1. Introduction
The Comoro Islands form an archipelago located in the Mozambique Channel between the east coast of Africa and the north-western coast of Madagascar. The formation of a huge submarine volcanic edifice since 2018, about 50 km offshore east of Mayotte, has focused the attention of the volcanological community with an increasing number of geophysical, petrological and geochemical studies [Bachèlery et al. 2019; Berthod et al. 2021a, b; Cesca et al. 2020; Feuillet et al. 2019; Lemoine et al. 2020; Liuzzo et al. 2021; Foix et al. 2021; Aiken et al. 2021].
The archipelago consists of four major volcanic islands from NW to SE: Grande Comore, Moheli, Anjouan, and Mayotte (Figure 1). Among them, Grande Comore hosts the frequently active basaltic shield Karthala volcano, whose last eruption occurred in 2007 [Thivet et al. 2022]. Subaerial Holocene volcanic activity related to alkaline magmas whose composition ranges from basanite to phonolite has been documented on the other islands [Bachèlery and Hemond 2016; Michon 2016; Tzevahirtzian et al. 2021; Quidelleur et al. 2022]. Based on a review of the existing morphological, geological and chronological data of The Comoro Islands, Tzevahirtzian et al. [2021] suggest that Mayotte and Moheli are the oldest islands, while Anjouan and Grande Comore are the most recent ones. The Comoro Islands are considered as part of the potentially diffuse Lwandle–Somali sub-plate boundary and possibly related to the SE extension of the East African Rift System [Michon 2016; Famin et al. 2020; Stamps et al. 2021].
Map of the Comoros archipelago located in the northern part of the Mozambique Channel between Africa and Madagascar. In (b) the map of the Petite Terre Island on the east coast of Mayotte hosting the two main areas of gas seeps on land and where all samples discussed in this paper have been collected. In (c) and (d) the Dziani Dzaha Lake and bubbling area Airport tidal flat (BAS area) respectively. Labeled spots correspond to the location of sampled bubbling pools.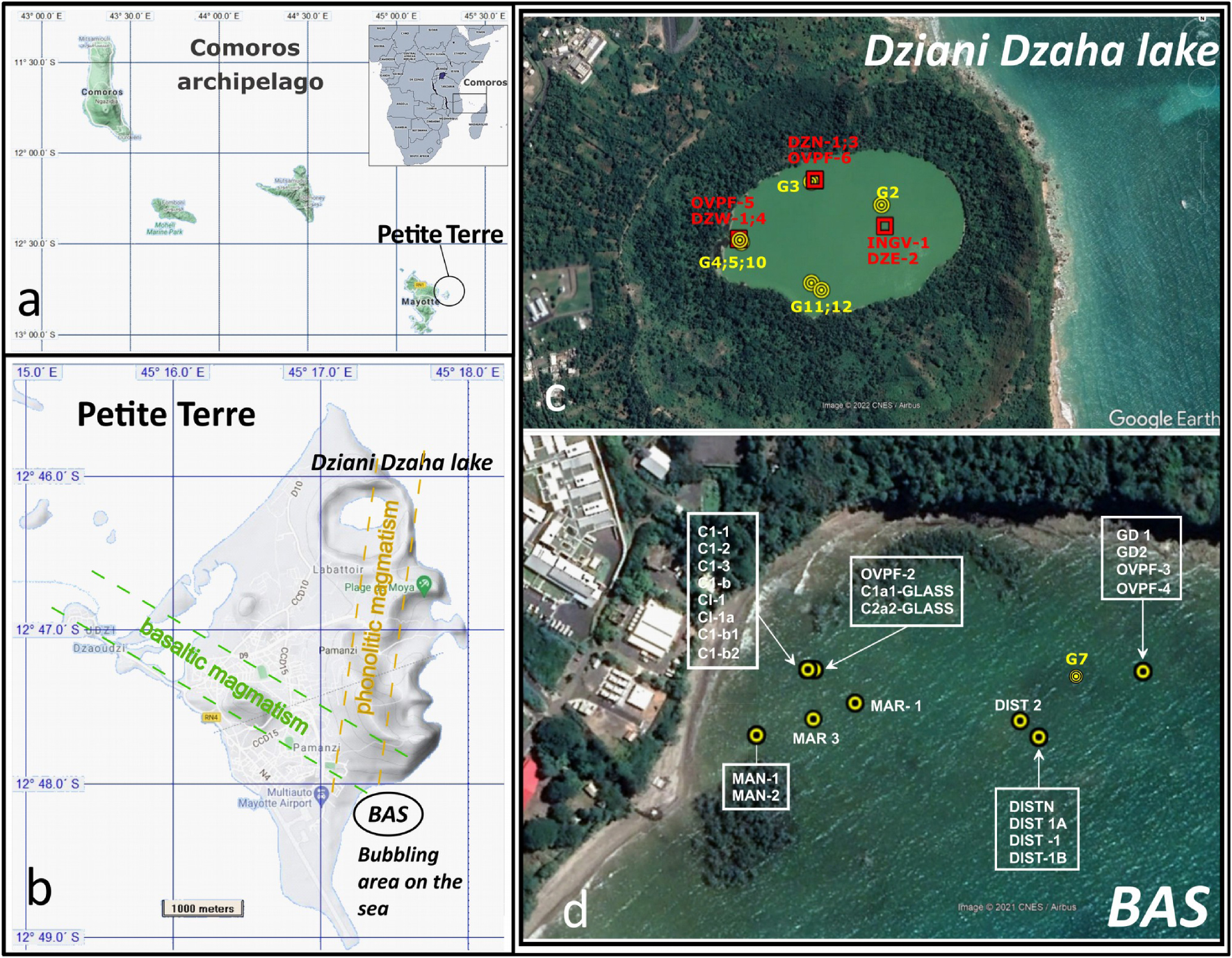
The islands of the Comoros archipelago are located within a particularly complex geodynamic region, whose volcanic and tectonic features are yet to be definitively constrained [Coffin et al. 1986; Gaina et al. 2013; Lemoine et al. 2020; Phethean 2016]. In this complex frame, only limited information exists on the signatures and sources of fluids and their potential link with the recent volcano-tectonic activity [Liuzzo et al. 2021].
At Mayotte, volcanic products becomes increasingly older moving from the eastern side (Petite Terre island), to the western main island (Grande Terre) [Nehlig et al. 2013]. The large-volume and long-lasting submarine eruption of Mayotte, the largest submarine event ever detected by monitoring networks [Berthod et al. 2021a, b; Cesca et al. 2020; Famin et al. 2020; Lemoine et al. 2020; Feuillet et al. 2021] is likely to have had a large scale impact on fluid emissions and compositions. Since 2018, about 6.5 km3 of evolved basanite lava have been emitted on the 3.5 km deep seafloor, 50 km east south-east from Mayotte from a deep source located in the upper lithospheric mantle [Bachèlery et al. 2019; Berthod et al. 2021a, b; Lemoine et al. 2020; Feuillet et al. 2021]. The new volcano grows on a N120° oriented volcanic ridge, which runs along the eastern submarine flank of Mayotte and its western subaerial tip is the small island of Petite Terre [Tzevahirtzian et al. 2021]. Since the beginning of the crisis, the region has experienced thousands of earthquakes with M > 3.5, related to deep (25–55 km depth) sources and distributed in two main swarms: a distal one (25 km away from Petite Terre) and a proximal one (5–15 km from Petite Terre) [Foix et al. 2021]. The important subsidence recorded by GNSS and Insar at Mayotte has been related to the drainage of a large magma reservoir located at mantle level [Lemoine et al. 2020], possibly located at a relatively small distance (13–22 km) from Petite Terre [Foix et al. 2021]. Mayotte Island is thus an ideal playground to study the possible influence of large mafic eruptions on the fluid emissions at regional scale.
On Petite Terre, recent volcanic activity has resulted in a set of Holocene basaltic scoria cones and phonolitic maars formed upon the existing coral reef [Zinke et al. 2001; Nehlig et al. 2013], as well as two main areas of low-temperature CO2-rich gas seeps (Figure 1). A first bubbling area occurs in the NE part of Petite Terre (Figure 1c) inside the crater lake hosted by the Dziani Dzaha phonolitic maar. Dziani Dzaha Lake is a meromictic lake with a maximum depth ranging between 4.5 m to around 18 m in a sub-central narrow depression and the bubbling emissions are heterogeneously distributed along the lake margins and in the central area, therefore upwelling through a variable water column in term of depth. Several CO2-rich and high-flux bubbling areas occur along the lake margins and a main CH4-rich spot made of myriads of small bubbles occurs close to the deepest part of the lake [Milesi et al. 2020]. A second bubbling area—first described in 1998 on the eastern tidal flat of Petite Terre—is located close to the locality named “Airport beach” [Figure 1d, BAS site; Traineau et al. 2006; Sanjuan et al. 2008]. There, tens of bubbling spots with variable flux occur at the southern base of the large “Vigie” phonolitic maar, on a muddy flat area exposed to significant tide and extended for about 250 × 300 m from the beach.
In this work, we focus on these two bubbling emission zones on Petite Terre Island, with the aim of characterizing their geochemical and isotopic signatures, by constraining their sources and assessing the potential influence of the still ongoing submarine volcano-tectonic activity.
2. Materials and methods
Since the beginning of the seismo-volcanic crisis in May 2018 at Mayotte [Feuillet et al. 2021], five campaigns including geochemical surveys were carried out, i.e. in December 2018, April 2019, September 2019, November 2020 and September 2021 (Tables 1a–1e).
