
As far as the presence of organic matrix is concerned, all calcifications, physiological or pathological, either human or animal, have a matrix. Apparently any biomolecule present at the site of calcification becomes incorporated into the organic matrix. However some, such as OPN and urinary prothrombin fragment 1, and perhaps inter-alpha-inhibitors are preferentially and specifically incorporated into the crystals. Thus crystals are actually not pure crystals with a protein coat but bio-crystal unit.
A review of the current literature about Randall’s plaques, plugs and idiopathic CaOx kidney stone and experimental data obtained through animal model and tissue culture studies discussed above indicates that: (1) at least the early steps of stone pathogenesis are more complicated than simple increase in urinary supersaturation; (2) there is oxidative stress, injury and inflammation at some stages during stone pathogenesis; (3) intratubular crystallization and parenchymal crystal deposition is a common occurrence. Based upon these observations, we have proposed that hypercalciuria, hyperoxaluria and hypocitraturia induce tubular crystallization activating many systems including renin–angiotensin system and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, leading to an increase in the production of reactive oxygen species (ROS). In response, exposed cells produce crystallization modulators such as OPN, matrix Gla protein (MGP), bone morphogenetic proteins (BMPs) and alkaline phosphatase (ALP). Activation of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome, causes the production of caspases and inflammatory cytokines that promote macrophage infiltration. Under normal conditions inhibitors keep the crystals small which are passed out during urination. Some of the crystals are taken in by the cells and are phagocytosed. Some are extruded into the interstitium where they attract macrophages and are taken care of by them. However, in the presence of continuing high calcium, oxalate and low citrate, as is possible in many kidney stone formers, there is development of oxidative stress and epithelial cells are unable to produce sufficient amounts of inhibitors. Epithelial cells undergo osteogenic changes, produce inflammatory cytokines attracting macrophages and their transformation from anti-inflammatory to pro-inflammatory. This leads to inflammation and deposition of collagen. Transformed epithelial cells, cellular apoptosis as well as macrophages produce calcifying vesicles. From then on, interstitial calcification progresses through the mineralization of collagen and other components of the extracellular matrix. Growing outward it reaches the sub-urothelial space on papillary surface and appears as Randall’s plaque. Exposure of the plaque to the pelvic urine might be brought about by metalloprotease mediated disruption of the tight junctions between cells of the covering urothelium. Involvement of matrix metalloproteinases (MMP) in crystal associated diseases of the kidneys has, so far not been investigated. MMPs are increased by oxidative stress and inflammation and a number of MMPs are known to play a role in a variety of kidney diseases [135, 136], including vascular calcification in chronic kidney disease [137]. Some of the proposed steps are summarized in Figure 9.
