1 Introduction
The synthesis of hybrid inorganic–organic porous solids or metal-organic frameworks (MOF-n) has recently grown exponentially, giving rise to the formation of new nanoporous materials with very high surface area [1–3]. The combination of the huge variety of possible organic linkers and the multiple physical properties of inorganic cations, allow a wide modulation of both the dimensions of the pores and the properties of the final porous compounds. In such structures, organic species (amines, carboxylates…) can act either as pillars, linkers and arrange, respectively, inorganic layers, chains or clusters of transition or rare-earth metals [4–9]. Usually rigid ligands containing aromatic ring are associated with mono or divalent metals, resulting in the construction of new architectures with different dimensionalities of pore system. Some of these materials exhibit significant properties in the field of gas adsorption [10,11]. For instance, the solid MOF-5 was recently found to be an excellent candidate for the methane [12] or hydrogen [13] adsorption.
We recently initiated a global study of the reactivity of trivalent metals (V3+ [14–18], Cr3+ [19], Fe3+ [20,21]) with aromatic dicarboxylic acids in water under mild hydrothermal conditions. We then extended this work to the p metal elements such as aluminum, gallium or indium. Up to now, the utilization of such cations was rarely reported in the literature since only the indium terephthalate In2(OH)3(bdc)1.5 [22] and the aluminum terephthalate MIL-53 [23] (Al(OH)(bdc)·xH2O, isostructural to the vanadium [14] and chromium [19]-based phases) were isolated (bdc = 1,4-benzenedicarboxylic acid). The latter was also tested for its hydrogen adsorption capacity and it can adsorb up to 3.8 wt.% of H2 under 16 MPa at 77 K [24]. This promising feature of MIL-53 incited us to continue our systematic study with other carboxylic acids and we report here the hydrothermal reaction of the another connector, 2,6-naphthalenedicarboxylic acid, with aluminum. This aromatic carboxylic acid was previously used with divalent metals such as Zn [25–27], Mn [26], Cd [26] and rare-earth metals such as Ce [28], Eu [28], Tb [28], Er [29], Yb [30]. The synthesis and the structural characterization of a new aluminum 2,6-naphthalenedicarboxylate called MIL-69 (Al(OH)(O2C–C10H6–CO2)·H2O are presented.
Indeed, simulations methods are particularly useful for predicting possible crystal structures of inorganic [31,32] and hybrid structures [33,34] and help the difficult process of structure determination. Here, an original simulation method is used in order to anticipate a possible crystal structure for MIL-69, starting with the knowledge of the existing MIL-53 structure and considering that very similar synthetic conditions than in MIL-53 were used. A structural model was proposed and further refined from the powder X-ray diffraction technique. The compound was further characterized by solid state NMR (27Al, 1H, 13C). MIL-69 exhibits a three-dimensional framework consisting of channels bound by infinite aluminum octahedra chains and 2,6-naphthalenedicarboxylate groups, encapsulating a water molecule.
2 Experimental procedure
2.1 Synthesis
Al(OH)(O2C–C10H6–CO2)·H2O (MIL-69) was synthesized hydrothermally in a 23 ml Teflon-lined bomb Parr under autogeneous pressure. The starting reactants were aluminum nitrate (Al(NO3)3·9 H2O, Carlo Erba Regenti, 98%), 2,6-naphthalenedicarboxylic acid (HO2C–C10H6–CO2H, Avocado, 98%, hereafter noted ndc), potassium hydroxide (KOH, Aldrich, 90%) and distilled water. The molar ratio was 1 Al (3.5 mmol, 1.314 g); 0.5 ndc (1.75 mmol, 0.3783 g); 1.2 KOH (4.35 mmol, 0.2440 g); 80 H2O (277.8 mmol, 5 ml). The reaction pH was 1–2. The mixture was placed in the autoclave at 210 °C for 16 h, which are the optimized conditions for the synthesis of the titled compound. For longer reaction times (24, 36 or 48 h), the formation of the aluminum oxyhydroxide AlO(OH) (boehmite form) was observed together with MIL-69. When the reaction time increases, the MIL-69 phase progressively disappears indicating its dissolution during the hydrothermal treatment and the aluminum re-crystallizes under the AlO(OH) form. A kinetic study of the hydrothermal reaction showed that maximal yield of formation of MIL-69 is observed for 16 h. It was 75% based on aluminum. For shorter reaction times, an additional unknown minor phase with Bragg peaks at 5.78 and 3.31 Å appears with MIL-69. The presence of KOH is required for setting the pH value around 1–2 otherwise a mixture of the phases MIL-69 and AlO(OH) is systematically observed. The obtained crystalline product was filtered off, washed with distilled water and dried at room temperature. Scanning electronic microscope examination showed that samples of MIL-69 consist of a very fine powder with grain sizes lower than 1 μm.