Chemical composition of major and minor gaseous components and isotopic values of CO2 and CH4 from bubbling area at Petite Terre (surveys 2018–2020)
| Sampling date | Sample | Lat | Long | Site | Major (Raw) | δ13C (‰) | δD (‰) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 (vol%) | CO (ppmv) | CH4 (ppmv) | N2 (vol%) | O2 (vol%) | H2 (ppmv) | He (ppmv) | CO2 | CH4 | CH4 | |||||
| 08/11/2020 | OVPF-1 | − 12.8002 | 45.2874 | BAS | 97.5 | 0.8 | 3859.0 | 0.7 | 0.1 | 18.0 | 26.0 | − 4.4 | − 18.9 | |
| 08/11/2020 | C1a1-glass | − 12.8002 | 45.2874 | BAS | 51.8 | 1.3 | 2046.0 | 36.9 | 10.0 | 3.8 | 13.0 | |||
| 08/11/2020 | OVPF-2 | − 12.8002 | 45.2874 | BAS | 97.2 | 0.6 | 3787.0 | 0.6 | 0.2 | 17.0 | 23.0 | − 4.3 | − 19.1 | |
| 08/11/2020 | C2a2-glass | − 12.8002 | 45.2874 | BAS | 0.9 | 1.4 | 16.0 | 76.4 | 20.4 | 4.0 | 4.7 | |||
| 08/11/2020 | OVPF-3 | − 12.8002 | 45.2895 | BAS | 97.7 | 36.0 | 5291.0 | 0.5 | 0.0 | 82.0 | 20.0 | − 4.2 | − 19.2 | − 135.0 |
| 08/11/2020 | GD-1 | − 12.8002 | 45.2895 | BAS | 49.5 | 1.5 | 2495.0 | 38.7 | 10.4 | 3.7 | 9.0 | |||
| 08/11/2020 | OVPF-4 | − 12.8002 | 45.2895 | BAS | 97.7 | bdl | 5145.0 | 0.6 | 0.1 | 218.0 | 19.0 | − 4.2 | − 19.9 | − 138.0 |
| 08/11/2020 | GD-2 | − 12.8002 | 45.2895 | BAS | 73.6 | bdl | 3600.0 | 20.7 | 5.4 | 2.4 | 13.0 | |||
| 10/11/2020 | C1-b1 | − 12.8002 | 45.28736 | BAS | 97.1 | bdl | 3958.0 | 0.7 | 0.1 | 28.0 | 28.0 | − 4.6 | − 19.1 | − 125.0 |
| 10/11/2020 | C1-b2 | − 12.8002 | 45.28736 | BAS | 98.8 | bdl | 3907.0 | 0.4 | 0.1 | bdl | 26.0 | |||
| 08/09/2019 | Dist N | − 12.8006 | 45.28883 | BAS | 97.1 | 2854.0 | 0.3 | 0.0 | 25.0 | − 4.1 | − 21.6 | |||
| 08/09/2019 | Dist N | − 12.8006 | 45.28883 | BAS | 98.5 | 2982.0 | 0.4 | 0.0 | 112.0 | 26.0 | − 4.0 | − 21.8 | ||
| 08/09/2019 | C1-2 | − 12.8002 | 45.28736 | BAS | 98.7 | 2444.0 | 0.3 | 0.1 | 29.0 | − 4.7 | − 21.0 | |||
| 08/09/2019 | C1-2 | − 12.8002 | 45.28736 | BAS | 97.3 | 2384.0 | 0.5 | 0.1 | 16.0 | 28.0 | − 4.7 | − 19.2 | ||
| 13/09/2019 | Dist 2 | − 12.8005 | 45.28871 | BAS | 98.3 | 1.2 | 2914.0 | 0.3 | 0.1 | 27.0 | − 3.8 | − 22.0 | ||
| 08/09/2019 | DIST-1 | − 12.8006 | 45.28883 | BAS | 18.0 | 390000.0 | 43.1 | 15.8 | 8.0 | 3558.0 | − 22.1 | − 137.8 | ||
| 08/09/2019 | C1-2 | − 12.8002 | 45.28736 | BAS | 4.1 | 455400.0 | 48.0 | 3.0 | 11.0 | 5528.0 | − 19.6 | − 118.1 | ||
| 06/04/2019 | Dist 1-A | − 12.8006 | 45.28883 | BAS | 97.1 | 1.2 | 2442.0 | 0.5 | 0.2 | <1 | 21.0 | − 3.7 | − 24.4 | |
| 06/04/2019 | Dist 1-B | − 12.8006 | 45.28883 | BAS | 95.8 | 2.4 | 2426.0 | 1.7 | 0.5 | <1 | 20.0 | − 3.6 | ||
| 06/04/2019 | Dist 2 | − 12.8005 | 45.28871 | BAS | 97.3 | 2.1 | 2406.0 | 0.3 | 0.1 | <1 | 19.0 | − 3.5 | − 21.4 | |
| 06/04/2019 | C1-1 | − 12.8002 | 45.28736 | BAS | 97.0 | 2.1 | 2088.0 | 0.8 | 0.2 | <1 | 23.0 | − 4.2 | − 19.0 | |
| 06/04/2019 | C1-3 | − 12.8002 | 45.28736 | BAS | 97.0 | 5.0 | 2036.0 | 0.9 | 0.2 | <1 | 23.0 | − 4.3 | − 19.0 | |
| 06/04/2019 | MAR 3 | − 12.8005 | 45.28740 | BAS | 96.5 | 10.0 | 2725.0 | 1.6 | 0.4 | <1 | 27.0 | − 4.2 | − 21.0 | |
| 16/12/2018 | MAR-1 | − 12.8004 | 45.28766 | BAS | 63.3 | 1.6 | 1209.0 | 27.8 | 7.5 | 2.2 | 7.0 | |||
| 16/12/2018 | MAR-1 | − 12.8004 | 45.28766 | BAS | − 4.8 | |||||||||
| 16/12/2018 | CI-1a | − 12.8002 | 45.28736 | BAS | 28.7 | 2.1 | 416.0 | 55.0 | 15.0 | <1 | bdl | − 4.5 | − 18.7 | |
| 16/12/2018 | C1-b | − 12.8002 | 45.28736 | BAS | 97.9 | 1.7 | 2130.0 | 0.7 | 0.1 | 318.0 | 23.0 | − 4.5 | ||
| 16/12/2018 | CI-1 | − 12.8002 | 45.28736 | BAS | − 4.9 | |||||||||
| 16/12/2018 | MAN-1 | − 12.8006 | 45.28705 | BAS | 95.5 | 0.7 | 4587.0 | 2.5 | 0.2 | <1 | 107.0 | − 5.1 | − 12.4 | |
| 16/12/2018 | MAN-1 | − 12.8006 | 45.28705 | BAS | − 5.6 | |||||||||
| 16/12/2018 | MAN-2 | − 12.8006 | 45.28705 | BAS | 83.5 | 8.0 | 4621.0 | 12.0 | 2.7 | <1 | 110.0 | − 5.0 | − 11.7 | |
| 16/12/2018 | MAN-2 | − 12.8006 | 45.28705 | BAS | − 5.7 | |||||||||
| 09/11/2020 | OVPF-5 | − 12.7708 | 45.2858 | DZIANI | 93.2 | bdl | 4707.0 | 3.3 | 1.2 | 11.0 | 41.0 | − 3.1 | − 27.1 | − 145 |
| 09/11/2020 | DZW-1 | − 12.7708 | 45.2858 | DZIANI | 97.3 | 0.3 | 4825.0 | 1.1 | 0.9 | 8.0 | 38.0 | − 2.6 | − 26.9 | − 161 |
| 09/11/2020 | DZW-4 | − 12.7708 | 45.2858 | DZIANI | bdl | 8.0 | 258400.0 | 40.8 | 31.0 | 392.0 | 2322.0 | − 26.9 | − 148 | |
| 09/11/2020 | DZW4-dupl | − 12.7708 | 45.2858 | DZIANI | ||||||||||
| 09/11/2020 | OVPF-6 | − 12.7694 | 45.2877 | DZIANI | 85.8 | bdl | 37800.0 | 8.5 | 0.3 | 12.0 | 1013.0 | − 0.9 | − 24.6 | − 124 |
| 09/11/2020 | DZN-1 | − 12.7694 | 45.2877 | DZIANI | 85.8 | bdl | 39700.0 | 9.1 | 0.5 | bdl | 958.0 | |||
| 09/11/2020 | DZN-3 | − 12.7694 | 45.2877 | DZIANI | bdl | bdl | 283500.0 | 64.5 | 2.6 | 24.0 | 7823.0 | − 24.8 | − 141 | |
| 09/11/2020 | DZN-3-dupl | − 12.7694 | 45.2877 | DZIANI | ||||||||||
| 09/11/2020 | INGV-01 | − 12.7710 | 45.2903 | DZIANI | 0.2 | 6.0 | 81900.0 | 73.6 | 16.5 | 39.0 | 478.0 | − 6.3 | − 38.4 | − 184 |
| 09/11/2020 | DZE-2 | − 12.7710 | 45.2903 | DZIANI | 0.3 | 1.2 | 3897.0 | 77.6 | 20.6 | 3.8 | 9.0 | |||
Isotopic values of the noble gases from bubbling area at Petite Terre (surveys 2018–2020)
| Sampling date | Sample | Lat | Long | Site | Noble gases isotopes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R/Ra | 4He/20Ne | [4He] (ppm) | [20Ne] (ppm) | Rc/Ra | 40Ar (ppm) | 38Ar (ppm) | 36Ar (ppm) | 40Ar∗ (ppm) | 40Ar (atm) | 40Ar/36Ar (corr) | 38Ar/36Ar (corr) | |||||
| 08/11/2020 | OVPF-1 | − 12.8002 | 45.2874 | BAS | 5.86 | 199.45 | 28.25 | 0.14 | 5.87 | 110.71 | 0.06 | 0.31 | 18.86 | 91.85 | 355.50 | 0.19 |
| 08/11/2020 | C1a1-glass | − 12.8002 | 45.2874 | BAS | ||||||||||||
| 08/11/2020 | OVPF-2 | − 12.8002 | 45.2874 | BAS | 5.92 | 136.09 | 24.49 | 0.18 | 5.94 | 91.41 | 0.05 | 0.25 | 18.86 | 72.55 | 371.61 | 0.19 |
| 08/11/2020 | C2a2-glass | − 12.8002 | 45.2874 | BAS | ||||||||||||
| 08/11/2020 | OVPF-3 | − 12.8002 | 45.2895 | BAS | 5.54 | 108.44 | 19.72 | 0.18 | 5.55 | 90.47 | 0.05 | 0.26 | 13.30 | 77.17 | 345.67 | 0.19 |
| 08/11/2020 | GD-1 | − 12.8002 | 45.2895 | BAS | ||||||||||||
| 08/11/2020 | OVPF-4 | − 12.8002 | 45.2895 | BAS | 5.46 | 78.22 | 19.89 | 0.25 | 5.48 | 98.17 | 0.05 | 0.29 | 13.31 | 84.86 | 341.19 | 0.19 |
| 08/11/2020 | GD-2 | − 12.8002 | 45.2895 | BAS | ||||||||||||
| 10/11/2020 | C1-b1 | − 12.8002 | 45.28736 | BAS | 5.53 | 99.83 | 26.99 | 0.27 | 5.55 | 72.16 | 0.03 | 0.18 | 19.54 | 52.62 | 404.40 | 0.19 |
| 10/11/2020 | C1-b2 | − 12.8002 | 45.28736 | BAS | 5.55 | 237.19 | 31.63 | 0.13 | 5.56 | 87.63 | 0.04 | 0.23 | 18.54 | 69.08 | 373.95 | 0.19 |
| 08/09/2019 | Dist N | − 12.8006 | 45.28883 | BAS | 6.86 | 329.38 | 24.41 | 0.07 | 6.87 | 59.28 | 0.03 | 0.15 | 14.92 | 44.36 | 392.00 | 0.19 |
| 08/09/2019 | Dist N | − 12.8006 | 45.28883 | BAS | 6.94 | 261.61 | 25.05 | 0.10 | 6.95 | 55.17 | 0.02 | 0.13 | 16.35 | 38.82 | 418.02 | 0.19 |
| 08/09/2019 | C1-2 | − 12.8002 | 45.28736 | BAS | 7.23 | 529.11 | 27.50 | 0.05 | 7.24 | 62.81 | 0.03 | 0.14 | 20.24 | 42.56 | 434.13 | 0.19 |
| 08/09/2019 | C1-2 | − 12.8002 | 45.28736 | BAS | 7.12 | 152.41 | 26.03 | 0.17 | 7.14 | 125.30 | 0.07 | 0.36 | 19.62 | 105.68 | 348.11 | 0.19 |
| 13/09/2019 | Dist 2 | -12.8005 | 45.28871 | BAS | 7.19 | 310.71 | 25.67 | 0.08 | 7.20 | 72.00 | 0.04 | 0.19 | 15.38 | 56.63 | 374.36 | 0.19 |
| 08/09/2019 | DIST-1 | − 12.8006 | 45.28883 | BAS | 6.90 | |||||||||||
| 08/09/2019 | C1-2 | − 12.8002 | 45.28736 | BAS | 7.17 | |||||||||||
| 06/04/2019 | Dist 1-A | − 12.8006 | 45.28883 | BAS | 7.07 | 167.74 | 21.20 | 0.13 | 7.08 | 87.28 | 0.05 | 0.24 | 15.06 | 72.22 | 354.85 | 0.19 |
| 06/04/2019 | Dist 1-B | − 12.8006 | 45.28883 | BAS | 1663.88 | |||||||||||
| 06/04/2019 | Dist 2 | − 12.8005 | 45.28871 | BAS | ||||||||||||
| 06/04/2019 | C1-1 | − 12.8002 | 45.28736 | BAS | 7.52 | 219.30 | 22.48 | 0.10 | 7.53 | 105.50 | 0.06 | 0.30 | 16.16 | 89.34 | 347.41 | 0.18 |
| 06/04/2019 | C1-3 | − 12.8002 | 45.28736 | BAS | 7.26 | 138.87 | 22.47 | 0.16 | 7.27 | 141.91 | 0.08 | 0.43 | 15.90 | 126.01 | 331.79 | 0.19 |
| 06/04/2019 | MAR 3 | − 12.8005 | 45.28740 | BAS | 7.24 | 107.84 | 27.16 | 0.25 | 7.26 | 238.96 | 0.14 | 0.75 | 17.44 | 221.52 | 318.11 | 0.19 |
| 16/12/2018 | MAR-1 | − 12.8004 | 45.28766 | BAS | 3.24 | 1.07 | 8.20 | 7.65 | 4.18 | 3346.63 | 2.15 | 11.53 | - | - | 290.73 | 0.19 |
| 16/12/2018 | MAR-1 | − 12.8004 | 45.28766 | BAS | ||||||||||||
| 16/12/2018 | CI-1a | − 12.8002 | 45.28736 | BAS | ||||||||||||
| 16/12/2018 | C1-b | − 12.8002 | 45.28736 | BAS | 7.13 | 200.34 | 23.19 | 0.12 | 7.14 | 75.08 | 0.04 | 0.19 | 18.51 | 56.57 | 390.28 | 0.19 |
| 16/12/2018 | CI-1 | − 12.8002 | 45.28736 | BAS | ||||||||||||
