1 Introduction
Alkylation of nitrogen heterocycles, such as imides, pyrroles, pyrazoles, indoles etc. is a crucial transformation. The derivatives so formed constitute an important class of organic compounds that finds wide applications in biology, [1,2] synthetic, and polymer chemistry [3–5]. Many routes are available for their synthesis, most of which involve direct N-alkylation of heterocycles using a base such as NaH, [6] KH/Et3N, [7] BuLi, [8] followed by reaction of the resulting salt with an alkylating agent in various solvents, for example, acetone, DMSO, [9] DMF, [10] HMPT, [11] etc. However, some of these methods are limited by harsh reaction conditions, low yields, use of toxic solvents or catalysts, etc. Some better methods reported for their synthesis involve the use of phase-transfer catalysts (PTCs) under non-conventional reaction conditions [12]. The use of phase-transfer catalysis is a technique that allows two reactants with immiscible phases to react more efficiently. Ionic liquids (ILs) have become increasingly used solvents and catalysts in “green” chemistry due to their unique properties [13–16]. Because of the fact that ILs are comprised of bulky organic cations, they seem to be an excellent substitute for PTCs along with quaternary ammonium salts, crown-ether derivatives, and phosphonium salts [17]. The most important technical problems in the industrial applications using soluble PTCs, are their recovery and subsequent reuse or disposal [18,19].
Immobilization of the catalyst on a polymeric matrix was thought to provide a simple solution to this problem, as the heterogeneous catalyst can be isolated at the end of the reaction by simple filtration and recycled for another run [20]. Recently, the preparation of a polystyrene immobilized dual acidic IL ([PS-IM(CH2)4SO3H][HSO4]) and its use as a catalyst in the Hantzsch synthesis of 1,4-dihydropyridines was reported [20c]. Polymer resins, the traditionally used supports for PTCs, require a conditioning period to widen their matrix pores, thus lowering activity, [21] whereas no such swelling occurs while using inorganic solid (silica, alumina, clay, etc.) immobilized PTC [22,23]. The reaction thus begins immediately, as it does with homogeneous catalysts. Moreover, silica-based supports such as commercial and amorphous silica, SBA-15, and MCM-41 have the advantage of having a porous structure, a large surface area, good mechanical strength, high thermal stability, and good physicochemical stability toward solvents and oxidizing agents. Luo et al. prepared a common silica gel immobilized IL to catalyse the synthesis of amidoalkyl naphthols from 2-naphthol, aldehydes and amides [23b]. Guan et al. successfully immobilized Brønsted acidic ionic liquid [(CH2)3SO3HVIM]HSO4 together with 3-mercaptopropyltrimethoxysilane and AIBN onto silica gel using TEOS as a silica source to generate the grafted catalyst with mesoporous structure, which was used for the esterification of alcohols [23c]. Also, such supported reagents efficiently induce reactions under simple conditions such as microwave irradiation, as the support does not restrict the transmission of microwaves [12]. Safri et al. have immobilized an IL on superparamagnetic nanoparticles for the synthesis of tetrasubstituted imidazoles [23d]. Ni ion-containing SILPs have also been utilized for various reactions recently [23e,f]. In continuation of our interest [24,25] in the development of an economic and rapid protocol, utilizing microwave energy, solid-supported catalysts and ionic liquids, [26–30] we report herein the examination of various immobilized and free ILs along with some traditional PTCs in a standard solid–liquid phase-transfer catalytic (SL–PTC) reaction. A comparison between various ILs (especially chemically immobilized ones) for their respective PTC activity has been made depending upon their cationic moiety, which imparts novelty to the work presented in this manuscript.
Some common ionic liquids were chosen for this work; tetraalkylammonium salts (nBu4NBr (1a), Et4NCl (1b)), imidazolium salts ([Bmim]Cl– (2a), [Bmim]Br– (2b), [Bmim]BF4– (2c)), and pyridinium salt ([Epy]Br– (3)) along with other non-conventional immobilized ionic liquids; SiO2-ammonium ILs ([SiO2–PTB]Cl– (4a), [SiO2–PTB]BF4− (4b), where PTB = propyl tributylammonium), SiO2-imidazolium ILs ([SiO2–Pmim]Cl− (5a), [SiO2–Pmim]BF4− (5b), where Pmim = propyl methylimidazolium), and SiO2–Pyridinium IL ([SiO2–Pmpy]Cl− (6), where Pmpy = propyl 4-methylpyridinium) (Scheme 1).

