1 Introduction
As an initial step toward a low carbon society by reducing the dependency and import of fossil resources, the Royal Thai Government has established the “Renewable and Alternative Energy Development Plan (AEDP 2012–2021)” targeting 25% share of renewable energy to Thailand's primary energy consumption [1]. According to the AEDP 2012–2021 project, the utilization of biofuels in the transportation sector will share 44% of fossil oil consumption in 2021, where the petro-diesel must blend with 10% of BDF corresponding to a large BDF market of 7.2 ML/day. However, potential consumers are becoming increasingly worried about the damage of vehicle engines and exhaust systems while they feed vehicles with petro-diesel blended with BDF more than 7%. Although the petro-diesel can be ideally blended with tens of percent of hydrogenated vegetable oil (HVO) without compromising fuel properties, HVO is currently so expensive that it can only share a small amount of diesel market in Thailand. It has pushed us to develop an innovative and cost-effective technology for upgrading of BDF in order to achieve the goal of the AEDP 2012–2021 project on blending of a great quantity of BDF with petro-diesel for use in flexible fuel vehicles without mechanical damage.
The physicochemical properties of BDF correlate closely with the structural configuration, composition and nature of individual FAME with various numbers of double bonds within the fatty acid chains [2,3]. Previous studies have demonstrated that cold flow properties increase in the order of saturated FAME < mono-unsaturated FAME < polyunsaturated FAME whereas the trend in oxidation stability is in reverse order [2–5]. Consequently, the mono-unsaturated FAME is a valuable component for high-quality BDF with good oxidation stability and cold flow properties, the cis-isomer especially [6,7]. Recent studies have attempted to tune the oxidation stability and cold flow properties of BDF derived from rapeseed oil, sunflower oil, soybean oil and animal fats by partial hydrogenation of polyunsaturated FAME into monounsaturated FAME over supported transition metal catalysts, i.e. Ni, Cu, Rh and Pd [7–10]. Partially hydrogenated fatty acid methyl esters (shortly termed H-FAME) for improvement in oxidation stability without any influence on cold flow properties can be obtained. Unfortunately, the oxidation stability of Thai palm BDF, predominately derived from refined, bleached and deodorized palm oil (RBDPO), is improved by adding large amounts of natural and artificial antioxidants [11,12]. The hydrotreating technology for the production of palm H-FAME for improvement in oxidation stability without any influence on cold flow properties is still ambiguous. Herein, the partial hydrogenation of palm BDF into palm H-FAME over supported Pd catalysts under mild reaction conditions is reported for the first time. Influences of the Pd dispersion and the acidic supports on the structural configuration, composition, oxidation stability and cold flow properties of palm H-FAME were thoroughly discussed.
2 Experimental
2.1 Synthesis of mesoporous silica materials
Siliceous SBA-15 as a supporting material was self-assembled under strongly acidic condition based on the modified procedures of Zhao et al. [13]. Typically, 4 g of Pluronic P123 triblock copolymer (Aldrich, Mn = 5800) and 2.36 g of NaCl (Wako) were completely dissolved in 160 g of 2 M HCl solution at 35 °C for 4 h. To this solution, 8.4 g of tetraethyl orthosilicate (TEOS, TCI, >96%) was rapidly added under stirring for 5 min. The gel composition was 0.017 P123: 1 TEOS: 2 NaCl: 7.9 HCl: 220 H2O. The synthesis gel sealed in a polypropylene bottle was aged at 35 °C for 24 h, followed by hydrothermal treatment at 100 °C for another 24 h. The as-made SBA-15 was obtained by filtration, washing and drying at 50 °C overnight. To remove the P123 template from the as-made sample, calcination was carried out at 500 °C in air for 12 h with a ramping rate of 1 °C min−1. As a result, the calcined SBA-15 material with surface area (SBET) of 792 m2 g−1, total pore volume (VTotal) of 1.10 cm3 g−1 and pore diameter (Φ) of 7.1 nm was obtained.