| 16/12/2018 | MAN-1 | − 12.8006 | 45.28705 | BAS | 6.40 | 222.22 | 102.00 | 0.46 | 6.41 | 497.76 | 0.26 | 1.41 | 81.33 | 416.43 | 352.77 | 0.19 |
| 16/12/2018 | MAN-1 | − 12.8006 | 45.28705 | BAS | ||||||||||||
| 16/12/2018 | MAN-2 | − 12.8006 | 45.28705 | BAS | 6.93 | 43.59 | 113.25 | 2.60 | 6.97 | 1762.78 | 1.07 | 5.71 | 74.57 | 1688.20 | 308.79 | 0.19 |
| 16/12/2018 | MAN-2 | − 12.8006 | 45.28705 | BAS | ||||||||||||
| 09/11/2020 | OVPF-5 | − 12.7708 | 45.2858 | DZIANI | 5.82 | 54.00 | 42.51 | 0.79 | 5.85 | 405.89 | 0.24 | 1.24 | 39.52 | 366.37 | 326.89 | 0.19 |
| 09/11/2020 | DZW-1 | − 12.7708 | 45.2858 | DZIANI | 5.28 | 48.62 | 42.45 | 0.87 | 5.31 | 281.37 | 0.16 | 0.85 | 31.40 | 249.97 | 331.99 | 0.19 |
| 09/11/2020 | DZW-4 | − 12.7708 | 45.2858 | DZIANI | 6.82 | 577.25 | 2322.33 | 4.02 | 6.83 | 9088.27 | 4.72 | 25.11 | 1669.59 | 7418.68 | 362.06 | 0.19 |
| 09/11/2020 | DZW4-dupl | − 12.7708 | 45.2858 | DZIANI | 6.74 | 575.22 | 2438.49 | 4.24 | 6.74 | 9634.94 | 5.01 | 26.56 | 1786.22 | 7848.72 | 362.81 | 0.19 |
| 09/11/2020 | OVPF-6 | − 12.7694 | 45.2877 | DZIANI | 6.67 | 628.12 | 1139.54 | 1.81 | 6.67 | 2055.13 | 0.82 | 4.42 | 749.02 | 1306.11 | 463.77 | 0.19 |
| 09/11/2020 | DZN-1 | − 12.7694 | 45.2877 | DZIANI | ||||||||||||
| 09/11/2020 | DZN-3 | − 12.7694 | 45.2877 | DZIANI | 6.42 | 1994.54 | 8223.72 | 4.12 | 6.42 | 15342.65 | 6.07 | 32.85 | 5636.66 | 9705.99 | 467.01 | 0.18 |
| 09/11/2020 | DZN-3-dupl | − 12.7694 | 45.2877 | DZIANI | 6.42 | 2122.31 | 8439.59 | 3.98 | 6.42 | 15764.01 | 6.22 | 33.62 | 5828.82 | 9935.20 | 468.76 | 0.19 |
| 09/11/2020 | INGV-01 | − 12.7710 | 45.2903 | DZIANI | 6.39 | 33.31 | 620.22 | 18.62 | 6.44 | 12340.99 | 7.63 | 40.26 | 444.49 | 11896.50 | 306.44 | 0.19 |
Corrected data for air contamination from the samples listed in Table 1a (surveys 2018–2020)
| Sampling date | Sample | Lat | Long | Site | Corrected for air contamination | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| He (ppm) | H2 (ppm) | O2 % | N2 % | CH4 (ppm) | CO (ppm) | CO2 % | |||||
| 08/11/2020 | OVPF-1 | −12.8002 | 45.2874 | BAS | 26.47 | 18.34 | 0.00 | 0.24 | 3932.82 | 0.81 | 99.37 |
| 08/11/2020 | C1a1-glass | −12.8002 | 45.2874 | BAS | 20.30 | 6.85 | 0.00 | −0.46 | 3949.21 | 2.28 | 100.07 |
| 08/11/2020 | OVPF-2 | −12.8002 | 45.2874 | BAS | 23.53 | 17.42 | 0.00 | 0.04 | 3881.17 | 0.61 | 99.57 |
| 08/11/2020 | C2a2-glass | −12.8002 | 45.2874 | BAS | −31.26 | 270.09 | 0.00 | 29.85 | 1112.06 | 89.66 | 70.00 |
| 08/11/2020 | OVPF-3 | −12.8002 | 45.2895 | BAS | 20.27 | 83.16 | 0.00 | 0.33 | 5365.96 | 36.51 | 99.11 |
| 08/11/2020 | GD-1 | −12.8002 | 45.2895 | BAS | 12.90 | 6.93 | 0.00 | −0.38 | 5033.43 | 2.78 | 99.88 |
| 08/11/2020 | OVPF-4 | −12.8002 | 45.2895 | BAS | 19.26 | 221.11 | 0.00 | 0.38 | 5218.42 | 0.00 | 99.07 |
| 08/11/2020 | GD-2 | −12.8002 | 45.2895 | BAS | 15.62 | 3.03 | 0.00 | 0.81 | 4825.20 | −0.09 | 98.70 |
| 10/11/2020 | C1-b1 | −12.8002 | 45.28736 | BAS | 28.60 | 28.63 | 0.00 | 0.27 | 4047.28 | 0.00 | 99.32 |
| 10/11/2020 | C1-b2 | −12.8002 | 45.28736 | BAS | 26.17 | 0.00 | 0.00 | 0.04 | 3936.47 | 0.00 | 99.57 |
| 08/09/2019 | Dist N | −12.8006 | 45.28883 | BAS | 25.62 | 0.00 | 0.00 | 0.15 | 2925.35 | 0.00 | 99.56 |
| 08/09/2019 | Dist N | −12.8006 | 45.28883 | BAS | 26.22 | 112.99 | 0.00 | 0.29 | 3008.39 | 0.00 | 99.39 |
| 08/09/2019 | C1-2 | −12.8002 | 45.28736 | BAS | 29.26 | 0.00 | 0.00 | 0.12 | 2467.35 | 0.00 | 99.63 |
| 08/09/2019 | C1-2 | −12.8002 | 45.28736 | BAS | 28.62 | 16.36 | 0.00 | 0.22 | 2438.25 | 0.00 | 99.53 |
| 13/09/2019 | Dist 2 | −12.8005 | 45.28871 | BAS | 27.39 | 0.00 | 0.00 | −0.06 | 2958.37 | 1.22 | 99.76 |
| 08/09/2019 | DIST-1 | −12.8006 | 45.28883 | BAS | |||||||
| 08/09/2019 | C1-2 | −12.8002 | 45.28736 | BAS | |||||||
| 06/04/2019 | Dist 1-A | −12.8006 | 45.28883 | BAS | 21.57 | 0.00 | −0.12 | 2512.60 | 1.23 | 99.87 | |
| 06/04/2019 | Dist 1-B | −12.8006 | 45.28883 | BAS | 20.74 | 0.00 | −0.26 | 2531.98 | 2.50 | 100.01 | |
| 06/04/2019 | Dist 2 | −12.8005 | 45.28871 | BAS | 19.47 | 0.00 | −0.07 | 2469.39 | 2.15 | 99.82 | |
| 06/04/2019 | C1-1 | −12.8002 | 45.28736 | BAS | 23.61 | 0.00 | 0.01 | 2148.01 | 2.16 | 99.77 | |
| 06/04/2019 | C1-3 | −12.8002 | 45.28736 | BAS | 23.56 | 0.00 | 0.14 | 2090.69 | 5.13 | 99.65 | |
| 06/04/2019 | MAR 3 | −12.8005 | 45.28740 | BAS | 27.74 | 0.00 | 0.22 | 2809.43 | 10.31 | 99.49 | |
| 16/12/2018 | MAR-1 | −12.8004 | 45.28766 | BAS | 8.10 | 3.18 | 0.00 | 1910.79 | 2.39 | 100.01 | |
| 16/12/2018 | MAR-1 | −12.8004 | 45.28766 | BAS | |||||||
| 16/12/2018 | CI-1a | −12.8002 | 45.28736 | BAS | 0.00 | 1483.76 | 6.87 | 102.53 | |||
| 16/12/2018 | C1-b | −12.8002 | 45.28736 | BAS | 23.32 | 322.78 | 0.00 | 0.38 | 2162.05 | 1.72 | 99.37 |
| 16/12/2018 | CI-1 | −12.8002 | 45.28736 | BAS | |||||||
| 16/12/2018 | MAN-1 | −12.8006 | 45.28705 | BAS | 109.57 | 0.00 | 1.72 | 4699.48 | 0.71 | 97.80 | |
| 16/12/2018 | MAN-1 | −12.8006 | 45.28705 | BAS | |||||||
| 16/12/2018 | MAN-2 | −12.8006 | 45.28705 | BAS | 127.26 | 0.00 | 2.27 | 5378.54 | 9.27 | 97.18 | |
| 16/12/2018 | MAN-2 | −12.8006 | 45.28705 | BAS | |||||||
| 09/11/2020 | OVPF-5 | −12.7708 | 45.2858 | DZIANI | 44.00 | 11.86 | 0.00 | −1.28 | 5089.07 | −0.02 | 100.76 |
| 09/11/2020 | DZW-1 | −12.7708 | 45.2858 | DZIANI | 39.50 | 8.34 | 0.00 | −2.26 | 5044.09 | 0.30 | 101.75 |
| 09/11/2020 | DZW-4 | −12.7708 | 45.2858 | DZIANI | |||||||
| 09/11/2020 | DZW4-dupl | −12.7708 | 45.2858 | DZIANI | |||||||
| 09/11/2020 | OVPF-6 | −12.7694 | 45.2877 | DZIANI | 1043.35 | 12.35 | 0.00 | 7.64 | 38935.48 | 0.00 | 88.36 |
| 09/11/2020 | DZN-1 | −12.7694 | 45.2877 | DZIANI | 984.77 | −0.01 | 0.00 | 7.63 | 40814.10 | −0.01 | 88.19 |
| 09/11/2020 | DZN-3 | −12.7694 | 45.2877 | DZIANI | |||||||
| 09/11/2020 | DZN-3-dupl | −12.7694 | 45.2877 | DZIANI | |||||||
| 09/11/2020 | INGV-01 | −12.7710 | 45.2903 | DZIANI | 2301.87 | 187.42 | 0.00 | 59.24 | 397823.18 | 28.19 | 0.73 |
| 09/11/2020 | DZE-2 | −12.7710 | 45.2903 | DZIANI | 257.75 | 219.69 | 0.00 | 54.91 | 260997.01 | 63.93 | 18.94 |
Chemical composition of major and minor gaseous components from bubbling area at Petite Terre (survey 2021)
| Sampling date | Sample | Lat | Long | Site | Major (Raw) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 (vol%) | CO (ppmv) | CH4 (ppmv) | N2 (vol%) | O2 (vol%) | H2 (ppmv) | He (ppmv) | |||||
| 5/9/2021 | C1-2 | −12.800117 | 45.2873 | BAS | 97.7 | 0.8 | 5099 | 0.25 | 0.0705 | bdl | 22 |
| 5/9/2021 | C1-1 | −12.80015 | 45.28736 | BAS | 97.45 | 0.9 | 5158 | 0.68 | 0.27 | bdl | 2021 |
| 5/9/2021 | MAN | −12.80064 | 45.28705 | BAS | 96.94 | 1.5 | 6654 | 1.59 | 0.45 | bdl | 38 |
| 5/9/2021 | MAR | −12.80045 | 45.28873 | BAS | 97.73 | 0.9 | 5838 | 0.43 | 0.23 | bdl | 15 |
| 5/9/2021 | DIST | −12.799917 | 45.28612 | BAS | 97.62 | 1.4 | 6637 | 0.5 | 0.24 | bdl | 2021 |
| 7/9/2021 | DZW | −12.770833 | 45.28582 | DZIANI | 97.28 | 1.1 | 6126 | 1.2021 | 0.73 | bdl | 54 |
| 7/9/2021 | DZN | −12.769403 | 45.28771 | DZIANI | 89.71 | 1 | 24,400 | 6.5 | 1.98 | bdl | 330 |
Corrected data for air contamination from the samples listed in Table 1d (survey 2021)
| Sampling date | Sample | Lat | Long | Site | Corrected for air contamination | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| He (ppm) | H2 (ppm) | O2 % | N2 % | CH4 (ppm) | CO (ppm) | CO2 % | |||||
| 5/9/2021 | C1-2 | −12.800117 | 45.2873 | BAS | 22.384 | 0.07 | 0.254368318 | 5188.096214 | 0.814 | 99.2 | |
| 5/9/2021 | C1-1 | −12.80015 | 45.28736 | BAS | 21.34 | 0.27 | 0.691000802 | 5241.444318 | 0.9146 | 98.5 | |
| 5/9/2021 | MAN | −12.80064 | 45.28705 | BAS | 37.834 | 0.45 | 1.583044892 | 6624.89353 | 1.4934 | 97.3 | |
| 5/9/2021 | MAR | −12.80045 | 45.28873 | BAS | 15.172 | 0.23 | 0.434934814 | 5904.998706 | 0.9103 | 98.7 | |
| 5/9/2021 | DIST | −12.799917 | 45.28612 | BAS | 21.28 | 0.24 | 0.506657787 | 6725.375469 | 1.4186 | 98.6 | |
| 7/9/2021 | DZW | −12.770833 | 45.28582 | DZIANI | 58.525 | 0.79 | 1.311395671 | 6639.347007 | 1.1922 | 97.2 | |
| 7/9/2021 | DZN | −12.769403 | 45.28771 | DZIANI | 1026 | 6.16 | 20.20949473 | 75863.33407 | 3.1092 | 65.9 | |
Bubbling gases were sampled using a steel funnel (on the tidal flat) or a floating plastic funnel (on the Dziani Dzaha Lake) connected to a three-way valve equipped with a syringe and a tube connected to two-stopcock glass bottles of 250 mL (for general chemistry and C–H isotopic analysis), two-stopcock steel bottles of 100 mL (noble gases elemental and isotopic analysis), and pre-weighed evacuated bottles containing 4N NaOH absorbing alkaline solution (for noble gases elemental and isotopic analysis) following the method of Giggenbach and Goguel [1989].