2.2 Simulations and structure solution
The structure of Al(OH)(O2C–C10H6–CO2)·H2O (MIL-69) was determined from structural computer simulation and laboratory powder X-ray diffraction data. The diffraction intensities were collected on a Siemens D5000 diffractometer (θ–2θ mode, step size: 0.02°(2θ), time acquisition/step: 65 s) using the Cu Kα radiation (λ = 1.5418 Å) at room temperature, between 6 and 76°(2θ). By using the DICVOL91 [35] software, a monoclinic cell with satisfactory figures of merit was found for MIL-69, with cell parameters of a = 24.6254 Å, b = 7.5285 Å, c = 6.5597 Å, β = 106.791°, V = 1164.27 Å3 (obtained with the figures of merit M11 = 56.9 and F11 = 57.6 (0.0035, 54)). The examination of the systematic extinctions led to the space group C2/c or Cc.
Then, lattice energy minimizations were used with the aim of anticipating the crystal structure of the MIL-69 compound. Considering that similar synthetic conditions were used than in for the synthesis of MIL-53, particularly regarding the organic: metal ratios, and also the similarity of cell parameters between MIL-53 and MIL-69, we assumed that a similar framework topology to that of aluminum terephthalate MIL-53 might occur in the aluminum naphthalate, MIL-69, with the aim of simply replacing the 1,4-bdc acid by the 2,6-ndc acid. Starting from the crystal structure of the as-synthesized aluminum terephthalate MIL-53 [23] in the C2/c space group, a model for the MIL-69 was derived as follows in the C2/c space group: the aromatic rings of the terephthalate molecules were removed from the MIL-53 structure file, while keeping the carboxylate functions. Such a modification leaved us with the infinite aluminum octahedra chains typical of the MIL-53 topology, where each aluminum atom is still fully coordinated with terminal water molecules and oxygen atoms of the carboxylic functions. The intermediate model was then submitted to a cell extension along the a axis (from 19.5 Å in MIL-53 [23], to 24.6 Å in MIL-69) which corresponds to the move of the chains away from each other, leaving the required space for the insertion of 2,6-naphthalenedicarboxylic acid. The naphthalene aromatic rings were inserted between the chains, while using the carboxylic functions as anchorage points so that the naphthalene molecule are placed in a similar way than in the MIL-53 topology. Finally, the constructed model was submitted to a full lattice energy minimization, allowing all atoms to relax while keeping the cell parameters fixed at the experimental values, using the universal forcefield [36] and Cerius2 software suite [37]. The minimizations rapidly converged towards a plausible crystal structure.
The atomic coordinates predicted by the simulations were then directly used as a starting model in our Rietveld refinement. The pattern matching procedures were performed with Fullprof2k using the WinPLOTR software package [38]. The final reliability factors including 47 refined parameters and 386 independent reflections are RP = 0.122, RWP = 0.140, RB = 0.0622 and RF = 0.0566 with the following cell parameters: a = 24.598(2) Å, b = 7.5305(6) Å, c = 6.5472(5) Å, β = 106.863(8)° and V = 1160.6(2) Å3. The corresponding Rietveld plot is reported in Fig. 1. Atomic coordinates and selected interatomic distances of MIL-69 (Al) are given in Tables 1 and 2, respectively. The chemical formula deduced from the structure determination is Al(OH)(O2C–C10H6–CO2)·H2O and is in agreement with the elemental analysis (CNRS Analysis Center, Vernaison, France): Al: obs.: 9.7% (calc.: 9.8%); C: obs.: 3.4% (calc.: 3.3%); H: obs.: 3.4% (calc.: 3.3%). Density measurement gave 1.606(5) g cm–3 (calc.: 1.529 g cm3).

Final Rietveld plot of Al(OH)(O2C–C10H6–CO2)·H2O (MIL-69). The observed data are shown by the open circles and the calculated data by the solid line.