The preparation of Zr-SBA-15 was similar to that of siliceous SBA-15, except the addition of zirconyl oxychloride octahydrate (ZrOCl2·8H2O, Wako) to the synthesis solution [14,15]. It should be noticed that the self-assembly process was carried out at 35 °C for 24 h under stirring before the hydrothermal treatment [16]. The gel composition was 0.017 P123: 1 TEOS: 0.05 ZrOCl2·8H2O: 2 NaCl: 7.9 HCl: 220 H2O. For the calcined Zr-SBA-15 material with a Zr loading of 0.64 mmol g−1, the SBET, Vp and Φ values were 785 m2 g−1, 1.26 cm3 g−1 and 8.3 nm, respectively.
2.2 Preparation of supported Pd catalysts
The Pd-supported catalysts were prepared by incipient wetness impregnation. Prior to the preparation, the calcined SBA-15 and Zr-SBA-15 materials were dried at 110 °C overnight. Typically, 0.0630 g of tetraaminodichloro palladium hydrate (Pd(NH3)4Cl2·H2O, 39.88 wt% of Pd) purchased from N.E. CHEMCAT Co. Ltd, Japan was completely dissolved in ca. 6.0–6.5 g of deionized water and stepwise dropped onto 5.0 g of SBA-15 or Zr-SBA-15 under vacuum (<0.8 torr). After 1 h, the impregnated samples were dried under microwave irradiation (1050 W, 50 Hz) for 15 min, pressed into a self-supporting disk and crushed into 425–100 μm chips, followed by calcination at 300 °C for 3 h under an atmosphere of O2. As a result, the 0.5wt%Pd/SBA-15 and 0.5wt%Pd/Zr-SBA-15 catalysts were prepared.
2.3 Characterization
The powder X-ray diffraction (XRD) patterns were recorded by using a Bruker AXS D8 advance diffractrometer using Cu Kα radiation (λ = 1.5418 Å) as a light source. The N2 physisorption isotherms were measured by using a BELSORP-28A instrument at 77 K. Before the measurement, the samples were degassed at 120 °C for 200 min under high vacuum (<20 Pa). The specific surface areas were calculated by a Brunauer-Emmett-Teller (BET) method in the P/P0 range of 0.05–0.25. The pore volumes were accumulated up to a P/P0 value of 0.95. The pore size distribution (PSD) curves were evaluated using a modified Broekhoff-de Boer method with the Frenkel–Halsey–Hill equation (BdB–FHH). The Pd dispersions were determined by pulse chemisorption of carbon monoxide (CO) using an Ohkura Riken R6015 instrument. For the pretreatment, the fresh Pd catalysts were in situ reduced at 300 °C for 4 h under an atmosphere of H2, followed by cooling to 50 °C under an atmosphere of He. For the CO chemisorption, the pretreated Pd catalysts were sequentially pulsed by a 10%CO/He flow until no more CO was adsorbed, where the palladium dispersions were calculated by a CO/Pd stoichiometric ratio of 1 [17,18]. The acid capacity and strength were determined with a pulse NH3 chemisorption instrument equipped with a micro-calorimeter (CSA-450G, Tokyo Riko, Co. Ltd., Tokyo) at 50 °C. The elemental contents in solids were analyzed by inductively coupled plasma-optic emission spectroscopy (ICP-OES) using a Thermo Scientific iCAP 6300 ICO instrument. The solid samples were completely dissolved in 25 mL of conc. HF-HCl mixed solution, followed by diluting with suitable amounts of Millipore water before the ICP-OES analysis. High-resolution scanning electron microscopy (HRSEM) images were taken by using a JEOL-1100 field emission SEM instrument. The samples in the form of fine powder were coated by a thin layer of gold before the measurement.