(Color online). (a) Preparation of immobilized ILs; (b) Structures of various immobilized ILs prepared.
2 Results and discussion
2.1 Preparation and characterization of the catalyst
Immobilized ionic liquids 4a, 4b, 5a, 5b and 6 were prepared as shown in Scheme 1.
Catalysts 4a and 4b were characterized using various techniques and the analytic data has been listed in Table 1.
Analytical data of silica gel and catalysts.
| Sample | Elemental analysis (%) | SBET (m2·g−1) | Pore volume (cm3·g−1) | Pore size (Å) | IR spectral data ν (cm−1) | ||
| C | H | N | |||||
| SiO2 | – | – | – | 392 | 0.70 | 60.0 | 796, 974, 1061, 1635, 1980, 3250 |
| [SiO2–PTB]Cl– (4a) | 17.68 | 3.28 | 1.40 | 199 | 0.58 | 53.9 | 699, 1061, 1348, 1462, 1630, 2347, 2878, 2962, 3254 |
| [SiO2–PTB]BF4– (4b) | 14.86 | 2.72 | 1.15 | 173 | 0.54 | 53.0 | 694, 1061, 1345, 1470, 1638, 2328, 2880, 2962, 3244 |
2.1.1 CHN analysis
Elemental analysis revealed that 0.97 mmol and 0.83 mmol of the organic moiety were grafted per gram of the SiO2 support in case of catalysts [SiO2–PTB]Cl– and [SiO2–PTB]BF4–, respectively.
2.1.2 BET surface area and pore size distribution
Compared with the parent SiO2, BET surface areas of immobilized ILs 4a and 4b significantly decreased from 392 to 199 and 173 m2·g–1, respectively. The N2 adsorption/desorption isotherms of 4a, 4b, and silica are shown in Fig. 1. Obviously, the immobilized ILs 4a and 4b maintain the characteristics of type-IV isotherms and show a uniform pore size distribution in the mesoporous region (Fig. 1). Compared to the support (silica gel), a pronounced decrease in the BET surface area, in the pore volume, and in the pore size occurred by the introduction of an organic moiety.

(Color online). N2 adsorption-desorption isotherms and pore size distribution profiles of immobilized catalysts (A, B) [SiO2–PTB]Cl−, 4a and (C, D) [SiO2–PTB]BF4−, 4b.
2.1.3 FT–IR spectroscopy
The FT–IR spectra of immobilized ILs (Fig. 2) exhibit characteristic peaks at 1348, 1462, 2347, 2878, 2962 cm−1, which are due to C–H vibrations of the methyl and methylene groups. An additional band in the fingerprint region at 699 cm−1 is due to C–H rocking of the methyl group. All these characteristic peaks of functionalized ILs cannot be observed in the IR spectrum of silica gel. The Si–O–Si stretching modes of silica gel can be observed as a strong peak at 1061 cm−1 and a broad feature seen at 3254 cm−1, related to Si–OH groups and water adsorbed on silica. Moreover, in the 3000–2800 and 1500–1300 cm−1 regions, the spectra of the immobilized ILs show some weak peaks, which should be due to the existence of organic moieties. The FT–IR spectrum of the reused catalyst 4a shows no significant change in its structure.
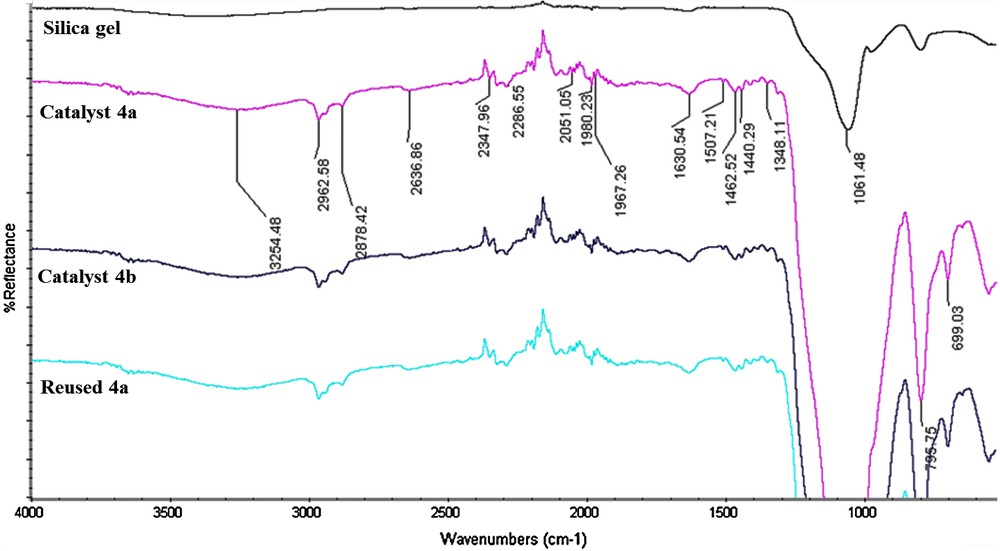
(Color online). FT-IR spectra of silica gel, fresh [SiO2–PTB]Cl−, [SiO2–PTB]BF4−and reused [SiO2–PTB]Cl−.