All gas samples were analyzed at the laboratories of the INGV (Istituto Nazionale di Geofisica e Vulcanologia; Section of Palermo), for their chemistry and for the isotopic compositions of noble gases (He, Ne, and Ar), C of CO2, and C and H of CH4, except for the last campaign of September 2021 for which only the chemistry of the gases was analyzed and discussed in this work. Results are reported in Tables 1a–1e.
The gas chemistry was determined using a gas chromatograph (GC, Agilent 7890 equipped with PPU and MS5A columns) associated with a MicroGC module (equipped with a PPU column) and a double detector (TCD and FID) using argon as carrier gas. The analytical errors were <3%.
The C-isotope composition of CO2 (expressed as δ13C‰ versus V-PDB) was determined using a continuous-flow isotope-ratio mass spectrometer (Thermo Delta Plus XP, Finnigan), connected to a gas chromatograph (Trace GC) and an interface (Thermo GC/C III, Finnigan). The gas chromatograph and its column (length = 30 m and i.d. = 0.32 mm; Poraplot-Q) were operated at a constant temperature of 50 °C using He as carrier gas. The analytical errors were <0.1‰. The same instrument was used for measuring the δ13C and δ2H of CH4, where a combustion interface (Thermo GC III, Finnigan) was used to produce CO2 from CH4 and a gas-chromatograph/thermal-conversion interface provided online high-temperature conversion of CH4 into H2. The SDs for the δ13C and δ2H measurements of CH4 were <0.2 and <2.5‰, respectively.
The He, Ne and Ar isotopic compositions were measured at the noble-gas laboratory of the INGV-Palermo. The 3He and 4He were measured into a split flight tube mass spectrometer (GVI-Helix SFT), after purification of the sample from the major gaseous species using four GP-50 Zr–Al getters and separation from the other noble gases with a trap filled with active charcoal and submerged in liquid nitrogen (for adsorbing argon), and a cryogenic trap equipped with a cold head (Janis Research) cooled down at a temperature of 10 K by a helium compressor and temperature controller that allows regulating the T in order to adsorb and release helium and neon. The 20Ne was measured by admitting Ne into a multicollector mass spectrometer (Thermo-Helix MC plus), after purification procedure into a stainless steel ultra-high vacuum line distinct from that used for He and Ar. The 3He/4He ratio is expressed as R and normalized to Ra, the atmospheric helium isotope ratio equal to 1.39 × 10−6. Analytical uncertainty (1𝜎) varied between 0.5 and 1.1%. Further details on the purification and analytical procedures can be found in Rizzo et al. [2019].
In the following section, we discuss the 3He/4He ratio corrected for atmospheric contamination (expressed as Rc/Ra) using the measured 4He/20Ne ratio (see Appendix A, Equation (.1) for the mathematical treatment). The Ar elemental and isotopic compositions (36Ar, 38Ar, and 40Ar) were quantified in a multicollector mass spectrometer (Helix MC-GVI). The analytical uncertainty (1𝜎) for single 40Ar/36Ar measurements was <0.1%. The 40Ar was corrected for air contamination (40Ar∗) in samples showing 40Ar/36Ar > 315 to exclude the most air contaminated samples, whose 40Ar correction could lead to over corrections (i.e., underestimation of 40Ar∗), assuming that the detected 36Ar was derived from atmosphere (Appendix A, Equation (.2)). The analytical uncertainty (1𝜎) of 4He/40Ar∗ and 4He/20Ne ratios is below 0.8 and 0.7%, respectively. Typical blanks for He, Ne, and Ar were <10−11, <10−12, and <10−10 cc STP, respectively, and are at least two orders of magnitude lower than the signals obtained during sample measurements. Further details on sample purification and analyses are described by Rizzo et al. [2019] and Boudoire et al. [2020].
3. Results
Tables 1a–1e show the analyses of all samplings, also including the analyses of some data that, by their nature, were affected by air contamination during acquisition operations, or are referable to emission sources that underwent secondary processes that altered their initial elemental and/or isotopic composition. In what follows, we will attempt to provide a description of these samples as well, however they will no longer be taken into account in the interpretative considerations (and related graphs).
3.1. Gas composition
With the exception of one CH4-rich bubbling spot in the Dziani lake (DZE), all studied samples of the Mayotte gas seeps are CO2-dominated. The Mayotte noble gas show a more variable composition for the Dziani Dzaha Lake than for the Airport tidal flat (BAS; Tables 1a–1e), with 4He ranging between 8.2 and 113 ppm at BAS and between 42.5 and 1139.5 for the Dziani Dzaha Lake. The 20Ne values for the BAS zone range from 0.1 to 7.7 ppm and between 0.8 and 18.6 for the Dziani Dzaha Lake. Finally, the 40Ar is in the range 55.2–3346.6 ppm in BAS and 281.4–2055.1 for the Dziani Dzaha Lake. Some of the samples in both Dziani and BAS areas have a significant air contamination showing concentrations of N2 and O2 up to 76.4% and 20.4% respectively (Tables 1a–1e, Figure 2a).
Relative proportion of He–Ar–N2 in Mayotte bubbling gases (a); the fields of composition of gases emitted in crustal and arc settings are also shown. Data collected at Petite Terre show variable degrees of air and ASW contamination. In (b) CO2–CH4–He ternary diagram shows that the CO2-rich Mayotte gases contain a significant amount of CH4 with the highest proportion of methane recorded in the Dziani Dzaha Lake. Areas in different shades of grey distinguish gases from arc- and crustal geodynamic environments—from literature data.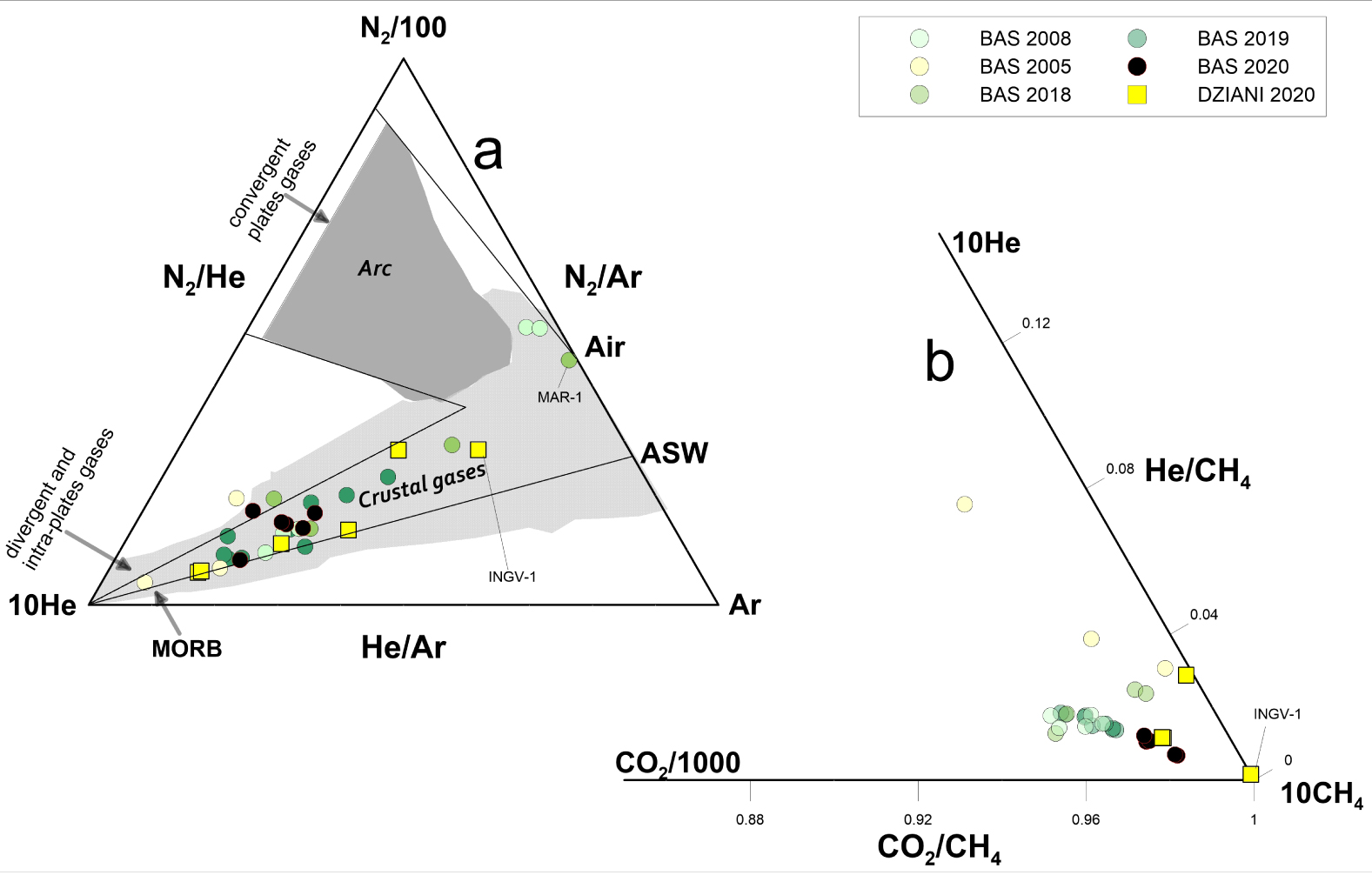
The BAS bubbling gases from the tidal flat show a CO2 dominated composition with concentrations up to 98.8% and a variable concentration in CH4 ranging between 16 and 5291 ppm with the lowest value related to an air-contaminated sample (C2a2-glass). The BAS gases show low concentrations in H2 and CO, ranging from below detection limit to 11 ppm for H2 and to 18 ppm for CO (higher values for H2 and CO in Tables 1a–1e refer to samples collected in steel samplers, which we do not consider reliable, as discussed in Section 4.3). At the Dziani Dzaha Lake, CO2 is also the dominant gas species in two of the three analyzed spots (DZW, DZN), with values up to 97.3%, while CH4 is variable between 3897 and 81,900 ppm, except in the third spot (DZE) where CH4 is the dominant gas. The H2 and CO are generally present in low concentrations.
The chemical composition of the Mayotte gases plotted in a N2, He and Ar ternary diagram (Figure 2a) follows a mixing trend between a He-rich component and an atmospheric component (air or air-saturated water—ASW). The bubbling gases from both areas at Mayotte show a variable degree of contamination by an atmospheric end-member, and its contribution is probably slightly higher for air than for ASW at least in BAS. On the whole, the He–Ar–N2 variability falls within a typical compositional range of gases emitted in intraplate or extensive tectonic settings. The two dominant mixing sources appear to be atmospheric and MORB-type mantle, and they are distinct from typical subduction-related gases, which have higher N2/Ar ratios due to the N2 excess released to the wedge by subducting sediments [e.g., Barry Hilton 2016]. With regard to the helium variability, the similarity between Dziani Dzaha Lake and BAS is interesting, as there seems to be no appreciable difference in the air-MORB mixing trends. This suggests that shallow processes do not significantly affect helium abundances at Petite Terre.
