1 Introduction
The development of environmental friendly and economically inexpensive methods for the synthesis of biologically active heterocycles using readily available reagents is an important objective of current organic synthesis [1]. Multicomponent reactions (MCRs), in which three or more different starting materials react to give a final product in a one-pot procedure, have been used as a powerful tool to achieve this goal [2]. These methodologies allow molecular complexity and diversity to be created by the facile formation of several new covalent bonds in a one-pot transformation. Because MCRs combine two major principles of organic synthesis, convergence, and atom economy, this class of reactions has found widespread applications in organic and medicinal chemistry [3].
1,3-oxazine derivatives have gained much attention due to varied biological properties like analgesic [4], anticonversant [5], antitubular [6], antibacterial [7] and anticancer [8] activities. Non-nucleoside reverse transcriptase inhibitor [9] trifluoromethyl 1,3-oxazine-2-one shows high activity against a variety of HIV-1 mutant strains [10]. In addition, naphthoxazine derivatives have exhibited therapeutic potential for the treatment of Parkinson's disease. They can also be used as an intermediate in the synthesis of N-substituted aminoalcohols [11]. Investigation and studies of 1,3-oxazine derivatives have been done via the three-component cyclocondensation of primary aliphatic and cyclic amines with formaldehyde and substituted phenols [12]. This process yielded several types of well-defined monomeric products depending on the particular amine, temperature nature and position of the substituent on phenol. Although several methods for the synthesis of 1,3-oxazine derivatives have previously been reported [13–15], only few of them have been established on the basis of the MCRs [16]. Therefore, the development of facile methodologies for the synthesis of highly functionalized 1,3-oxazine derivatives is still challengeable in the field of multicomponent reactions.
In view of combined environmental and economical demands, the literature reports applications of metal-ion-free and environmentally safe reagents in multicomponent reactions. In recent years, an increasing attention is being focused on the development of green synthetic methods in aqueous medium. Water is one of the most abundant, cheapest, and environmentally friendly solvents. Indeed, water exhibits unique reactivity and selectivity, which is different from that of conventional organic solvents [17]. Thiamine hydrochloride (VB1) is a naturally occurring, water soluble, non-toxic and biodegradable reagent. The structure of VB1 contains a pyrimidine ring and a thiazole ring linked by a methylene bridge (Fig. 1). The use of VB1 analogs as powerful catalysts for various organic transformations has been well documented [18]. Recently, Hu et al. have reported several VB1-catalyzed reactions for the synthesis of various heterocyclic compounds, such as benzo[4,5]imidazo[1,2-a]pyrimidine and [1,2,4]triazolo[1,5-a]pyrimidine derivatives [19], dihydropyridines [20], 1,2-dihydro-naphth[1,2-e][1,3]oxazine-3-one [21], and pyrimidinones [22]. According to previous reports, VB1 exhibits similarities with phase transfer catalysts (PTC), and can catalyze varieties of chemical reactions [23–26].

Structure of thiamine hydrochloride (VB1).
In continuation of our ongoing research on the development of novel methodologies for the multicomponent synthesis in water as a green reaction medium [27], we disclose herein the synthesis of 1,3-oxazine derivatives from α- or β-naphthol, anilines and formaldehyde via a one-pot, multicomponent strategy (Scheme 1).
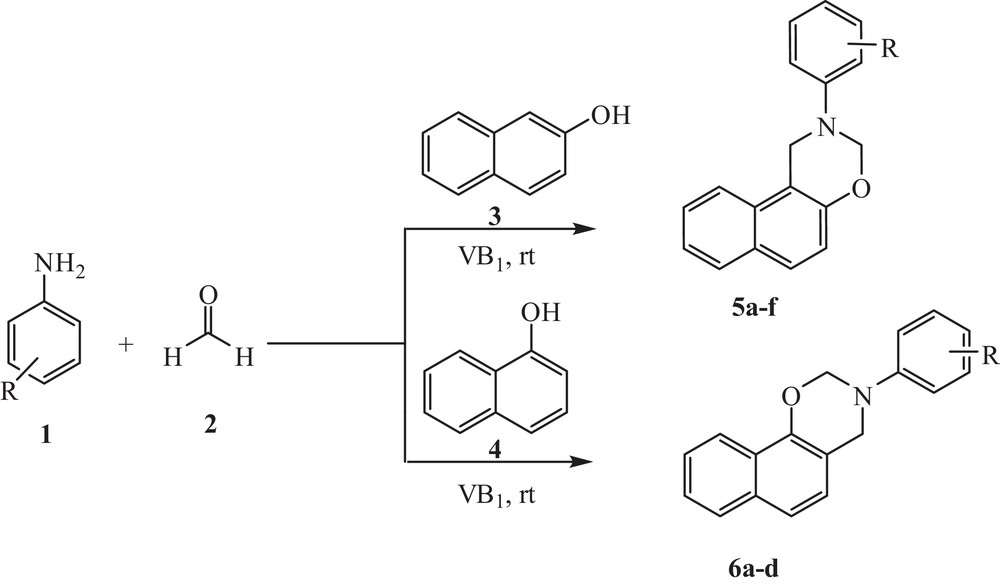
VB1-catalyzed synthesis of 1,3-oxazine derivatives in water.