2.1.4 Thermogravimetric analysis
The stability of immobilized ILs 4a and 4b was determined by thermogravimetric analysis (Fig. 3). The first step in the TG curve indicates an initial weight loss of 2.4% up to 120 °C due to the adsorbed water on the inner and outer surfaces of silica. The second step shows the complete loss of the IL covalently grafted onto silica at 190 to 750 °C.

TG analysis of [SiO2–PTB]Cl−, 4a and [SiO2–PTB]BF4−, 4b.
2.1.5 Scanning electron microscopy
The shape and surface morphology of the samples were investigated by SEM. As shown in Fig. 4, the particle size of the silica gel-supported IL is similar to that of silica gel, which demonstrates that the particles of silica gel had a good mechanical stability during the immobilization step. However, the surface morphology of these two samples is different. Fig. 4 shows that the surface of SiO2 was very slick, whereas a small aggregate on the surface of silica gel-supported ILs, 4a and 4b, was obvious. The SEM graph of the reused catalyst, 4a, gives evidence that no leaching of the catalyst took place during the reaction. On the basis of all these studies and the related literature [31,32], we may conclude here that the organic moiety has been covalently grafted onto the surface of silica gel.

Scanning electron micrographs (SEM images) for (A) silica gel, (B) [SiO2–PTB]Cl−, (C) [SiO2–PTB]BF4−and (D) six-time reused catalyst.
2.2 Comparative study of various PTCs
Microwave-assisted solvent-free N-benzylation of phthalimide using K2CO3 (inorganic solid phase) with benzyl chloride (organic liquid phase) was carried out under SL–PTC conditions (biphasic ones with traditional PTCs or free ILs and triphasic ones with immobilized ILs) to determine the PTC activity of various ILs (Scheme 1). The reaction conditions were optimized to obtain relatively high yields, involving the best minimum reaction time and the amount of the catalyst. Progress of the reaction was monitored by using thin layer chromatography (TLC).
A blank reaction in the absence of catalyst was carried out. Since the solubility of potassium carbonate in organic phase is negligible, this baseline reaction should take place at the interface between the solid phase and the liquid one. The role of phase-transfer catalysts in such cases is to enhance the rate of reaction by exchanging the alkali metal cation for a soluble quaternary ammonium ion. Hence, from Table 2, it can be seen that a much higher yield is obtained by using as little as 3 mol% of catalyst. To study the effect of immobilization on the reactivity of the triphase catalyst, reactions were carried out with immobilized and homogeneous catalysts, keeping their amounts constant.
Screening of various PTCs for the N-benzylation of phthalimide.
| Entry | PT catalyst | Mole% of catalyst | MWI | |
| Time (min) | Yield (%)a | |||
| 1 | Blank | – | 20 | 4 |
| 2 | nBu4NBr (1a) | 4 | 4 | 87 |
| 3 | Et4NCl (1b) | 5 | 10 | 60 |
| 4 | [Bmim]Cl− (2a) | 10 | 20 | 18 |
| 5 | [Bmim]Br− (2b) | 10 | 20 | 22 |
| 6 | [Bmim]BF4− (2c) | 10 | 20 | 17 |
| 7 | [Epy]Br− (3) | 10 | 20 | 10 |
| 8 | [SiO2–PTB]Cl− (4a) | 3 | 3 | 96 |
| 9 | [SiO2–PTB]BF4− (4b) | 3 | 3 | 90 |
| 10 | [SiO2–Pmim]Cl− (5a) | 7 | 15 | 34 |
| 11 | [SiO2–Pmim]BF4− (5b) | 7 | 15 | 32 |
| 12 | [SiO2–Pmpy]Cl− (6) | 8 | 20 | 25 |
a Isolated yields.