The chemical composition in relation to the plot of CO2–CH4–He (Figure 2b) highlights that low temperature gas seeps of Petite Terre have a general high CH4 concentration. A relatively higher abundance of CH4 in Dziani than in BAS tidal flat is likewise evident. Since the beginning of the seismo-volcanic crisis, the CO2-rich bubbling spots of the two sites show comparable abundances of CH4, and Dziani still hosts the only CH4-dominated bubbling area. The INGV-1 sample in Dziani is the most enriched in methane (Figure 1c); however, it should also be noted that this specific sample is also affected by air contamination.
3.2. Systematics of noble gas isotope ratios, CO2 and CH4 isotopes
Tables 1a–1e report the isotopic compositions of noble gases, CO2 and CH4 in the sampled gases from the two bubbling areas of Petite Terre. Regarding the measured 3He/4He ratios, these vary between 5.45 and 7.5 Ra in BAS, and between 5.3 and 6.8 Ra in Dziani Dzaha Lake (Figure 3). It is worth noting that the range of R/Ra values is very similar between BAS and Dziani, although it should be noted that less data is available for Dziani and that they are only related to the 2020 campaign. In both BAS and Dziani, a few samples show a clear air contamination as recorded by relatively high content of N2 and O2. It is possible that these samples underwent some issues during the sampling operations or during the storage and transport to the laboratory that fractionated the 3He/4He, (for example MAR-1 Figure 3) leading us to exclude them for further discussion. The majority of samples do not show significant air contamination as indicated by both the chemistry of gases and the noble gas systematics (Tables 1a–1e). For instance, the 4He/20Ne ratios is at least 2 orders of magnitude higher than the ratio in air (0.318) reaching 1663 in the BAS (this study) and up to 2750 in the survey carried out by BRGM in 2008 [Sanjuan et al. 2008], while is reaches up to 2122 in the Dziani Dzaha Lake.
4He/20Ne versus 3He/4He (R/Ra) ratios in Mayotte bubbling gas. Continuous and dashed black lines correspond to mixing trend between air and magmatic gases. Magmatic signature is constrained using the analyses of mineral crushing of Class et al. [2005] from La Grille and Karthala volcanoes [see also Liuzzo et al. 2021]. The two solid red and green bars correspond to the range of the R/Ra variability in La Grille and Karthala datasets [Class et al. 2005]. La Grille is the older and rarely active volcano in the north of Grande Comore (last dated eruption: 1029–1424 CE) [Bachèlery and Hemond 2016, and references therein], while Karthala is the most active volcano of Grande Comore (last eruption: 2007).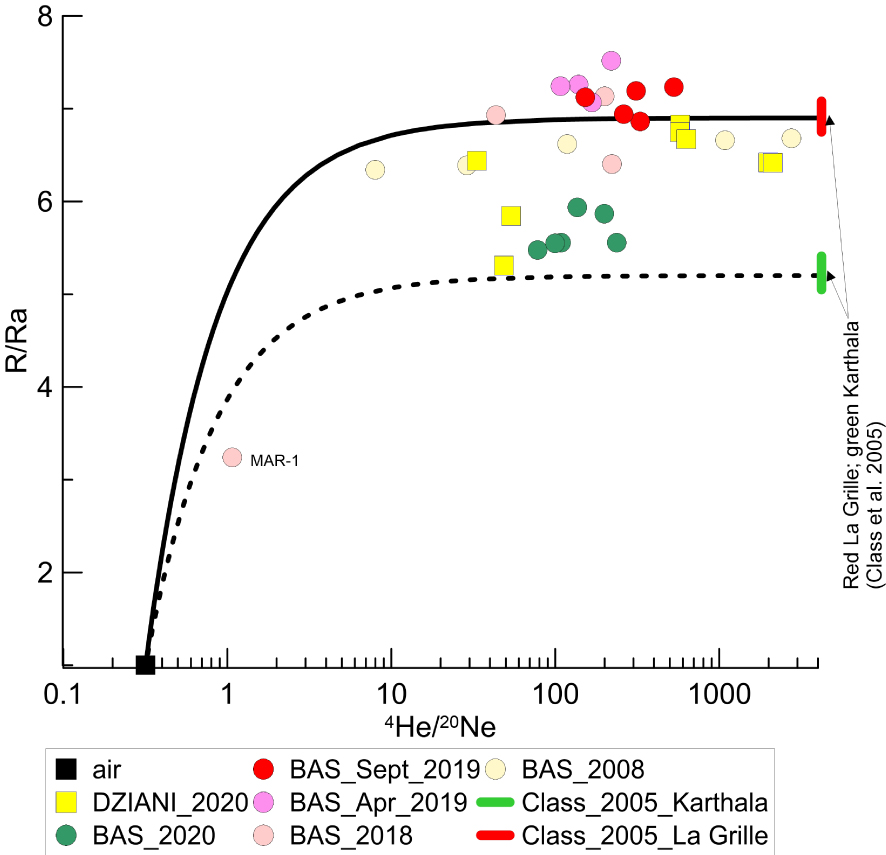
The 40Ar/36Ar values range from the atmospheric ratios up to 434 in BAS, and up to 468 in the Dziani Dzaha Lake. The few air contaminated samples have lower ratios, like MAR-1 and MAN-2 in BAS site (40Ar/36Ar = 290 and 308; and 4He/20Ne = 1.07 and 43.59, respectively), and INGV-01 in the Dziani Dzaha Lake (40Ar/36Ar = 306 and 4He/20Ne = 33.3).
With the exception of a few air-contaminated samples, the difference between R/Ra and Rc/Ra is almost negligible.
The 4He/40Ar∗ ratio of BAS gases ranges between 1.3 and 1.7, with a general overlap of values between different sampled pools and sampling periods, and they are within 1.1 and 1.5 in Dziani Dzaha Lake, irrespective of the variable CH4/CO2 ratios. These values fall within the typical range of mantle production ratio [4He/40Ar∗ = 1–5; Marty 2012] and magmatic values from other geodynamic settings [e.g., Reunion hot spot, Boudoire et al. 2018; Eger Rifţ intra-plate in Europe, Bräuer et al. 2011; Etna intra-plate, Paonita et al. 2012; Kolumbo arc volcano in Greece, Rizzo et al. 2019].
The 4He/40Ar∗ variability is commonly used to track magmatic degassing processes due to the ∼7–10 times lower solubility in silicate melts of Ar than He [e.g. Burnard 2001; Paonita et al. 2012; Barry et al. 2014; Boudoire et al. 2018]. 4He/40Ar∗ ratios at Petite Terre do not show systematic variations as a function of location or time. Liuzzo et al. [2021] suggest that this homogeneous signature reflects that all bubbling spots are related to a single degassing source and pressure, likely related to magmas stored close to the mantle-crust underplating depth (15–20 km depth), as observed in other magmatic systems [e.g., Reunion, Boudoire et al. 2018]. This depth range corresponds to the shallower part of the magmatic plumbing system below Mayotte, where evolved basanitic magma differentiate to form phonolitic melts [Berthod et al. 2021a, b; Foix et al. 2021].
The C-isotope compositions of CO2 (δ13CCO 2) at BAS gases vary from − 5.7‰ and − 3.5‰, where the most negative ratios are those measured in samples from MAN-1 and MAN-2 a sampling pool with relatively low gas flux and located close to a dense area of Mangrove trees on the airport flat. At Dziani Dzaha Lake the variability is much wider with δ13CCO2 spanning between − 0.9 and − 6.3‰ (data from this work), where the most negative value is that of the sample INGV-01 from the CH4-rich plume (DZE). Interestingly, previous data collected in 2016 from Milesi et al. [2019] show an even higher variability with 13CCO2 between − 0.3 and − 20.1, where the most negative correspond to G2 sample in Milesi et al. (2019) collected in the same area of DZE. Figure 4a shows the δ13CCO2 distribution and highlights a significant separation of the C isotope signature between the two bubbling areas, where Dziani is systematically more positive than BAS (with the exception of G2 not represented in the plot) and where the δ13CCO2 in BAS are confined to a narrow range of about 2 delta units.
Comparison of carbon isotopic variability in CO2 (left) and CH4 (right) and elemental composition of the two main bubbling areas of Mayotte–Petite Terre (BAS tidal flat and Dziani Dzaha intracrateric lake).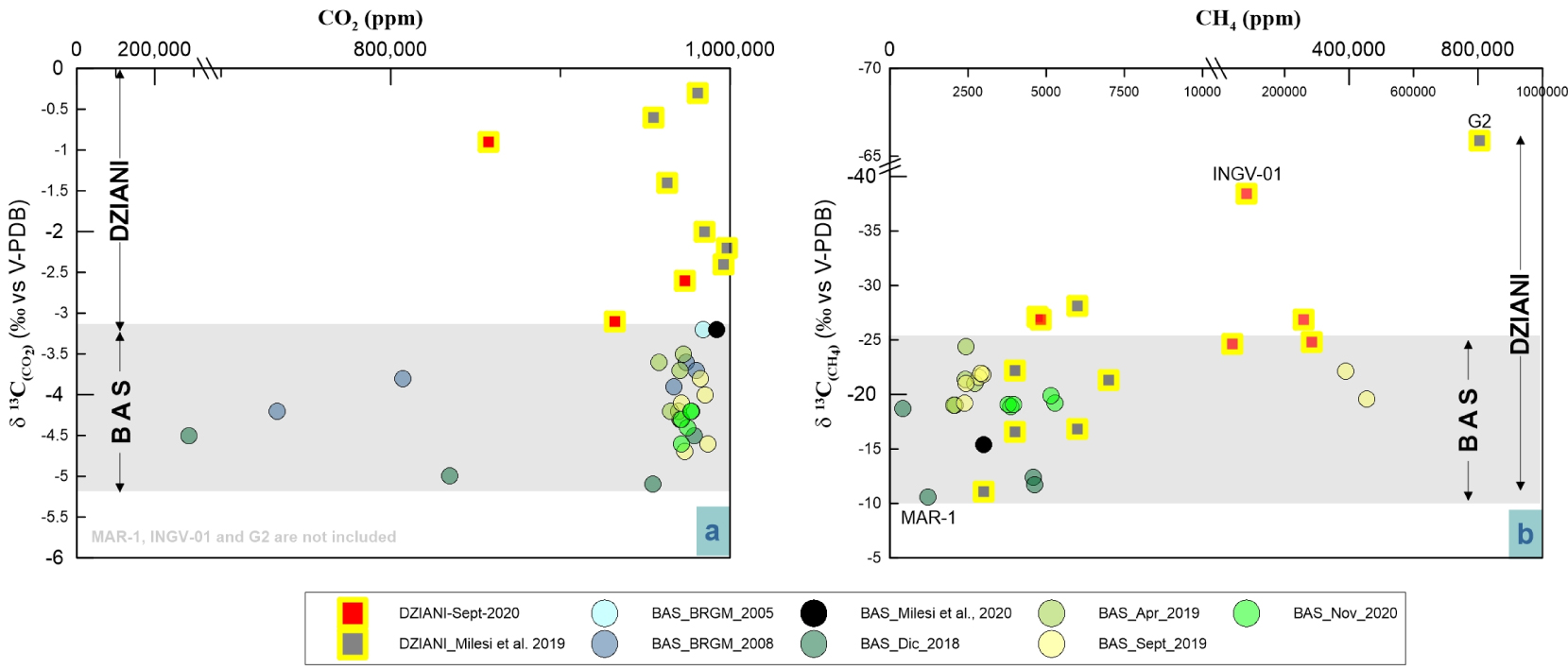
On the contrary, the distribution of the C-isotope composition of CH4 (δ13CCH 4) (Figure 4b) partly overlaps in BAS and Dziani areas, with the BAS site showing again a fairly narrow range of variability, (from − 25‰ to − 10‰). At BAS, the least negative values are found in the MAN low-flux pool located close to the mangrove area (MAN-1 and MAN-2 samples) that is considered affected by gas–water dissolution by Liuzzo et al. [2021]. The site of the Dziani Dzaha Lake shows a wider variation in δ13CCH4 fluctuating from minimum values of more than − 65‰ (DZE area) to maximum values of − 11‰ (DZW area). The different isotopic variability of carbon in a δ13CCO2 and δ13CCH4 space is evident in Figure 5, where the two markers are linearly and negatively correlated (R2 = 0.7) in the BAS site and positively correlated in the Dziani Dzaha Lake. Interestingly our new dataset on Dziani Dzaha Lake clearly shows more negative δ13CCH4 values with respect to that of Milesi et al. [2020]. Regarding hydrogen isotopic values in CH4 (δDCH 4), our samples yielded a δD of − 118.1‰ and − 137.8‰ V-SMOW, respectively in BAS and − 124‰ and − 184‰ V-SMOW in Dziani.
