1 Introduction
Classification and nomenclature are the bases of any knowledge. In the periodic table of the elements [1] – a remarkable example of classification – each column contains a series of elements which have the same number of valence electrons. Nevertheless it is clear that, in the column 1, hydrogen is quite different from the alkali metals. Similarly, each of the columns 13–16 contains several elements which strongly differ from one another. Therefore, it seems that an arrangement of the elements in several classes, according to their main common properties is of a scientific and practical interest and deserves to be studied and carried out.
2 The metals and the metallic state
The metals form more than the three quarters of the elements. Their physical and chemical properties strongly vary according to the number of their valence electrons. But they have several common properties that define the metallic state and characterize a class [2].
2.1. The metals crystallize in three-dimensional (3D) compact lattices. The number of strong bonds between one atom and their neighbors (number of coordination NC) is 12 in the cubic face centered and hexagonal compact lattices. In the cubic centered lattice each atom exchanges with its neighbors eight short bonds and six bonds longer of about 15%. Most metals crystallize in these three systems. Many crystallize in two structures, according to the temperature. The physical properties of these metallic structures are the same in any directions: they are isotropic. Moreover they contain a single type of strong bond, the metallic one: they are homodesmic, according to the classification of Evans [3].
The structures of zinc and cadmium are close to the hexagonal compact one, but the distance between the hexagonal atomic planes is 190 nm instead of 163 in the perfect structure. Therefore these elements are not quite isotropic and homodesmic. Similarly the quadratic stable variety of tin is partly anisotropic and heterodesmic. Nevertheless these elements, which possess the other characters of the metallic state belong to the metals.
2.2. The number of coordination in the metals is always higher than the number of valence electrons, so that the lattice cohesion arises from a resonance phenomenon between the different bonds. Consequently, the metallic bond is no directional, and the valence electrons are delocalized.
These characters allow the sliding of the atomic planes with respect to their neighbors – by the intermediate of dislocations – without breaking the crystalline lattice. Therefore, the metals are able to accept a “plastic deformation”, they are malleable and ductile.
On the other hand, the delocalization of the valence electrons gives to the metals a high electrical conductivity. It decreases by increasing temperature, on account of the thermal oscillations of the atoms, which hinder the electron motion.
2.3. The most general chemical property of the metals is their capacity for giving up electrons and becoming cations. The best electron donors are the alkali and the alkaline earth metals from which the electronegativity X in the Pauling’s scale is close to 1. That capacity decreases by increasing the number of valence electrons, and becomes very small for the metals of the second and third triads of the periodic table (X = 2.2) and much more for gold from which the electronegativity equals that of selenium (2.4). Nevertheless these elements which are more electronegative than hydrogen (X = 2.1) are not excluded for the metal class.
The metallic character of an element more depends on its structure and its physical properties than on its chemical ones. This remark will be also valid in other element classes.
3 The inert gases
These non-metallic elements which occupy the column 18 of the periodic table form a second definite class, although they differ from one another in their densities and their boiling points which increase with the atomic number. Indeed, they are the least active of the elements, even if the three heaviest are able to react with fluorine and other very oxidizing reagents. Therefore they are monoatomic and crystallize at low temperature in highly symmetrical lattices by the effect of no directional Van der Waals attractions (NC = 0).
4 A large series of ‘non-metals’: the panelements
The elements others than the metals and the inert gases are hydrogen and a series of 18 elements of the columns 13–17 (the unstable astatine excluded). For a long time they were named ‘metalloids’. But this term, which suggests some analogies with the metals, is not valid for elements such as oxygen, nitrogen, and halogens.
Therefore, these 19 elements are ranged without any order in the lumber-room of the ‘non-metals’. This expression is not inexact but does not say anything about their activity which is of a fundamental importance in the whole chemistry: indeed all the chemical compounds – except the intermetallic ones – contain one, several or many of these elements. Of course, they are present in all the minerals from which the metals are extracted. Therefore, these ‘non-metals’ deserve to be named ‘panelements’ (from the Greek τo´ pãn, the whole).
In the solid state, the panelements form lattices much less compact than the metals. Silicium and germanium have the cubic type diamond homodesmic structure (NC = 4). All the other panelements contain both covalent and Van der Waals bonds, thus they are heterodesmic with NC between 3 and 1.