2.4 Partial hydrogenation and oil analysis
Partial hydrogenation of palm BDF derived from RBDPO, a commercially available BDF in Thailand, over supported Pd catalysts was performed by using a stainless steel fixed-bed reactor at 100 °C and 0.3 MPa under an atmosphere of H2 (150 Ncc min−1). The supported Pd catalysts mixed with 1.4–1.8 g of quartz sand (Wako) were finely packed inside the stainless steel reactor, followed by assembling to the partial hydrogenation system (Supplemental Scheme S1). The weight hourly space velocity of palm BDF (shortly termed WHSVBDF) was maintained at 111 h−1 for the 0.5wt%Pd/SBA-15 and 0.5wt%Pd/Zr-SBA-15 catalysts and decreased to 37 h−1 for the commercial 0.5wt%Pd/γ-Al2O3 catalyst. Before partial hydrogenation, the supported Pd catalysts were in situ reduced by a H2 flow of 50 mL min−1 at 300 °C for 2 h. To start the partial hydrogenation reaction, the palm BDF was pumped into the stainless steel fixed-bed reactor at 100 °C under an atmosphere of H2 (0.3 MPa). At the specific reaction period, the upgraded palm BDF, i.e. H-FAME, was collected and quantitatively analyzed by an Agilent gas chromatograph 6890N instrument equipped with a flame ionization detector (GC-FID) and a HP-88 capillary column (100 m in length, 0.25 mm in diameter and 0.25 μm in thickness). The pour and cloud points were measured by a Tanaka mini pour/cloud point tester model MCP-102 instrument. The oxidation stability was determined in terms of the induction period using Rancimat equipment (model 743, Metrohm, Switzerland) according to the EN14112 standard. The soap forming elements, phospholipids and undesirable components of palm BDF and H-FAME were analyzed by the EN14538 method.
3 Results and discussion
3.1 Characterization of the Pd-supported catalysts
In Figs. 1 and 2, the XRD patterns and N2 adsorption–desorption isotherms show that the 0.5wt%Pd/SBA-15 and 0.5wt%Pd/Zr-SBA-15 catalysts possess at least three diffraction peaks of (100), (110) and (200) planes in the small-angle region and a classical type IV isotherm with a steep H1 hysteresis loop in a relatively high P/P0 region. It suggests that the well-ordered 2D-hexagonal P6mm channeling pores with large pore mouths and narrow pore size distribution are maintained after the impregnation. Table 1 shows that the SBET, Vp and Φ values are around 569–702 m2g−1, 0.916–1.06 cm3g−1 and 6.9–8.1 nm, respectively. By contrast, the commercial 0.5wt%Pd/γ-Al2O3 catalyst with crystalline framework gives relatively low SBET and Vp values, but slightly large Φ value distributed in a wide range.

Powder XRD patterns of (a) 0.5wt%Pd/SBA-15, (b) 0.5wt%Pd/Zr-SBA-15 and (c) commercial 0.5wt%Pd/γ-Al2O3 catalysts. The asterisked peaks represent γ-Al2O3.