Carbon isotopic composition in CO2 and CH4 in the two gas seeps areas of Mayotte (BAS tidal flat and Dziani Dzaha Lake). Milesi et al. [2020] data were collected in 2016, before the beginning of the seismo-volcanic crisis in 2018.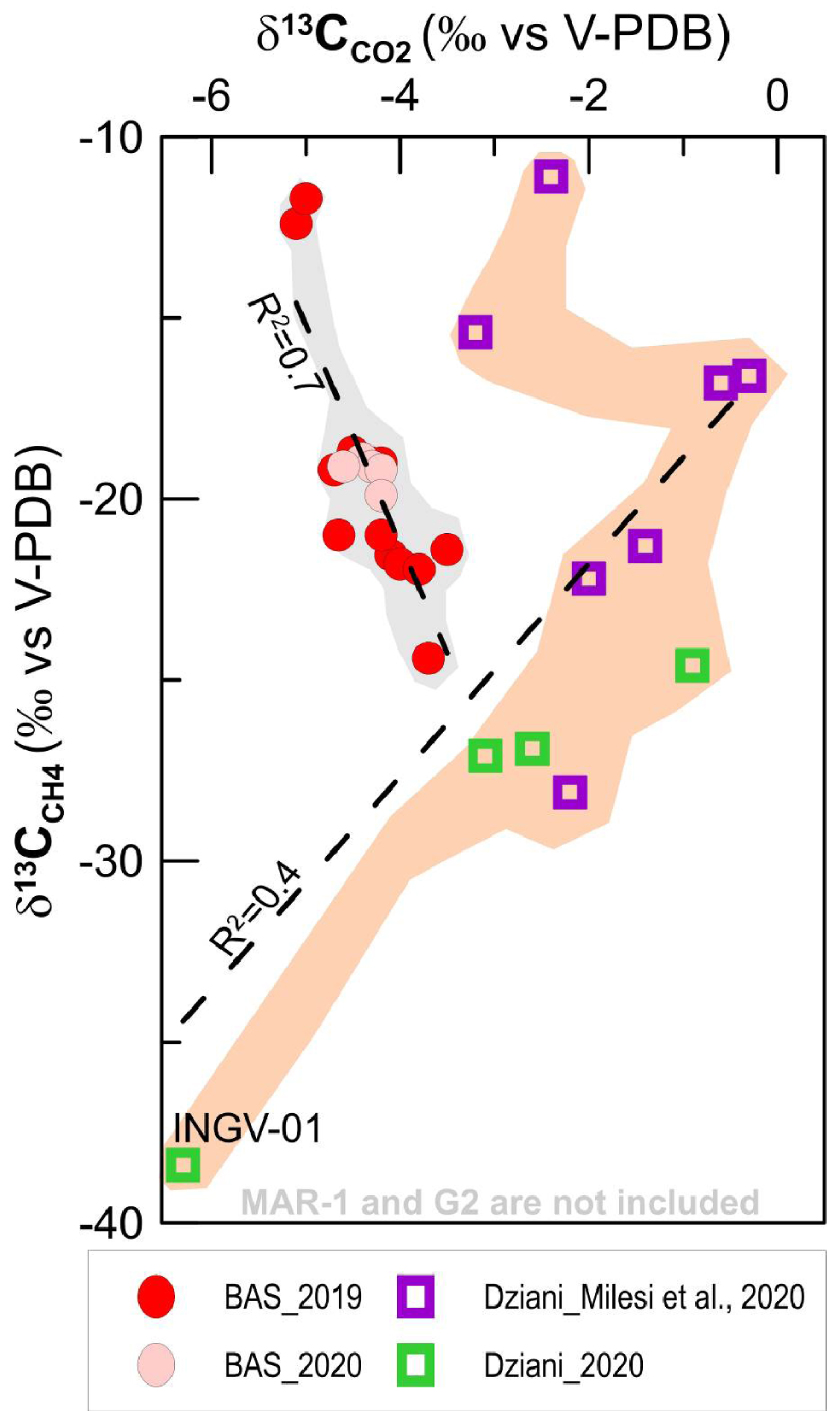
4. Discussion
4.1. CO2 origin and gas–water interaction
In order to assess the origin of CO2 rich gas emissions in Mayotte, we followed the approach used in Sano and Marty [1995] and we extended the dataset from the BAS area discussed in Liuzzo et al. [2021] with new gas sampling made in 2020 in BAS and in the Dziani Dzaha Lake. In order to discriminate the possible contributions from various sources (magmatic, organic and marine sediments), we considered the isotopic variability of carbon in CO2 versus CO2/3He ratio (Figure 6). The two mixing curves were modeled between the local mantle endmember resulting from the average values of our data (in which we only considered data that were not modified by secondary processes), data from literature [CO2/3He = 5.0 × 109 and δ13C = −4.3‰, Liuzzo et al. 2021] and an organic (δ13C = −25‰) and limestone endmember (δ13C = 0‰) from Hoefs [2015]. For both organic and limestone carbon endmembers, a value of CO2/3He = 1.0 × 1013 is assumed. Finally, in order to evaluate the secondary processes of gas–water interaction, we have considered data corrected for air only for samples having N2 < 22%, following the approach of Liuzzo et al. [2021].
In (a) δ13C of CO2 versus CO2/3He diagram of bubbling gases at Petite Terre (Mayotte). In (b) He/CO2 versus Rc/Ra. Diagram 6a shows that gases at Petite Terre are in the field of mantle-like sources with no evident organic or limestone contributions. Solid lines are mixing curves between organic, mantle and limestone endmembers. The dashed lines indicates Rayleigh fractionation (Rf) trends related to gas dissolution in water under four different pH–T conditions (see text for discussion and interpretation). Diagram 6b displays the effect of variable degree of water-gas interaction controlling CO2/He variability.
The distribution of the data for the two degassing areas at Petite Terre shows clear differences. With the exception of the MAN-1 and MAN-2 samples, the BAS area shows little variability in bulk chemistry and C isotopy and are reasonably interpretable as being related to an outgassing process from a deep magmatic source. Conversely, the gas samples from the Dziani Dzaha Lake area have a larger scattering.
Gas–water interaction effects previously highlighted for some samples from the BAS area by Liuzzo et al. [2021] likely play an even more important role in the Dziani Dzaha Lake. We modeled four possible Rayleigh fractionation curves by assuming gas dissolution in water under equilibrium conditions (Appendix A, Equation (.3)). However, whereas in the case of the BAS zone a simple dissolution process can be assumed (MAN 1-2 curve Rf-1) [Liuzzo et al. 2021], in the case of Dziani, a single dissolution step cannot reproduce the measured large scattering. Moreover, when considering the typical parameters of the Dziani Dzaha Lake (pH = 9 and temperature of 36 °C from Milesi et al. [2020]) the corresponding curve Rf-2 clearly does not fit with any Dziani Dzaha Lake data. The pH value of up to 9 in Diazni Dzaha Lake can potentially facilitate calcite precipitation. However, the precipitation of calcite, by subtracting the heavier isotope, would produce a more negative δ13C component in the gas phase (resulting in a curve that would overlap with Rf-2), but in our case the trend of the values measured in the lake is the opposite. In order to fit the Dziani Dzaha Lake gases in the range of temperatures of 32–36 °C, much more acid pH of about 5.2–5.7 are needed (Rf-3 path). If, on the other hand, fractionation in equilibrium with the lake’s pH of about 9 is considered, the temperature required to obtain a good fit on the data (Rf-4 path) should be about 102 °C. However, these high temperatures are not realistic, nor have such temperature anomalies ever been found in any of the surveys performed in the lake before or after the beginning of the seismic crisis. Modeling seems to suggest that simple fractionation does not occur in the water column of the Dziani Dzaha Lake, on the other hand if the fractionation process occurs in the aquifer and does not undergo major changes in the lake, the Rf-3 model is likely the most plausible.
Nevertheless, the variability of He/CO2 versus Rc/Ra shown in Figure 6b, evidences that a partial dissolution in water did play a role in modifying the composition of gas bubbling streaming through the Dziani Dzaha Lake, and appears to be notably extreme in the CH4-rich INGV-1 sample. In particular, this sample is collected in correspondence of the deepest portion of the lake (Figure 1c), and as already noted for sample G2 in Milesi et al. [2020], may have been significantly affected by the gas transfer and residence in the higher water column.
The current dataset does not permit to exclude that gaseous emissions from Dziani Dzaha Lake undergo gas–water interaction processes under non-equilibrium conditions and/or the occurrence of other fractionation processes than those here modeled. Among these, isotope exchange in Dziani between δ13CCO2 and δ13CTDIC could lead to the less negative isotopic values measured at the Dziani Dzaha Lake with respect to BAS, if we consider that water lake reaches DIC concentrations up to 0.2 mol/L and δ13CTDIC up to + 13‰ [Cadeau et al. 2020]. The acquisition of more data on both exsolved and dissolved gases in the future is thus needed in order to better constrain the processes controlling the variability of the carbon isotopic signature in the Dziani Dzaha Lake. All of these effects need to be evaluated and eventually filtered out in order to calculate the thermobarometric conditions of the hydrothermal system feeding the gas seeps (see next section), as it has been recognized in other studies of hydrothermal gases [Capasso et al. 2005; Gilfillan et al. 2009; Rizzo et al. 2019]. In fact, besides δ13C isotopic signature, the CO2/3He, He/CO2, CH4/CO2 ratios can also be potentially modified by oxydation/reduction reactions and/or by gas–water interaction in which CO2 dissolves preferentially with respect to the other species.
4.2. CH4 origin and δ13C versus δ2H variability
For the samples collected in the 2019–2020 campaigns, it was possible to define the isotopic signature of carbon and hydrogen in methane in both BAS and the Dziani Dzaha Lake sites and compare them with previous data of Dziani Dzaha Lake from Milesi et al. [2020]. The results are plotted in the classification diagram proposed by Schoell [1980] (Figure 7). It must be stressed that distinguishing between methanogenesis processes of biological origin and thermogenic processes at the origin of CH4 [Mazzini et al. 2011; Schoell 1980; Welhan 1988] is complicated by the possible mixing between endmembers with different isotopic signatures [Taran et al. 2010a, b], or by the occurrence of oxidation processes [e.g., Batista Cruz et al. 2019].