2 Results and discussion
From an economical and environmental point of view, we have studied initially the three-component reaction of β-naphthol, aniline and formaldehyde (1:1:3) in the presence of VB1 (5 mol%) in water at room temperature for 1 h to obtain the desired 1,3-oxazine derivative 5a in quantitative yield (65%). However, no product formation was observed when the mixture was stirred under similar reaction conditions in the absence of VB1, even after prolonged stirring (Table 1, entry 1). Inspired by the result, we further investigated the effect of catalyst loading on the product formation and reaction time; results are summarized in Table 1. When the reaction was carried out in the presence of 2 mol% of VB1, very small amounts of product (5a) were observed after 2 h. An increase in the catalyst loading from 5 mol% to 10 mol% not only decreased the reaction time, but also increased the product yield from 65% to 92% (Table 1, entry 4). However, further increase in the amount of VB1 (15 mol% and 20 mol%) did not improve the yields. Therefore, 10 mol% of VB1 was sufficient to push the reaction forward. The catalytic activity of the recycled VB1 was also examined according to the typical experiment conditions. After completion of the reaction (TLC), the desired product 5a was isolated by extraction with ethyl acetate. Then, the aqueous solution containing the catalyst was further recycled for three consecutive runs, which yielded product 5a in 92%, 86%, 80% yields, respectively (Table 1, entry 4). Thus, VB1 could be effectively used as a reusable catalyst for the multicomponent synthesis of oxazines.
Effect of catalyst loading on model reaction.
| Entry | Catalyst (mol%)a | Time (min) | Yield (%)b |
| 1 | 0 | 180 | – |
| 2 | 2 | 60 | 20 |
| 3 | 5 | 60 | 65 |
| 4 | 10 | 30 | 92, 86, 80c |
| 5 | 15 | 30 | 92 |
| 6 | 20 | 30 | 90 |
a Reaction conditions: β-naphthol (1 mmol), aniline (1 mmol), formaldehyde (3 mmol) in water (2 mL).
b Isolated yield.
c Catalyst was reused three times.
We have studied further the effect of various solvents on the above reaction. The results displayed in Table 2 indicate that the solvent affected the efficiency of the reaction. Poor yields were obtained in aprotic solvents such as CHCl3, DMF, CCl4 and CH3CN (Table 2, entries 5–8). However, the best results were obtained in protic solvents such as ethanol, methanol and water (Table 2, entries 2–4). The same reaction was also carried out under solvent-free conditions, but very poor yields of product 5a were obtained, even after 5 h (Table 2, entry 1). Therefore, the best reaction conditions were obtained by using 10 mol% of VB1 as the catalyst in H2O at room temperature.
Effect of solvents on product formation.
| Entry | Solventa | Time (min) | Yield (%)b |
| 1 | Solvent-free | 300 | 27 |
| 2 | H2O | 30 | 92 |
| 3 | EtOH | 30 | 78 |
| 4 | MeOH | 30 | 80 |
| 5 | CHCl3 | 30 | 50 |
| 6 | CCl4 | 30 | 42 |
| 7 | CH3CN | 30 | 34 |
| 8 | DMF | 30 | 36 |
a All the reactions were carried out at room temperature.
b Isolated yields.
In order to explore the scope and generality of the present method, wide varieties of aromatic amines with electron-releasing as well as electron-withdrawing groups were reacted with α- or β-naphthol with three equivalent of formaldehyde to afford the corresponding 1,3-oxazine derivatives in excellent yields (Table 3). Aromatic amines carrying either electron-donating or electron-withdrawing substituents could react efficiently to give the corresponding products without significant difference. In the present work, the primary aromatic amines are of particular interest, since their chemical behavior can be modified significantly by ring substitution and they are capable of undergoing nuclear condensation with formaldehyde.
Synthesis of 1,3-oxazine derivatives catalyzed by VB1 in water.
| Entry | Amine | Product | Time (min) | Yield (%)a | M.P. (°C) [Ref] |
| 1 | 5a | 30 | 92 | 48–50 [12] | |
| 2 | 5b | 20 | 88 | 88–90 [12] | |
| 3 | 5c | 40 | 90 | 111–112 [12] | |
| 4 | 5d | 30 | 87 | 75–76 [13] | |
| 5 | 5e | 35 | 92 | 116–118 [12] | |
| 6 | 5f | 25 | 90 | 75–77 [13] | |
| 7 | 6a | 40 | 80 | 110–112 [14] | |
| 8 | 6b | 30 | 78 | 195–196 [14] | |
| 9 | 6c | 30 | 85 | 300 (d) [14] | |
| 10 | 6d | 45 | 75 | 75–77 [14] |
a Isolated yield; Compounds were characterized by IR, 1H-NMR and mass spectroscopy.