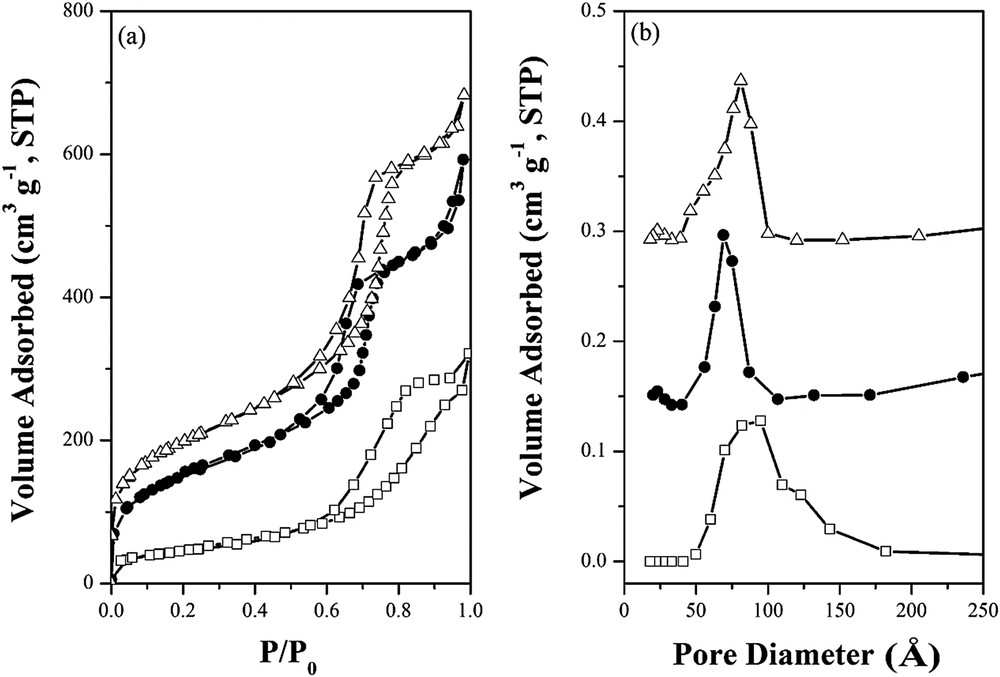
(a) N2 adsorption–desorption isotherms and (b) PSD curves of (●) 0.5wt%Pd/SBA-15, (▵) 0.5wt%Pd/Zr-SBA-15 and (□) commercial 0.5wt%Pd/γ-Al2O3 catalysts.
Structural properties and catalytic activity of the Pd-supported catalysts.
| Catalysts | Pd nanoparticlesa | SBET (m2 g−1) | Vp (cm3 g−1) | Ф (nm) | Acidic sites (mmol g-cat−1)b | ||||
| (%) | (nm) | Total | Strong | Moderate | Weak | ||||
| 0.5wt%Pd/SBA-15 | 69 | 0.71 | 569 | 0.916 | 6.9 | 1.81 | 0.220 | 0.571 | 1.02 |
| 0.5wt%Pd/Zr-SBA-15 | 86 | 0.57 | 702 | 1.06 | 8.1 | 2.38 | 0.674 | 0.746 | 0.970 |
| 0.5wt%Pd/γ-Al2O3 | 30 | 1.6 | 166 | 0.469 | 9.5 | 0.416 | 0.203 | 0.600 | 0.153 |
a Based on the pulse CO chemisorption.
b Based on the pulse NH3 chemisorption.
The Pd dispersions and sizes were determined by the pulse CO chemisorption technique [17,18]. Table 1 shows that the Pd dispersions of the 0.5wt%Pd/SBA-15 and 0.5wt%Pd/Zr-SBA-15 catalysts are around 69–86%, indicating that ultra-small Pd nanoparticles in the range of 0.57–0.71 nm have been impregnated onto the pore surfaces. By contrast, the commercial 0.5wt%Pd/Al2O3 catalyst with low surface area and pore volume has a low Pd dispersion of 30%, corresponding to relatively large Pd nanoparticles (1.6 nm).
The optical photos show that the 0.5wt%Pd/SBA-15 and 0.5wt%Pd/Zr-SBA-15 catalysts are pale brown particles (Supplemental Fig. S1). The commercial 0.5wt%Pd/Al2O3 catalyst is black spheres. The microstructure of supported Pd catalysts examined by HRSEM photos further demonstrates that the 0.5wt%Pd/SBA-15 and 0.5wt%Pd/Zr-SBA-15 catalysts are the aggregates of thin platelets with ca. 1 μm in width and ca. 300 nm in thickness (Fig. 3). It is noteworthy that the lengths of channeling pores are equal to the thicknesses of the platelets [15]. In other words, the 0.5wt%Pd/SBA-15 and 0.5wt%Pd/Zr-SBA-15 catalysts contain short channeling pores on a sub-micrometer scale, which would be a great benefit to the molecular diffusion during heterogeneous catalysis. By contrast, the commercial 0.5wt%Pd/Al2O3 catalyst shows large and irregular particles at the micrometer level.