5 Metalloids and antimetals [4]
The name ‘panelements’ corresponds to their activity in the whole chemistry. Moreover they are very diverse and – except hydrogen – they may be divided in two well-characterized classes:
- ● the elements highly electronegative (X ≥ 2.5 in the Pauling’s scale) will be named ‘antimetals’: indeed, whereas the metals are electron donors, the antimetals are electron acceptors; they attack the metals by capture of their valence electrons and become anions whereas the metals become cations;
- ● for the elements of middle electronegativity (2 < X ≤ 2.5), the ancient term ‘metalloid’ is valid: it situates the corresponding elements, which have some analogies with the metals between these latter and the antimetals.
Both classes differ from one to another not only in their electronegativity, which affects their chemical properties, but also in their crystalline lattices: the antimetals form molecular rather similar lattices, whereas the metalloid lattices are macromolecular and very diverse.
6 Commentary on tables
The data contained in tables which are classical have been mainly derived from the Refs. [5–7]. Scheme 1 situates the seven antimetals and the metalloids in the periodic table.
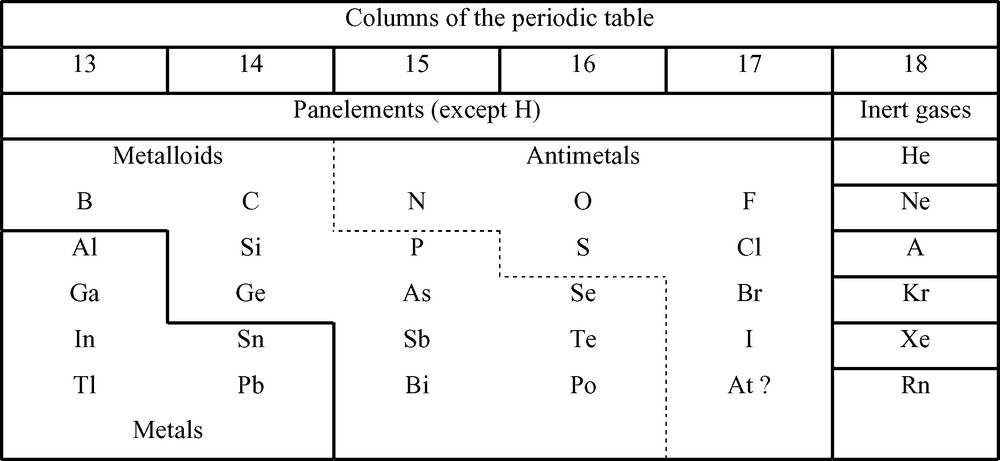
Situation of the metalloids and antimetals in the periodic table.
Tables 1 and 2 summarize some physical and chemical properties of the antimetals (on the right) and the metalloids (on the left).
- ● Tables 1 and 2 show that in each class the elements have a series of common properties which differ from one class to the other. Therefore the division of the panelements in antimetals and metalloids is valid, even if several elements have not all the properties of their class.
- ● Remarks relative to several elements:
- ○ graphite. The carbon electronegativity (X = 2.5) equals those of sulfur and iodide, so that several ionic carbides (acetylides) such as Li2C2 and (Ca, Ba, Sr) C2 may be obtained by direct synthesis. But graphite, the stable variety of carbon, crystallizes in a lamellar structure which is refractory and conductive of electricity: thus it is evidently a metalloid;
- ○ nitrogen. In account of its volatility, its electronegativity equal to that of chlorine (X = 3) and the existence of ionic nitrides (Li3N, Ca3N2…) obtained by direct synthesis, nitrogen is an antimetal. Its weak chemical reactivity, much less than that of chlorine, is a consequence of the strong stability of the N2 molecule due to its triple bond;
- ○ phosphorus is generally known in its white molecular form (P4) which is volatile and very reactive and, therefore may be compared to an antimetal. Nevertheless, it has to be ranged in the metalloids: indeed its electronegativity (X = 2.1) is too small for an antimetal. Moreover, its stable black variety is rhombohedral and conductive of electricity as the following elements of the column 15;
- ○ Selenium and sulfur have almost the same electronegativity (X = 2.4 for Se, 2.5 for S) and their chemical activities are comparable. But the stable structure of sulfur is molecular (S8) and insulating, whereas the stable selenium form (silver-gray or ‘metallic’ selenium) is macromolecular hexagonal as tellurium. Its electrical conductivity, very small in the dark rapidly increases with the light intensity. Therefore sulfur has to be ranged in the antimetals, and selenium in the metalloids.