δ13CCH4 versus δDCH4 classification diagram (modified from Schoell [1980]). G2, G3 and G7 data acquired in 2016 from Milesi et al. [2020]. Most samples collected in the Mayotte gas seeps fall in the typical abiogenic fields, while a clear microbial signature is found in the sample collected by Milesi in 2016. The sample INGV-01 collected in the same area of G2 have an intermediate composition between biotic and abiotic end-members.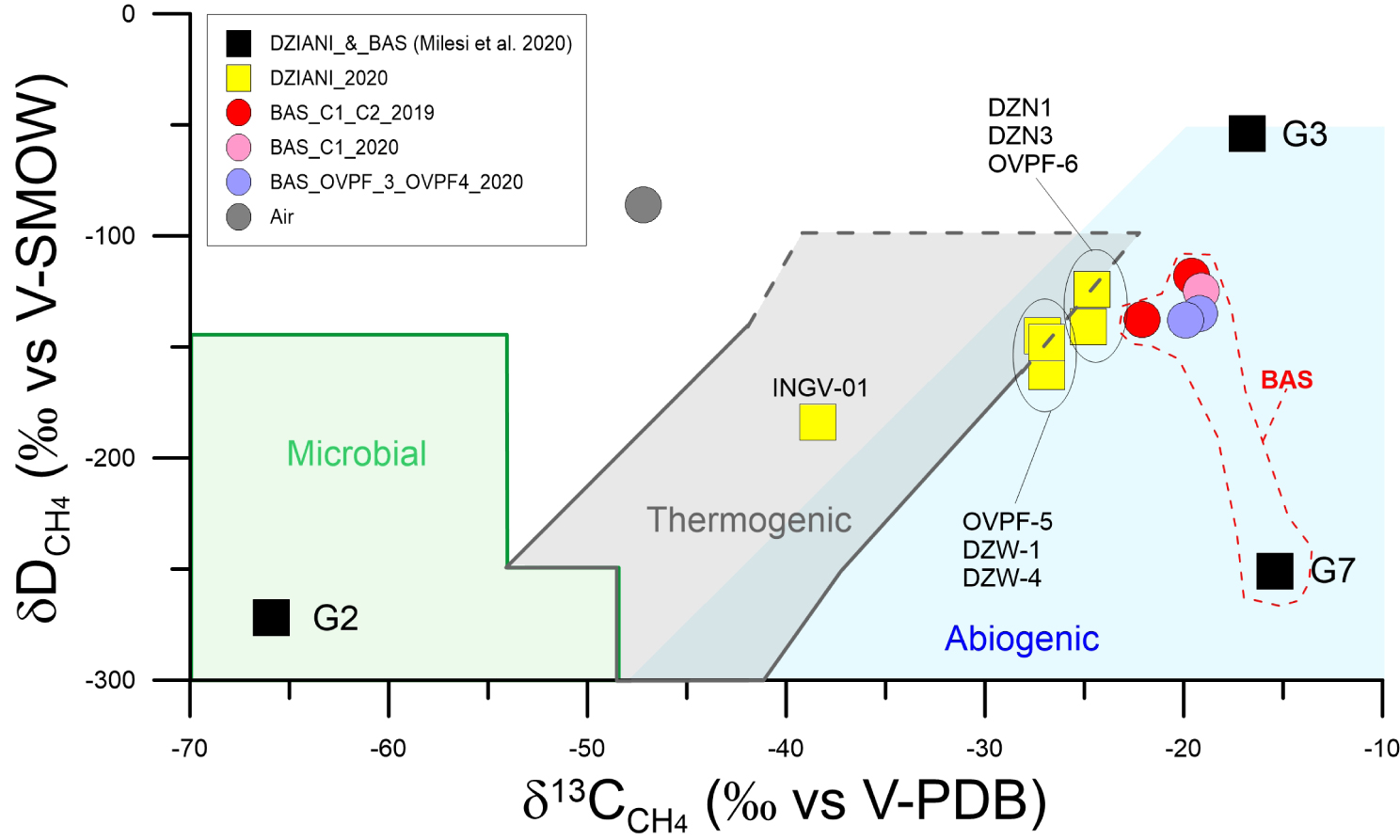
Although with some relative variability, the isotopic signature of methane in BAS falls in the field of abiogenic origin, together with sample G7, acquired in 2016 in this area by Milesi et al. [2020]. This confirms that outgassing in the BAS tidal area originates from a homogeneous and unique source, particularly if we also take into account the stable and low variability of Rc/Ra and δ13C‐CO2 values (Figures 3–5). The situation in the Dziani Dzaha Lake is more complex. The CH4-rich G2 sample collected near the deepest part of the lake corresponds to a gas that has clearly undergone a reduction process by microbial methanogenesis, as already highlighted in Milesi et al. [2020]. Our INGV-01 sample from this area (Tables 1a–1e), records a possible strong interaction in water or in the sediments (Figure 6b). The isotopic characteristics of methane for this sample can be consistent with a thermogenic origin or with mixing between abiogenic and biogenic endmembers. In the Dziani Dzaha Lake, the isotopic signature of the CH4-rich bubbling is thus distinct from that of the CO2-rich bubbling (ex. G3), which have abiogenic signature similar to the samples in BAS, but with a less negative proportion of δ2H. The remaining samples from the 2020 campaign in Dziani have a signature intermediate between abiogenic and thermogenic fields (DZN 1-2; OVPF-5; DZW 1-4). The variability of methane isotopic characteristics in distinct outgassing areas in Dziani thus appears to be much more complex than measured in BAS, which is confirmed also by the wider variability in δ13C signature in CO2 (Figures 4, 5). This again suggests that several processes may operate in the lake, including abiotic degassing of gases, microbial production and oxidation of methane, and partial dissolution of CO2 in water, all of which contribute to varying degrees to the greater chemical and isotopic variability compared to the more homogeneous BAS area.
It is therefore clear that further data are needed to better constrain the origin of methane in the Dziani Dzaha Lake, and we are aware that the debate on methane origin is open within the scientific community, in particular in order to justify its possible abiotic origin assuming a “magmatic” or “late magmatic” origin [Etiope and Sherwood Lollar 2013]. However, answering this question is not trivial, considering that the recent submarine eruption only 50 km off-shore from Petite Terre represents by far the largest known submarine eruption until now and that intense seismicity occurs at variable distance from the island [Feuillet et al. 2021; Berthod et al. 2021a, b; Foix et al. 2021]. In this context, a further clue is given by the isotopic signature of helium involved in the outgassing process at Petite Terre, which can be useful in discerning and assessing the deep origin of the gas. Therefore, we considered the isotopic variability of methane by comparing it with the percentage of mantle-related helium, following the approach indicated in Etiope and Sherwood Lollar [2013] (Figure 8). In our case, the Petite Terre gases fall within the area where magmatic CH4 inputs are clearly recognized (EPR—East Pacific Rise, Socorro, Lost City [Proskurowski et al. 2008; Taran et al. 2010a; Welhan and Craig 1983]). This leads us to conclude that input of gas of magmatic origin contributes to the general outgassing at Petite Terre, and specifically even regarding a not negligible contribution of methane, which in turn, particularly in the Dziani Dzaha Lake area, has undergone further transformation processes by microbial activity.
δ13C and δD in CH4 versus the proportion of He sourced from the mantle (%He). The Petite Terre data are compared with a larger dataset from various geodynamic origins from Etiope and Sherwood Lollar [2013], and appear consistent with environments where methane of magmatic origin is clearly recognized.
4.3. Equilibrium temperature of hydrothermal gases
The elemental composition of the gases of Petite Terre shows a general low concentration of H2 and CO in both BAS and Dziani Dzaha Lake areas. Therefore, although H2 and CO are considered useful geo-indicators for equilibrium temperature and pressure in the hydrothermal system, we did not consider these species to be suitable for thermobarometric purposes in the study of Mayotte fluids. On the contrary, the amounts of CO2 and methane (Tables 1a–1e) are high enough to have good analytical precision in both concentration and isotopic data. Therefore, we used the approach adopted by Liuzzo et al. [2021], assuming that in the hydrothermal system an equilibrium is attained between the dominant species H2O–H2–CO2–CO–CH4, in which the formation of methane is favoured by the decreasing temperature from the reaction (.4) indicated in Appendix A.4. For this system, the temperature has been calculated assuming a condition of thermal equilibrium between CH4 and CO2 by (.5) in Appendix A.4 as proposed by Giggenbach [1992]. Moreover, in order to further constrain the possible evidence of recent input of deep fluids in the Mayotte hydrothermal system, we evaluated the thermal equilibrium in combination with their isotopic signatures based on their δ13C isotopic fractionation factor between CO2 and CH4. To this aim, we combined the temperatures obtained from (.5) with the temperatures calculated using (.6) proposed by Bottinga [1969], valid for temperatures ranging between 0–700 °C (Appendix A.4), following the approach proposed in Ono et al. [1993], and recently applied in the Comoros area by Liuzzo et al. [2021], finally obtaining (.7). On this basis, the curves of thermal equilibrium were modeled assuming that both chemical and isotopic equilibrium is maintained with a fixed δ13CC02 representative of the possible range of magmatic signature; here we considered a range of δ13CC02 magmatic signatures from − 4‰ to − 8‰ when coupling (.5) and (.6).
The results are shown in Figure 9, which allows to extend the preliminary study made by Liuzzo et al. [2021], taking into account recent gas sampling in 2020 at both BAS and Dziani Dzaha Lake areas of Petite Terre. The data considered are those in which there are no obvious secondary variations and/or gas–water interactions.
δ13C in CO2 (red) and CH4 (green) versus log(XCH 4/XCO 2) for Petite Terre bubbling gases. Red and yellow areas correspond to the range of equilibrium temperature of the hydrothermal gas samples collected in the BAS area between 2018 and 2020, respectively. The blue dashed line corresponds to the CH4 and CO2 thermal equilibrium expressed in (.5) in Appendix A.4 [Giggenbach 1992]; the continuous black lines are calculated following (.7) in Appendix A.4 by assuming isotopic and chemical equilibrium between CH4 and CO2 for two possible end-members of δ13C (CO2) at −4‰ and −8‰, which in turn are indicated as horizontal black dashed lines.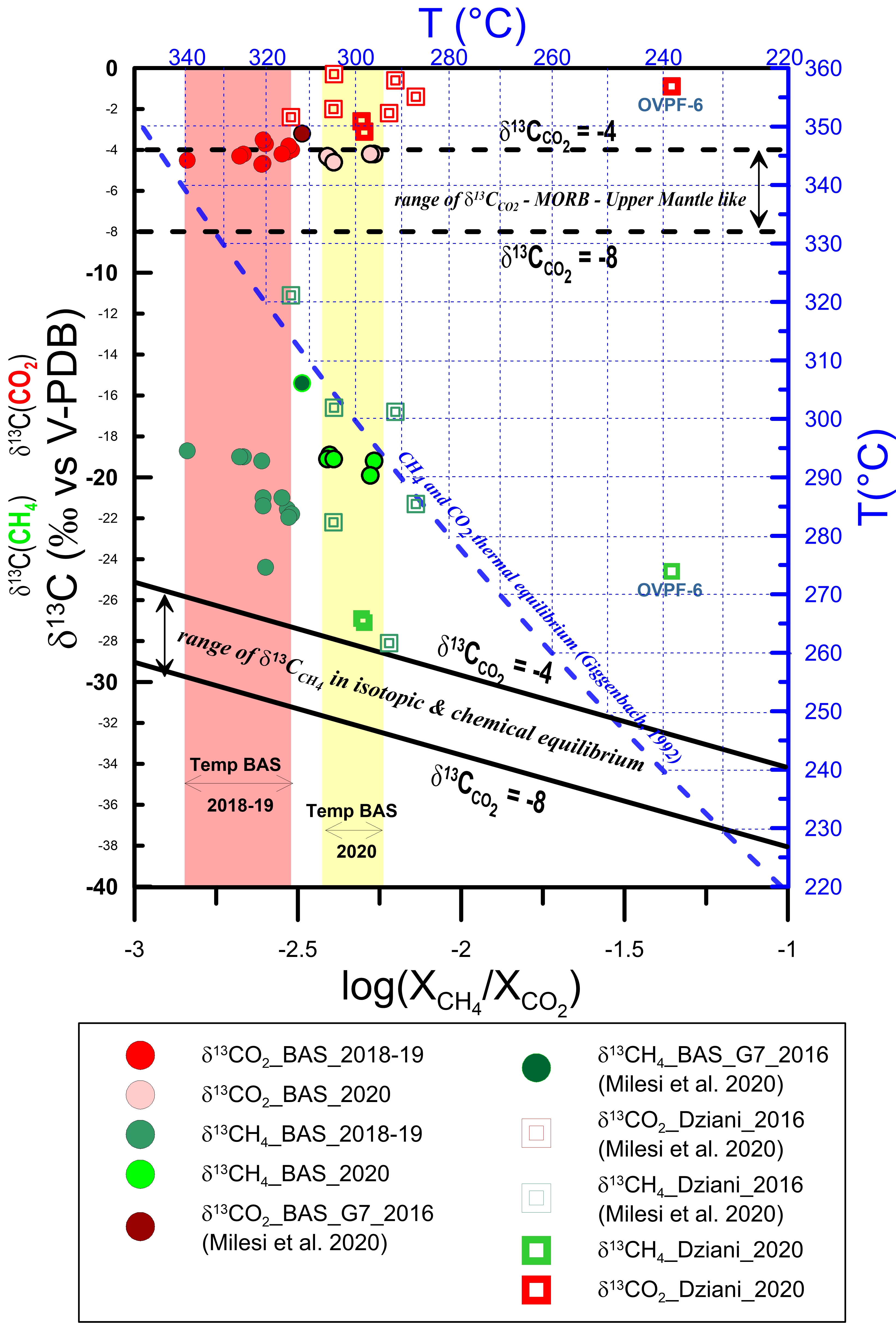
The methane-rich gases at the Dziani Dzaha Lake are certainly conditioned by the microbial activity in lacustrine waters and sediments; the range of variability of δ13C(CH4) and δD is high, and the values are generally scattered. The variability of δ13C(CO2) is less than for δ13C(CH4); however, δ13C(CO2) of gases bubbling through the Dziani Dzaha Lake show a wider range of variability than BAS. As noted in the previous sections, this leads us to conclude that outgassing in Dziani Dzaha Lake is probably affected by several physicochemical processes and are related to several sources. Their isotopic variability is related to variable degrees of mixing between organic and abiotic components [Milesi et al. 2020]. Figure 9 also shows that isotopic equilibrium is not reached between CO2 and CH4, as all sampled gases at Dziani Dzaha Lake have variable δ13C(CH4) values above the calculated equilibrium curves. Bacterial oxidation of CH4 likely controls isotopic fractionation, determining an increase in the isotopic ratio in the residual methane [Baker and Fritz 1981; Coleman et al. 1981; Horita 2001], and this process is expected to be significant in the Dziani Dzaha Lake, where an extensive microbial activity has been well documented.