Atomic coordinates of Al(OH)(O2C–C10H6–CO2)·H2O (MIL-69)
| Atom | Wyckoff position | x | y | z |
| Al | 4a | 0 | 0 | 0 |
| O1 | 4e | 0 | –0.099(1) | 3/4 |
| O2 | 8f | 0.0465(3) | –0.825(1) | 0.654(1) |
| O3 | 8f | 0.0678(4) | –0.8618(9) | 0.984(1) |
| Ow | 4e | 0 | –0.458(1) | 3/4 |
| C1 | 8f | 0.1442(3) | –0.769(2) | 0.857(2) |
| C2 | 8f | 0.1835(3) | –0.812(2) | 0.068(1) |
| C3 | 8f | 0.2803(4) | –0.818(2) | 0.290(2) |
| C4 | 8f | 0.0870(4) | –0.824(2) | 0.828(2) |
| C5 | 8f | 0.242(4) | –0.797(2) | 0.080(2) |
| C6 | 8f | 0.3390(3) | –0.794(2) | 0.307(2) |
Selected interatomic distances (Å) in Al(OH)(O2C–C10H6–CO2)·H2O (MIL–69)
| Al–O1 = 1.780(4) | C1–C2 = 1.48(1) |
| Al–O2 = 1.845(8) | C1–C6 = 1.34(1) |
| Al–O3 = 1.994(9) | C2–C5 = 1.44(1) |
| O2–C4 = 1.28(1) | C5–C3 = 1.43(1) |
| O3–C4 = 1.28(1) | C5–C5 = 1.40(2) |
| C4–C1 = 1.43(1) | C3–C6 = 1.43(1) |
| Ow…O1 = 2.70(1) |
2.3 Solid-state NMR
NMR spectra were acquired on a Bruker Avance 500 spectrometer with a 11.7 T field, equipped with a Bruker 4 and 2.5 mm probes, with resonance frequencies for 1H, 13C and 27Al of 500.13, 125.77, and 130.32 MHz, respectively. Typically, π/4 pulse widths of 2.1 μs, repetition times of 2 s, and spectral widths of 100 kHz were used for single pulse 1H MAS experiments. The MAS speed employed was 30 kHz. The Hahn echo pulse sequence was performed for 27Al MAS experiment using selective pulses with a π/2 pulse length of 1 μs corresponding to a radiofrequency strength of 83 kHz, and a relaxation delay of 1 s. The experiment was synchronized with rotation speed (30 kHz), for which an echo delay of 2.33 ms corresponding to the fid length was set. 13C MAS spectra were acquired using a 4-mm ZrO2 rotor under conditions of high-power proton decoupling using a pulse length of 5.9 μs (π/2), a repetition delay of 20 s, a rotation rate of 12.5 kHz, and decoupling power of 9 kHz. All spectra were referenced relative to standards tetramethylsilane for 1H and 13C NMR and Al(NO3)3 aqueous solution for 27Al using adamantane (1.74 ppm for 1H and 38.3 ppm for 13C) and NH4Al(SO4)2·12 H2O (–0.6 ppm for 27Al) as secondary references. Decomposition of spectra was achieved using the dmfit2004 NMR simulation software [39].