Tetraalkylammonium-based reagents resulted in high yields after 4 min of irradiation because of the improved transfer of base into the organic phase, as shown in the plausible mechanism (Scheme 2). nBu4NBr gave better yield of 87% as compared to Et4NCl. A lower activity of Et4NCl may be due to a lower organophillicity because of shorter chain lengths. All imidazolium salts (2a–c), and the pyridinium salt (3) could not result in any more significant conversion than the blank reaction. The lack of catalytic activity was noted for all imidazolium compounds, regardless of their anionic character.

(Color online). Plausible mechanism.
Probable factors influencing this observation could be the accessibility of the positive charge of the nitrogen cation to form a complex with the reacting anion, the distribution of the ion pairs/complex in the organic phase and the polarizability of the positive charge of the cation (i.e., through resonance in the case of imidazolium and pyridinium). The tetravalent ammonium heterocycles (2a–c, 3, 5a–b and 6) incorporate the nitrogen cation into a conjugated ring system and the cation might be resonance stabilized enough so that it is unable to exchange for the carbonate ion, thus resulting in low conversion in all cases.
2.3 Catalytic abilities of the immobilized ILs 4a and 4b
It was observed that reaction yields were higher in the case of immobilized PTCs in comparison to their homogeneous counterparts. Because the same organic moiety is present in these catalysts, this difference in catalytic activity should originate from the nature of the inorganic moiety used as the support. Thus, silica is not just acting as a support for the catalyst, but actually it is playing an important role in the reaction. By providing a third immiscible phase, silica enhances the anion exchange between two immiscible phases over its surface.
While conventional PTCs (nBu4NBr, Et4NCl) catalysed the reaction in this work as expected; their use suffers from certain limitations such as decomposition at relatively low temperatures (< 80 °C) and non-recoverability. Identifying immobilized tetraalkylammonium-based PTCs, such as [SiO2–PTB]Cl−, [SiO2–PTB]BF4−, which have much higher decomposition temperatures (as determined by TG analysis, Fig. 3) and remarkable reusability, may expand the scope of PTC reactions and well overcome the limitations of conventional homogeneous PTCs.
Further, N-alkylation reactions of heterocyclic compounds were carried out using immobilized SL–PTCs, 4a and 4b, as shown in Table 3.
N-Alkylation of phthalimide and succinimide.
| S. No. | Substrate | Halide | Product 7a–e, 8a, 8b | Time (min) | Yield (%) |
| 1 | Benzyl chloride | 3 3 | 96a 90b | ||
| 2 | Hexyl bromide | 5 6 | 93a 94b | ||
| 3 | Allyl bromide | 4 5 | 95a 95b | ||
| 4 | 1,5-Dibromo pentane | 6 6 | 88a 86b | ||
| 5 | Geranyl bromide | 9 9 | 83a 80b | ||
| 6 | Benzyl chloride | 3 4 | 94a 95b | ||
| 7 | Hexyl bromide | 4 4 | 93a 93b |
a Time of irradiation and isolated yields for the reactions carried out with catalyst 4a.
b Time of irradiation and isolated yields for the reactions carried out with catalyst 4b.
2.4 Catalyst recyclability testing
The effectiveness of recycled immobilized IL was studied for the N-benzylation of phthalimide only in the case of 4a. Catalyst 4a was found to be sufficiently active, even after repeated use for several times (Table 4).
Recyclability study of catalyst [SiO2–PTB]Cl−, 4a.
| Run | 1 | 2 | 3 | 4 | 5 | 6 |
| Time (min) | 3 | 3 | 3 | 3 | 4 | 4 |
| Yield (%)a | 96 | 96 | 93 | 90 | 90 | 88 |
a Isolated pure products.
3 Conclusions
In conclusion, we have successfully achieved the synthesis of immobilized ILs i.e. [SiO2–PTB]Cl− and [SiO2–PTB]BF4−, which behaved extremely well as SL–PTCs for carrying out the N-alkylation of nitrogen heterocycles. These catalysts proved to be far better than conventional homogeneous SL–PTCs in terms of their stability, easy recovery, and reusability. The results of this investigation also demonstrate that cation-structure-dominated catalytic behaviour, as the catalysts with fixed cations (quaternary tetraalkylammonium salts) offered very high catalytic activity for the above-specified N-alkylation reaction, whereas the compounds with a cation that is part of a π-bonding system (imidazolium- and pyridinium-based salts) were poorly active. Although the mechanism of PT-catalyzed reactions is already well established, nowhere in the literature, to the best of our knowledge, such comparison between homogeneous and heterogeneous PTCs depending upon their cation part has been made so far.