Proposed scheme for the development of Randall’s plaques based upon experimental and clinical data as discussed. Urinary supersaturation with respect to the CaOx and CaP, which is mainly a result of high calcium (Ca) and oxalate (Ox) and lower crystallization inhibitory potential (CIn), is increased. Transient increase produce small crystals which are excreted in the urine. Some of the crystals are endocytosed, moved into the interstitium leading to their clearance. Persistent but mild increase in supersaturation and continued production of intraluminal crystals provokes an immunological response. There are osteogenic changes and production of inflammatory and crystallization modulatory molecules. There is production of matrix vesicles through budding from the epithelial cells into the basement membrane, as well as degradation of interstitial elements and macrophages. Calcification continues with the mineralization of collagen, matrix vesicles and cellular degradation products, eventually reaching the base of the urothelium. It is now ready to be exposed to the pelvic urine through the urothelial loss and become a nidus for the development of a stone.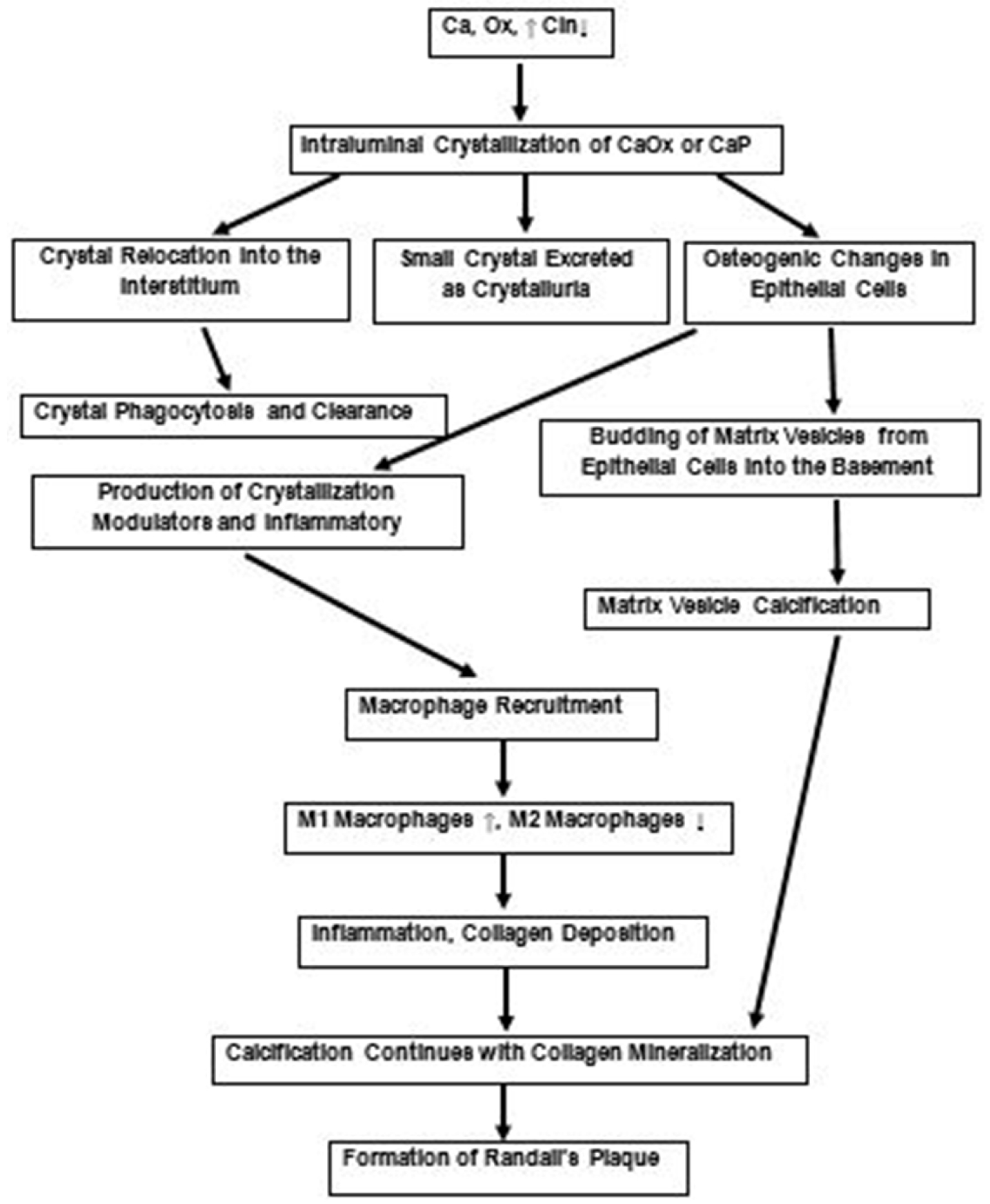
Once RP’s become exposed to the urine, stones develop by the addition of CaOx crystals which can either happen by crystal nucleation on the exposed plaque or by accretion of CaOx crystals formed independently in the surrounding urine. Crystallization will depend upon the presence of calcium, and oxalate as well as various crystallization modulators present in th urine. It should, however, be recognized that CaOx crystals will not be depositing or nucleating on CaP crystals. Both CaOx and CaP crystals as well as the plaque are covered by an organic matrix. Therefore CaOx crystals would most probably nucleate on the organic material present on the CaP crystals or plaque. If there is any specificity of interaction between the two types of crystals, it is based upon the specific macromolecule present on crystal surfaces. For example, OPN shown to be specifically incorporated into the CaOx crystals is well known for its crystal nucleation capabilities when adsorbed onto a surface.
The formation of ductal plugs is dependent upon the supersaturation. Acute and or persistent supersaturation will result in excessive crystallization reducing their movement with the urine. Slowing will allow the aggregates to increase their mass and further reduce their pace through the tubules eventually blocking their lumens. Bends in the tubules as well as changes in their luminal diameter will also hamper movement of crystal aggregates. Blockage of the openings of the terminal collecting ducts would create a lesion exposed to the pelvic urine. From this point, further growth will most likely be similar to that what happens on the exposed Randall’s plaque discussed above. Crystalline overgrowth and development of stone will depend upon the concentration of calcium, oxalate, citrate as well as many macromolecular modulators of crystallization present in the pelvic urine.
5. Conclusions
Kidney’s immune system responds to the abnormal excretion of stone forming ions and salts by producing macromolecules to control and inhibit crystallization within the renal tubules. Most of the small crystals are expelled as crystalluria. Others are dispatched into the interstitium where macrophages are responsible for their disposal. Failure of the normal appropriate renal response leads to injury, inflammation and the formation of the plaques and plugs. Many of the calcium binding macromolecules produced to modulate and inhibit crystallization, become incorporated into the crystals and become part of the organic matrix of the stones. The matrix is neither a glue nor a mortar binding individually-produced inorganic crystals but a component of the bio-crystals, biological CaP or CaOx.




 CC-BY 4.0
CC-BY 4.0