3 Description of the structure
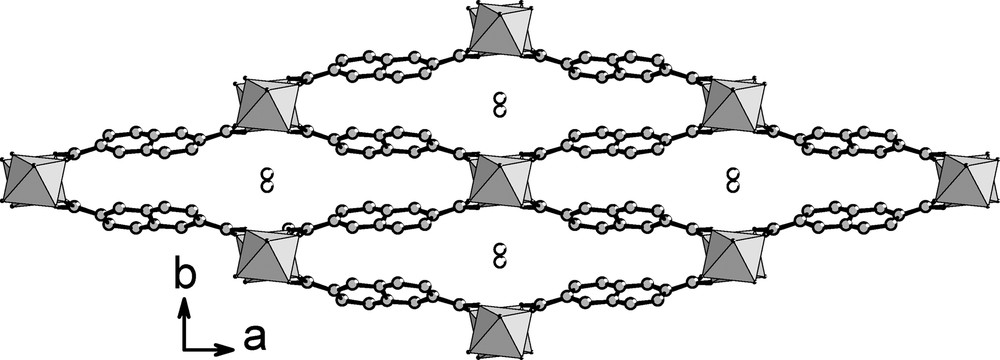
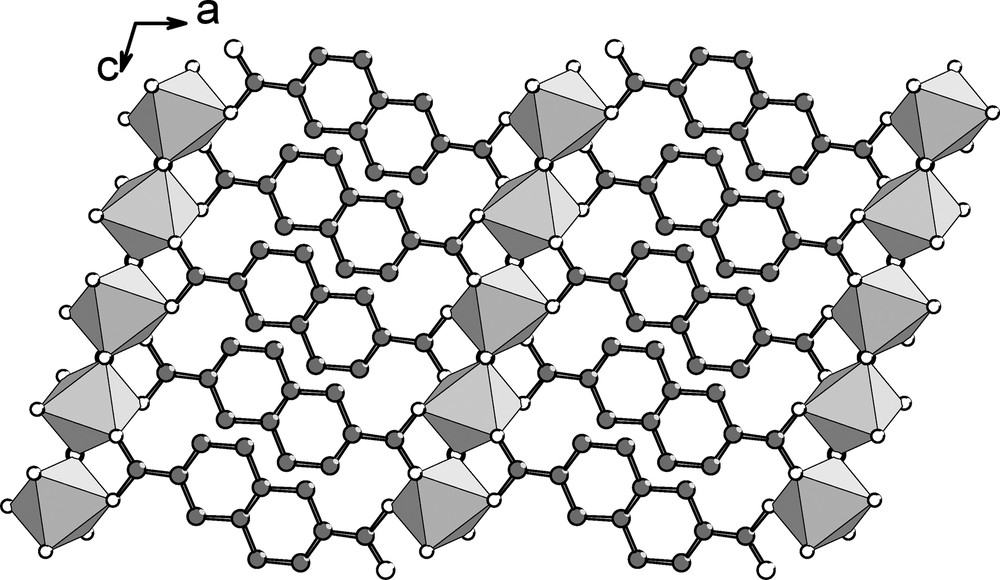
The structure of Al(OH)(O2C–C10H6–CO2)·H2O (MIL-69) consists of a three-dimensional framework built up from the connection of infinite chains of corner-sharing octahedral AlO4(OH)2 units with naphthalate ligand ndc (Fig. 2). The aluminum cation (on special position 4a) is octahedrally coordinated to four oxygen atoms from the carboxylic function and two hydroxyl groups located in trans position. The Al–O distances are 1.845(8) and 1.994(9) Å, and Al–OH one is 1.780(4) Å (Fig. 3). Bond valence calculations [40] give values of 1.41 and confirm the occurrence of a μ2-hydroxide anion for the bridging oxygen O1. The resulting AlO4(OH)2 units are linked to each other through the two opposite hydroxyl groups and this generates an infinite chain of corner-sharing octahedra running along the c axis. The files of octahedra are connected to each other through the naphthalenedicarboxylate molecules. The carboxylate ion, R-CO2–, acts as a bridging bidentate ligand adopting an usual syn–syn configuration [41,42]; the oxygen atoms of each carboxylic acid function are linked to two consecutive aluminum atoms (Fig. 4). This particular connection mode gives rise to the formation of a three-dimensional organic-inorganic network with flat channels running along the c axis, parallel to the AlO4(OH)2 chains. The accessible pore size of this one-dimensional tunnel is approximately 2.7 × 19.4 Å (it was taken into account the oxygen radius of 1.35 Å) and does not include significant void. However, a water molecule is trapped nearby the center of the tunnels (at the special position 4e, 0 0.458(1) 3/4). It mainly interacts with the bridging hydroxyl groups (dOw…O1 = 2.70(1) Å) and the oxygen atoms of carboxylic acids dOw…O2 = 3.12(1) Å). Weak π–π interactions (dC…C ≈ 3.9 Å) are also observed between the aromatic rings of two adjacent naphthalate species along the b axis. The occurrence of the hydrogen bond interactions together with the π–π ones contributes to the shrinkage of the channels along the b axis. A similar flat tunnel shape is also observed in the ytterbium-ndc framework CUmof-9 [30].
Representation of the structure of Al(OH)(O2C–C10H6–CO2)·H2O·(MIL-69) showing the channels running along c and encapsulating water molecules (open circles). Grey octahedra: AlO4(OH)2.

Coordination surrounding with atoms labels for the unique crystallographically aluminum cation.