A plausible mechanism for the VB1-catalyzed synthesis of 1,3-oxazine derivative ‘5a’ is depicted in Scheme 2. Mannich-type condensation of aniline with formaldehyde in the presence of VB1 gives imine 7, which was then attacked by the electron-rich centre of β-naphthol to form intermediate 8. Intermediate 8, via a second Mannich-type condensation with a second molecule of formaldehyde, gives intermediate 9, which through intramolecular cyclization afforded 1,3-oxazine derivative 5a.
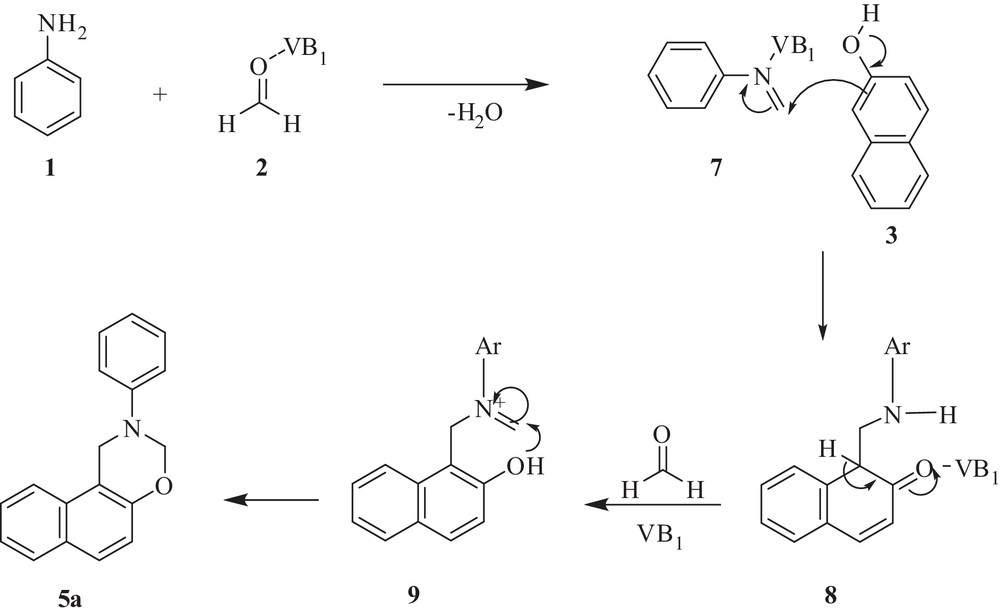
Plausible mechanism for the formation of the 1,3-oxazine derivative (5a).
3 Conclusion
In conclusion, we have developed an efficient and convenient method for the synthesis of a variety of 1,3-oxazine derivatives via a one-pot, three-component condensation of anilines, formaldehyde and α- or β- naphthol catalyzed by VB1 in aqueous medium. The use of a biodegradable catalyst in a universal solvent – water –, operational simplicity and mild reaction conditions make this method attractive for the synthesis of polysubstituted oxazine derivatives.
4 Experimental
4.1 General procedure for synthesis of 1,3-oxazine derivatives (5a–f, 6a–d)
A mixture of α-or β-naphthol (1 mmol), formaldehyde (3 mmol) and VB1 (10 mol%) in water (2 mL) was stirred at room temperature for an appropriable time (Table 3). After completion of the reaction (TLC), the product was extracted with ethyl acetate (2 × 5 mL). The organic layer was washed with brine and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to afford solid or viscous 1,3-oxazine derivatives in excellent yield.
4.2 Spectra data of the representative compounds
4.2.1 2-Phenyl-2,3-dihydro-1H-naphtho[1,2-e][1,3]oxazine (5a)
IR (KBr, cm−1): 1610, 1590, 1450, 1215, 1032; 1H-NMR (200 MHz, DMSO-d6): δ = 5.06 (s, 2H, Ar–CH2–N), 5.70 (s, 2H, N–CH2–O), 7.01–7.89 (m, 11H, Ar–H); MS (ESI): m/z 262 (M + 1).
4.2.2 3-Phenyl-3,4-dihydro-2H-naphtho[2,1-e][1,3]oxazine (6a)
IR (KBr, cm−1): 1605, 1585, 1208, 1025; 1H-NMR (200 MHz, DMSO-d6): δ = 4.88 (s, 2H, Ar–CH2–N–), 5.62 (s, 2H, N–CH2–O), 6.89–7.78 (m, 11H, Ar–H); MS (ESI): m/z 262 (M + 1).



Vous devez vous connecter pour continuer.
S'authentifier