4 Experimental
4.1 Materials and apparatus
1H NMR (300 MHz), 13C NMR (75 MHz) and 29Si NMR (57 MHz) spectra were recorded (δ/ppm) on a JEOL A1 300F spectrometer for solutions in CDCl3 with tetramethylsilane (TMS) as an internal standard. IR spectra were recorded on Nicolet iS50 FT–IR spectrometer. A focused monomode microwave oven was used (Plazmatronika RM 2001 PC, 800 W). Elemental analyses were recorded on Flash 2000 Organic Elemental Analyzer CHNS-O. The melting points were recorded on a Büchi R-535 apparatus. BET studies were recorded on a Quantachrome Instruments Nova 2200e surface area and pore size analyzer (all calculations were done using NovaWin software). TGA graphs were recorded on a TA SDTQ600 instrument. SEM images were recorded on Carlo ZEISS EVO 5 SEM instrument. Silica gel (100–200 mesh) was purchased from Merck and purified with hot piranha solution (3:1, conc. H2SO4: 30% H2O2) for 1 h, rinsed with water and dried under vacuum at 140 °C. All other chemicals (AR grade) were used without any further purification.
4.2 Preparation of the catalyst
The synthesis of 1-(3’-triethoxysilylpropyl)-tributylammonium chloride (IL-Cl−) was carried out using a reported method [33] by refluxing tri-N-butylamine and 3-chloropropyltriethoxysilane (1:1 ratio) under an atmosphere of nitrogen at 90 °C–95 °C for 24 h (Scheme 1). Further, the pretreated silica gel (3.30 g, 55 mmol) and IL-Cl− prepared above (2.13 g, 5 mmol) were refluxed in dry toluene (50 mL) for 24 h under a nitrogen atmosphere. The resultant product was filtered and washed with a 1:1 mixture of Et2O: DCM to obtain [SiO2–PTB]Cl− (4a). Anion exchange using a known procedure [34] was carried out by immersing [SiO2–PTB]Cl− in a mixture of acetone: acetonitrile (9:1) containing 1.1 equiv of NaBF4 at room temperature for 60 h to give [SiO2–PTB]BF4−(4b).
4.3 General procedure for preparation of N-alkyl phthalimide (7a–e, 8a, 8b)
A mixture of phthalimide (4.8 mmol, 0.70 g), alkyl halide (6.0 mmol), 4a or 4b (0.3 mmol, 3 mol%) and potassium carbonate (18.8 mmol, 2.6 g) was heated in a commercial microwave oven in an open Erlenmeyer flask at 60 °C–65 °C for the required time (as shown in Table 3) according to a 50:10-s heating: cooling cycle, each at an 80% power level. The completion of the reaction was monitored using TLC. After cooling to r.t., the reaction mixture was extracted with methylene chloride (2 × 25 mL). Then the extracts were dried over anhydrous Na2SO4, filtered, and the solvent was evaporated to dryness to give the crude product. Solids were purified through recrystallization in absolute EtOH, and liquids were purified over a silica gel column using hexane: EtOAc (96:4) as the eluent. The successful formation of the products was confirmed by 1H NMR, 13C NMR and FT–IR spectra.
4.4 Recycling of the immobilized IL, [SiO2–PTB]Cl−(4a)
Residual immobilized IL, 4a, was washed with methylene chloride (2 × 10 mL) to remove any organic impurity, dried under vacuum for further use. Recycling was done till minimum yields up to 88% were obtained (Table 4).