Representation of the structure of Al(OH)(O2C–C10H6–CO2)·H2O (MIL-69) showing the infinite chains of AlO4(OH)2 units (gray octahedra) connected via the 2,6-naphtalenedicarboxylate ligand along c.
Such an atomic arrangement of octahedra-based chains is obviously reminiscent to that encountered in the compound series synthesized in the presence of 1,4-benzenedicarboxylic acid with aluminum [23] (MIL-53), chromium [19] (MIL-53) or vanadium (MIL-47 [14] and MIL-68 [17]). In these compounds, identical infinite MO4(OH)2 files are observed and are connected to each other via the terephthalate ligand instead of 2,6-naphthalenedicarboxylate one. The same structure of MIL-69 was also obtained with vanadium replacing aluminum [43].
TG experiments were performed under nitrogen with heating rate of 3 °C min–1 between room temperature and 800 °C using a TA Instrument TG2050 apparatus. The thermogravimetric analysis (Fig. 5) indicates that water can be removed upon heating below 100 °C (obs.: 6.1%; calc.: 6.9%). The water weight loss is reversible and the product rapidly re-adsorbs water in air atmosphere. The solid is thermally stable up 450 °C and then a second weight loss is observed. It is assigned to the departure of the 2,6-naphthalenedicarboxylate ligand together with the collapse of the structure (obs.: 67.2%; calc.: 74.0%). At 800 °C, the XRD pattern shows that the product is amorphous. The vanadium [43] analog of MIL-69 showed no cell parameter modification on water removal. This is in contrast with the breathing phenomenon encountered in the parent series of MIL-53 (Cr [19], Al [23]). Flat lozenge-shape tunnels (2.6 × 13.6 Å2) were observed in the hydrated form, in which a water molecule is engaged in the hydrogen bond interactions with anions of the hybrid network. In the anhydrous form, the tunnels become larger (8.5 × 8.5 Å2) and exhibit a square-like shape. The origin of these two different structural behaviors might come from the existence of π–π interactions between the aromatic ligands, which are stronger for the naphthalene-based molecule than those of the single aromatic ring of the terephthalate ligand. Concerning the MIL-69 (Al) phase, a study of its thermal behavior is currently in progress in order to characterize the cell expansion when water molecules are removed from the structure pores.
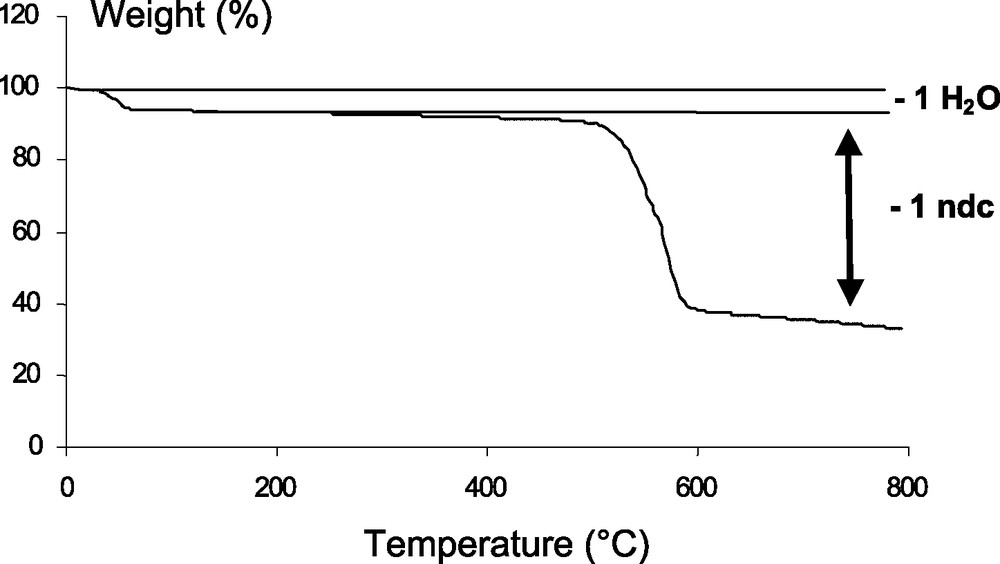
TG curve of Al(OH)(O2C–C10H6–CO2)·H2O or MIL-69 under N2 (heating rate 3 °C min–1).