- ● In brief the important differences between the metalloids and the antimetals mainly depend on their structures: covalent or macromolecular for the former, molecular for the latter. Consequently the proposed division of the panelements in metalloïds and antimetals becomes evident.
Comparison between metalloids and antimetals: structures and physical properties
| Metalloids | Antimetals |
| ● Many allotropies between the molecular and the stable structures for P, As, Sb, Se, Te | Molecular structures |
| ● Stable covalent or macromolecular structures | ● Diatomic molecules, except S8 |
| ○ Homodesmic 3D: Si, Ge, cubic diamond type (NC = 4) | ● NC = 1 except N2 (3), O2 and S8 (2) |
| ○ Heterodesmic 2D: C hexagonal with plane sheets and B, P, As, Sb, Bi rhombohedral with folded sheets (NC = 3) | ● In solid state, Van der Waals no directional bonds between the molecules, leading to compact heterodesmic lattices |
| ○ Heterodesmic 1D: Se, Te hexagonal with helicoïdal fibers (NC = 2) | |
| High reticular energies | Lattices of small reticular energy |
| ● Crystalline lattices hard and brittle | ● Solids of small hardness |
| ● Densities lower than those of the neighbor metals but higher than those of the neighbor antimetals | ● Densities higher than those of the neighbor inert gases, but much lower than those of the neighbor metalloids |
| ● Melting points strongly higher than those of the neighbor antimetals and metals | ● Low melting points and boiling points: only iodine and sulfur are solid at room temperature |
| ● No or very small (Te) solubility in the homopolar solvents | ● Solubility in the homopolar solvents small for N2 and O2, but high for the other elements |
| Electrical conductivities small or very small, (much lower than those of the neighbor metals) increasing with temperature by electron transfer in the conduction band | Electrical insulators |
Comparison between metalloids and antimetals: chemical properties
| Metalloids | Antimetals |
| Moderate to weak reactivity, which often requires high temperatures | High reactivity increasing with electronegativity |
| Compounds with metals | Compounds with the metals |
| ● Ionic carbides (acetylides) of Li, Ca, Sr, Ba, obtained by direct synthesis | 1. Direct exothermic reactions often very brisk leading to a very high number of ionic and ionocovalent compounds diverse in their properties |
| ● Many ionocovalent, homopolar and interstitial compounds, refractory for the carbides and borides, generally fusible above 700 °C for the others | 2. Melting points and boiling points decreasing along the series O–N–S–F–Cl–Br–I |
| ● Alloys: carbon and silicium steels – alloys with fusible metals for Sb and Bi | Besides many refractory oxides, the halides of a high oxidation state are easily volatile and sometimes liquid. |
| Compounds with hydrogen | Compounds with hydrogen |
| ● Direct synthesis possible at high temperature but not used | 1. Direct exothermic synthesis (A + H2) always possible (but not always used) leading to gaseous or liquid (HF, H2O) molecules |
| ● Indirect synthesis for all the metalloids | 2. Acid functions for 5 of the 7 antimetal compounds: weak for H2S, middle for HF, strong for HCl, HBr and HI |
| ● Acid functions only for H2Se (weak) and for H2Te (middle) | 3. Hydrogen polysulfides in chains synthesized by indirect way (H/S ratio < 2) |
| ● Compounds in chains and cycles for B, C, Si (H/M > 2) | |
| Higher oxides leading to weak Brönsted acids, except H3PO4 (middle) and H2SeO4 (strong) | Higher oxides of N, S, Cl, Br, I leading to strong Brönsted acids |
7 Use of the antimetals and metalloids
All the panelements are chemically active. In account of their low melting and boiling points, due to their molecular structure, the antimetals are only used as chemical reagents. On the contrary their macromolecular structure allows to use the metalloids as materials. For instance graphite is used for its mechanical and thermal properties and also as a conductor of electricity, silicium and selenium for their electronic properties. These data confirm the difference between these two element classes.