Regarding the BAS area, the range of variability of δ13C(CH4) is consistent with an abiogenic source [Schoell 1980], and the data also show moderate variability in their isotopic signature. Therefore, a single degassing source produces the bubbling observed at BAS. In the 2020 samples, however, a significant shift of the methane toward heavier isotopic concentrations, as observed in Liuzzo et al. [2021] is still evident. Although a carbon isotopic fractionation of methane cannot be excluded in BAS area as in Dziani, Liuzzo et al. [2021] have shown that isotopic signature of the hydrothermal gases in the BAS area likely records a quenching effect. In this interpretation, CO2 and CH4 are considered initially in isotopic equilibrium during outgassing of the deep (mantle level) magmatic source. In this interpretation, isotopic disequilibrium is a consequence of fast ascent of the gases to shallow crustal layers, with little time for isotopic re-equilibration. Such a quenching effect is expected because of the much faster rate of re-equilibration (about 100 times) of the elemental reaction with respect to the isotopic one [Giggenbach 1982]. Our new dataset confirms that isotopic disequilibrium between CO2 and CH4 previously shown in 2018–2019 samples, is still well recorded by the 2020 samples. An important consequence of the disequilibrium is that the actual temperatures could be higher than those calculated from the equilibrium obtained from (.5), as discussed by Liuzzo et al. [2021]. This is because, having numerically simulated a thermal isotopic-chemical equilibrium between CO2, CH4 and their d δ13C, the corresponding δ13C values of methane would be in equilibrium if they intersected the corresponding curve. However, the δ13C(CH4) values would have to shift to the left to meet the equilibrium curve and thus would have even higher temperatures.
The occurrence of isotopic disequilibrium implies that the hydrothermal system at BAS may have recently received new input of deep-hot CO2 and CH4-rich gases possibly before or at the beginning of the volcanic activity in 2018.
This hypothesis is further supported by the time evolution of the equilibrium temperatures (calculated with (.5)) over years in both the BAS and Dziani areas (Figure 10). In the BAS area, a well-defined trend of cooling can be observed between 2018 and 2021. The equilibrium temperature of gases collected in the Dziani Dzaha Lake follows the same trend even if the dataset is smaller and the gases have been variably affected by secondary processes. The cooling trend recorded by equilibrium temperatures mimics that of progressive decrease of the seismic activity and magma extrusion rate over the same time period [Bachèlery et al. 2019; Berthod et al. 2021a, b; Cesca et al. 2020; Feuillet et al. 2021; Lemoine et al. 2020; REVOSIMA 2019; REVOSIMA Monthly Bulletin, December 2021]. It is therefore reasonable to assume that a deep fluid input, which was somehow related to the most intense phase of submarine eruptive activity, may have reached the outgassing areas of Petite Terre, resulting in the initial increase in equilibrium temperature and isotopic disequilibrium in the hydrothermal system, which then over time fell steadily, as the seismo-volcanic crisis declined. The existence of this link is expected as most of the seismicity and the deep sources of magma feeding the distal submarine eruption are located close to Petite Terre (5–15 km) [Foix et al. 2021] and the involved volumes of magma are huge and CO2-rich [Feuillet et al. 2021; Berthod et al. 2021a, b].
(a) Time evolution of equilibrium temperature in hydrothermal fluids of Mayotte (Petite Terre) calculated by using (.4) from Giggenbach 1992 (Appendix A.4). In box (b), overlaid in time, the declining rate of seismicity (event/day for M > 3.5 earthquakes; from REVOSIMA Monthly Bulletin—December 2021]) since the beginning of the volcano-tectonic crisis in 2018.
5. Conclusion
This study has investigated the geochemistry of the two main areas of low-T gas seeps occurring at Mayotte (Petite Terre): the Airport tidal flat (BAS) and the intracrateric Dziani Dzaha Lake. The chemical and isotopic study has permitted to constrain both the sources and the secondary processes controlling the signature of the bubbling hydrothermal fluids.
The CO2-rich bubbling gases streaming through Petite Terre Island are sourced by the shallower part of the deep magmatic plumbing located near the Moho at about 17 km b.s.l. [Foix et al. 2021; Berthod et al. 2021a, b] (Figure 11a). This inference stems from the homogeneous and low He/Ar∗ ratios of Mayotte gases and the absence of crustal signature in their chemical and isotopic signatures. The seismically very active magmatic system is located close to the island (5–15 km) and has likely provided the CO2–He rich fluids percolating through the whole deep magma plumbing system, whose deeper part is located in the mantle (around 36–50 km depth), has a basanitic composition and is expected to be very CO2 rich [Berthod et al. 2021a, b]. Petrological and geophysical data show that both the deep (mantle) and the shallow (crustal underplating) parts of the plumbing systems were drained to feed the recent submarine eruptive activity responsible for the construction of the huge volcanic edifice about 50 km offshore Petite Terre [Berthod et al. 2021a; Cesca et al. 2020; Feuillet et al. 2019; Lemoine et al. 2020]. Seismic and eruptive activity have steadily declined since the beginning of the crisis in 2018. This evolution is likely recorded by the decrease over time of equilibrium temperatures in Petite Terre hydrothermal fluids.
(a) Conceptual diagram of the deep and shallow magma and fluid plumbing system [modified from Berthod et al. 2021a, b] beneath the eastern flank of Mayotte Island. (b) Detail of the signatures and transfer of magmatic fluids reaching the two gas seep areas of Petite Terre (BAS and Dziani Dzaha Lake).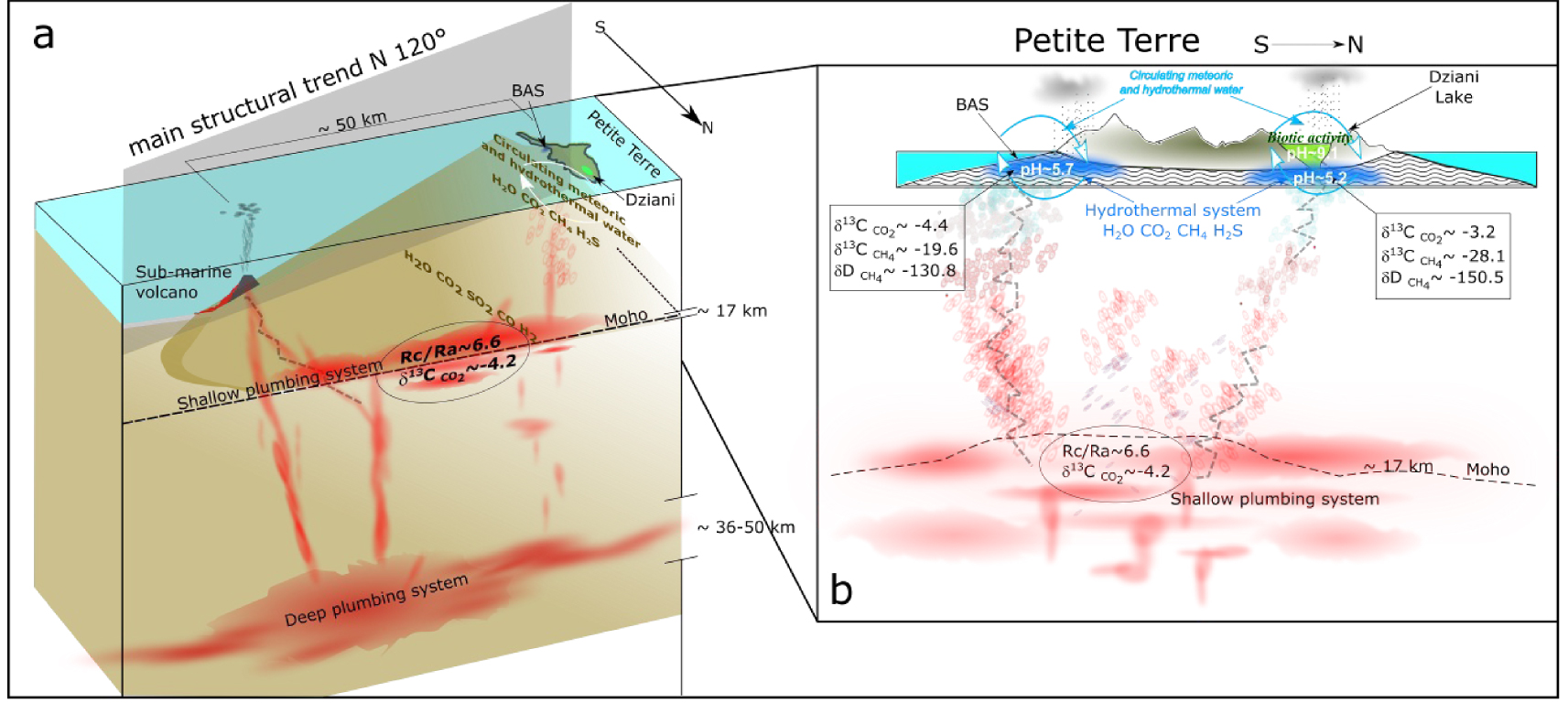
Hydrothermal gases bubbling in both the BAS and Dziani Dzaha Lake reflect primarily the signature of deep gases in terms of geochemical tracers such as R/Ra δ13C in carbon and methane (Figure 11b), however their chemical and isotopic composition is partly affected by secondary processes that produce some variability between the two areas. The Dziani Dzaha Lake hosts the only CH4-dominated bubbling area and gases streaming through the lake are variable and in some cases significantly affected by microbial activity in a meromictic lake environment. In the BAS tidal area, the influence of the microbial activity and of the gas–water interaction is certainly less significant. Secondary processes in the Dziani Dzaha Lake explain the significant difference in the methane isotopic signature. Conversely, the He content and isotopic signature is much less affected by late-stage processes and preserved the signature of the pristine deep source.
The recognition in the BAS area of deep gases related to the several stages of outgassing from the magmatic plumbing system and not affected by secondary processes, make this area the most suitable area for volcano monitoring purposes.
Conflicts of interest
Authors have no conflict of interest to declare.
Acknowledgements
This work is part of the PhD (XXXIV cycle) of Marco Liuzzo at the University of Ferrara. The work has been partially funded by INGV (GECO project Fondi Ricerca libera 2019 INGV) and by REVOSIMA Consortium (IPGP, CNRS, BRGM, IFREMER) for fieldwork and and by the Interreg “Hatari” (IPGP/OVPF) for analytical activities. The authors are very thankful to INGV, Sezione di Palermo, for allowing the access to the analytical facilities and to the BRGM Mayotte for their support during fieldwork.
A.1. Rc/Ra calculation
The 3He/4He ratio is expressed as R/Ra (being Ra the He isotope ratio of air and equal to 1.39 × 10−6) with an analytical uncertainty (1𝜎) below 0.3%. The 3He/4He ratio corrected for atmospheric contamination has been calculated using the measured 4He/20Ne ratio following Sano and Wakita [1985] and is reported in units of Rc/Ra, as follows:
| (A1) |
A.2. Argon correction
40Ar was corrected for air contamination (40Ar∗) in samples showing 40Ar/36Ar > 315 assuming that the 36Ar present derived from atmosphere, as follows:
| (A2) |
A.3. δ13CCO2 calculation in a Rayleigh fractionation under dissolution equilibrium
In order to constrain the pristine C isotopic signature of CO2 in Mayotte, we modeled a Rayleigh fractionation assuming dissolution under equilibrium conditions based on the approach used in Rizzo et al. [2019] and Liuzzo et al. [2021] for the application in the previous study for Comoros archipelago. The Clark and Fritz [1997] equation is as follows:
| (A3) |
A.4. Equilibrium temperature
Assuming that in the hydrothermal system an equilibrium is attained between the dominant species H2O–H2–CO2–CO–CH4, methane can form inorganically from the Sabatier reaction [Hulston and McCabe 1962]:
| (A4) |
| (A5) |
The equilibrium temperature for the isotopic fractionation of δ13C between CO2 and CH4 was calculated using the equation proposed by Bottinga [1969] valid for temperatures ranging between 0–700 °C:
| (A6) |
In Figure 7 the thick black lines were modeled assuming that both chemical and isotopic equilibrium is maintained with a fixed δ13CC02 corresponding to the range of magmatic signature ( − 4‰ and − 8‰; dashed black lines) by coupling the Equations (.5) and (.6):
| (A7) |




 CC-BY 4.0
CC-BY 4.0