4.5 Product characterization data
4.5.1 1-(3’-Triethoxysilylpropyl)-tributylammonium chloride (IL-Cl−)
1H NMR (CDCl3): δ 0.73–0.75 (m, 2H, –CH2–Si–), 0.94–0.99 (m, 9H, 3 × CH3–(CH2)3–N–), 1.18–1.23 (m, 2H,–N–CH2–CH2–CH2–Si–), 1.33–1.41 (m, 9H, 3 × –O–CH2–CH3), 1.60–1.68 (m, 6H, 3 × CH3–CH2–(CH2)2–N–), 1.69–1.79 (m, 6H, 3 × CH3–CH2–CH2–CH2–N–), 3.26–3.40 (m, 8H, 4 × –CH2–N–), 3.49–3.53 (m, 6H, 3 × –O–CH2–CH3). 13C NMR (CDCl3): δ 10.31, 13.65, 18.22, 19.74, 20.14, 24.15, 25.13, 26.53, 47.19, 52.13, 58.40, 58.95. 29Si NMR (CDCl3): δ –46.6. IR (cm−1, neat) ν 645, 739, 925, 1045, 1100, 1269, 1382, 1459, 1690, 2874, 2959.
4.5.2 N-Hexyl phthalimide (7b)
Yellow oil. Bpt = 110 °C. 1H NMR (CDCl3, 300 MHz): δ 0.87–0.89 (m, 3H,–CH2–CH3), 1.30–1.40 (m, 6H,–(CH2)3–CH3), 1.65–1.67 (m, 2H,–N–CH2–CH2–), 3.64 (t, 2H, J = 7.2 Hz,–N–CH2–), 7.68–7.80 (m, 4H, ArH). 13C NMR (CDCl3, 75 MHz): δ 13.5, 22.1, 26.1, 28.1, 30.9, 37.4, 122.5, 131.8, 133.2, 167.5. IR (cm−1, neat) ν: 1062, 1172, 1396, 1615, 1711, 1772, 2931.
4.5.3 1,5-N,N-Diphthalimide pentane (7d)
See reference number [35a].
4.5.4 N-Geranyl phthalimide (7e)
White solid. Mpt = 57–58 °C. 1H NMR (CDCl3, 300 MHz): δ 1.48 (s, 3H,–CH3), 1.54 (s, 3H, –CH3), 1.74 (s, 3H,–CH3), 1.91–1.99 (m, 4H,–CH2–CH2–), 4.19 (d, 2H, J = 6.9 Hz,–CH2–N–), 4.94 (t, 1H, J = 6.9 Hz,–CH = C[Me]2), 5.18 (t, 1H, J = 6.9 Hz, =CH–CH2–N–), 7.59–7.62 (m, 2H, ArH), 7.73–7.75 (m, 2H, ArH). 13C NMR (CDCl3, 75 MHz): δ 16.34, 17.63, 25.69, 26.28, 35.73, 39.46, 118.00, 123.07, 123.82, 131.56, 132.36, 133.67, 140.53, 167.93. IR (cm−1, neat) ν: 946, 1085, 1171, 1395, 1653, 1711, 1770, 2929.
4.5.5 N-Benzyl succnimide (8a)
White solid. M. pt = 78–81 °C. 1H NMR (CDCl3, 300 MHz): δ 2.65 (s, 4H,–CO–[CH2]2–CO–), 4.61 (s, 2H,–N–CH2–Ar), 7.21–7.35 (m, 5H, ArH). 13C NMR (CDCl3, 75 MHz): δ 28.1, 42.3, 127.9, 128.6, 128.8, 135.2, 135.7, 176.8. IR (cm−1, neat) ν: 1082, 1166, 1399, 1654, 1703, 1774, 2928.
4.5.6 N-Hexyl succinimide (8b)
Colourless oil. Bpt = 300 ± 11 °C. [35b] 1H NMR (CDCl3, 300 MHz): δ 0.74–0.77 (m, 3H,–CH2–CH3), 1.15–1.17 (m, 6H,–(CH2)3–CH3), 1.43–1.45 (m, 2H,–N–CH2–CH2–), 2.59 (s, 4H,–CO–(CH2)2–CO–), 3.38 (t, 2H, J = 7.2 Hz,–N–CH2–). 13C NMR (CDCl3, 75 MHz): δ 13.9, 22.4, 26.4, 27.6, 28.0, 31.2, 38.8, 177.2. IR (cm−1, neat) ν: 1096, 1173, 1403, 1698, 1772, 2930.
Acknowledgements
We are grateful to the Council of Scientific and Industrial Research (CSIR) and University Grants Commission (UGC) for financial support. We also thank the CSIR, India, for the award of research fellowship.
Appendix A Appendix A. Supplementary data
Spectral data for intermediate IL-Cl–and the compounds 7a–e, 8a and 8b are available as online supplementary information at http://dx.doi.org/10.1016/j.crci.2015.02.004.