8 Relations between metals and metalloids
From the top to the bottom of the columns 13–16 of the periodic table the electronegativity of the elements decreases and their metallic character increases. So, in the column 13, aluminum, the second element is a metal. In the column 14, it is only tin, the fourth element (in its white stable form) which is a metal. What about the columns 15 and 16? Antimony and bismuth are sometimes considered as metals for their ability to form alloys with the former. But they do not possess any of the main properties of the metallic state (see Section 2) and therefore have to be ranged beyond the metalloids. Owing to the evolution from the column 13 to the column 15, polonium is certainly a metalloid too.
9 Hydrogen
On account of the chemical activity of the molecule H2, hydrogen is evidently a panelement. But owing to its physical properties it is not a metalloid. Moreover on account of its low electonegativity (X = 2.1) and its ability to become a cation, it cannot take place beyond the antimetals. This primordial element and the most present in the universe is well situated alone at the beginning of the periodic table.
10 An arrangement of the elements according to their electrical conductivity
The electrical conductivity measurements naturally leads to divide the elements in three classes: the metals, the ‘semi-metals’ and the ‘non-metals’. What are the relations of these classes with those clearly defined previously?
- ● Actually the semi-metals of the physicists correspond almost exactly to the metalloids and thus confirm that these elements form a class. Nevertheless the metalloids are very diverse: for instance bismuth is closer to the metallic state than arsenic. Thus the term metalloid seems to be preferable to the expression semi-metal which suggests that the relation metal–semi-metal is quantitative (semi = 1/2), and is the same for all the semi-metals.
- ● The elements other than the metals and semi-metals are isolating, and therefore may be ranged in the non-metals. But this ‘class’, valid for the electrical conductivity studies cannot be generalized: indeed, neglecting the chemistry, it mixes the antimetals and the inert gases: thus the most active and the least active elements of the whole chemistry.
11 Others arrangements of the elements in several periodic tables
During the last 15 years, a series of inorganic and physical chemistry books have been published which contain various element arrangements inside the periodic table.
- ● The periodic table contained in [8] situates hydrogen in the line 1, over and between the columns 7 and 8 in agreement with its middle electronegativity (2.1) and its ability to become both anion or cation. Helium is placed on the top of the column 18. The other elements are divided in metals and non-metals. These latter are situated on the right and over the broken line Al, Ge, Sb Po. Therefore Ge, Sb, Bi and Po are included in the metals: a debatable arrangement!
- ● The periodic table contained in [9,10] differ in the situation of hydrogen and helium. In [9] hydrogen is situated in line 1 over and between the columns 8 and 9, for the same reasons as in [8], and helium is on the top of column 18, in agreement with its atomic number and its physical and chemical properties.
In [10] hydrogen is situated on the top of the column 1 and helium in the top of column 2, both in agreement with their electronic structures. The distribution of the other elements, the same in the both periodic tables is the following:
- ● block s: columns 1 and 2: typical elements;
- ● block d: columns 3–12: transition elements;
- ● block p: columns 13–18: post-transition elements.
(The block f concerns the lanthanides and actinides which do not interest the present article).
It is clear that this arrangement of the elements, founded on the electron distribution round the nucleus, is valid and is of a scientific and pedagogic interest. It is clear too, that it is quite different from the arrangement proposed in the present article which is founded on the element structures and also on their physical and chemical properties.
12 Summary and conclusion
The present text shows that, although they are very diverse, the chemical elements possess in their structures and physical properties and also in their chemical ones a series of common characters, so that they may be ranged in four definite classes: the metals, the inert gases, the metalloids and the antimetals. This classification is founded on a series of well known experimental data relative to the elements in their stable allotropic forms. Only hydrogen does not belong to any of the four classes. This primordial element and the most present in the universe is well situated alone at the beginning of the periodic table.
The neologism “antimetal” designates the elements of which the chemical activity is opposite to that of the metals, and which attack the metals by capture of their valence electrons.
Metalloid is an ancient term, at present unusual, but which is quite valid for a series of elements which have some analogies with the metals. It seems to be preferable to the synonymous expression semi-metal often used by the physicists, which appears too quantitative (semi = 1/2) and uniform for a series of very diverse elements.
Finally this text proposes a rational solution of an old unsolved problem: the arrangement of the elements in several classes. It set in order the elements other than the metals and the inert gases. According to their presence in the whole chemistry they deserve to be named ‘panelements’.
Of course as shown in Scheme 1 the domains and the names of the different classes may be easily introduced in the periodic table, which therefore gives about those a general view.



Vous devez vous connecter pour continuer.
S'authentifier