4 Solid-state NMR of MIL-69
The 27Al 30 kHz-MAS NMR spectrum indicates a unique resonating signal with a powder pattern indicative of second-order quadrupolar interaction (Fig. 6). Simulation of the line shape (bottom pattern in Fig. 6) results in a quadrupolar coupling constant of 11.1 MHz, an asymmetry parameter of 0.17 and an isotropic chemical shift value of 3.1 ppm, confirming aluminum is octahedrally coordinated. These findings strictly agree with structure analysis for which, only one crystallographic site is found for aluminum with an octahedral coordination.

Experimental Hahn echo 27Al MAS NMR spectrum (top) of MIL-69 acquired at a spinning speed of 30 kHz. The simulated spectrum (bottom) using a powder model with an isotropic chemical shift δiso of 3.1 ppm, a quadrupolar coupling constant CQ of 11.1 MHz, and an asymmetry parameter η of 0.17.
The 1H 30-kHz MAS NMR spectrum exhibits a broad signal, which can be decomposed into four components (Fig. 7). The most intense signal at 7.0 ppm is assigned to hydrogen belonging to the benzene rings of the 2,6-naphthalenediacarboxylate, as expected for this chemical shift value. The peaks at 5.3 and 4.4 ppm could correspond to the hydrogen of the water molecules. Only one water species is observed in the structure and the occurrence of the two contributions for water may result from two locally inequivalent environments for the encapsulated molecule. The fourth signal is assigned to the μ2-hydroxyl group, bridging the aluminum cations to each other. It is noted that no resonating line is observed in the range 10–15 ppm relating to the protonated carboxylic acid R-CO2H, as expected from the structure analysis.
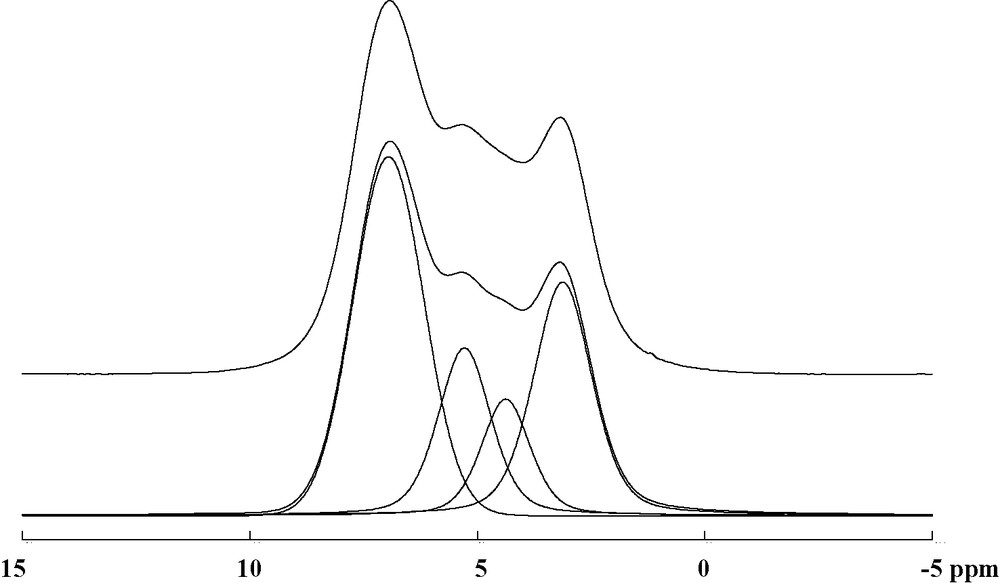
Experimental 1H MAS NMR spectrum (top) of MIL-69 collected at a spinning speed of 30 kHz. The simulated spectrum and its decomposition are included (bottom) showing four components at 7.0 ppm (46%), 5.3 ppm (18%), 4.4 ppm (11%) and 3.1 ppm (27%).
The 13C{1H-decoupled} MAS NMR spectrum shows two groups of signal at 176 and 134 ppm (Fig. 8). The peak resonating at 176 ppm corresponds to the carbon of the carboxylate R-CO2– and the broader line at 134 ppm is assigned to the different carbon atoms of the two aromatic rings of the naphthalene species.

13C{1H} high-power decoupling MAS spectrum of MIL-69 acquired at a spinning speed of 12.5 kHz. The star denotes satellite side band.
Acknowledgements
The authors thank Dr. C. Serre (Institut Lavoisier, University of Versailles) for fruitful discussions.