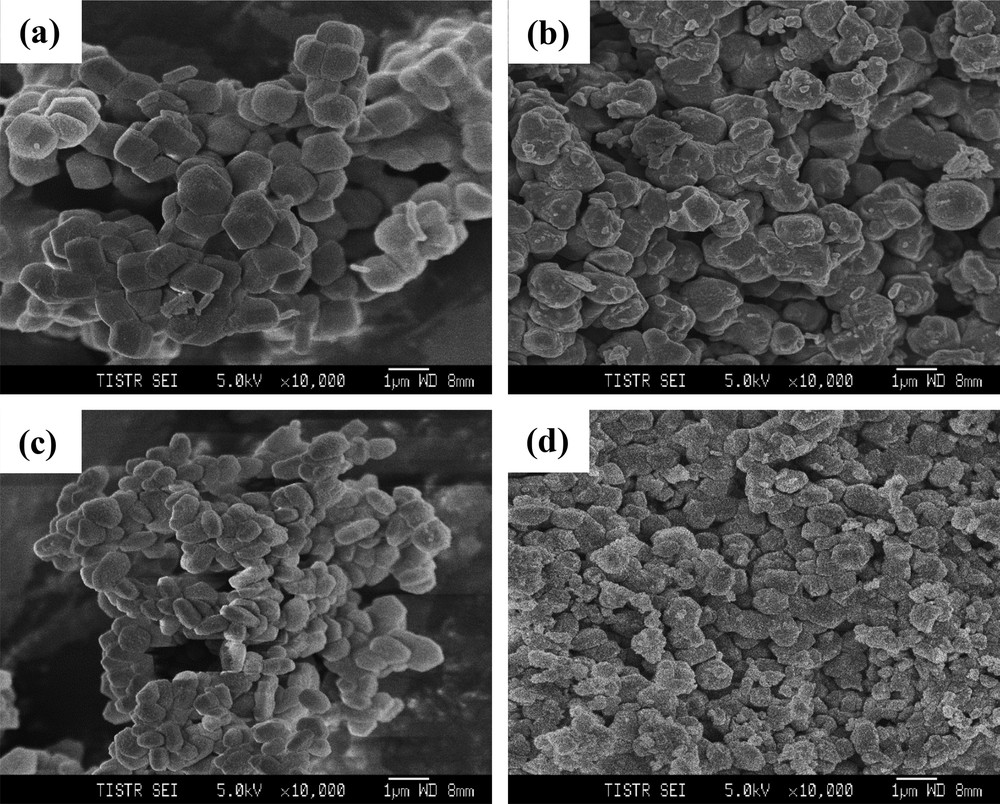
HRSEM photos of (a) SBA-15, (b) 0.5wt%Pd/SBA-15, (c) Zr-SBA-15, (d) 0.5wt%Pd/Zr-SBA-15 catalysts.
3.2 Analysis and partial hydrogenation of palm BDF
The composition and fuel properties of palm BDF kindly supplied by a commercial scale BDF manufacturer in Thailand are tabulated in Table 2. Regarding the composition, the palm BDF is composed of 0.19 wt% of tri-unsaturated FAME, 9.54 wt% of di-unsaturated FAME, 41.01 wt% of mono-unsaturated FAME and 49.07 wt% of saturated FAME. The cis- and trans-mono-unsaturated FAMEs are 40.90 and 0.11 wt%, respectively. The soap forming elements and phosphorus in palm BDF can meet the specification of the EN14214 standard. It implies that the commercially available palm BDF in Thailand can be probably used without either the formation of soap or the damage of the exhaust system to be concerned. For the undesirable components, a little amount of silica is detectable, probably leached from the glass container during storage. Regarding the fuel properties, the cloud and pour points of palm BDF used in the present study are similar to the literature reports whereas the oxidation stability is surprisingly high [3,5,11, 19,20]. It was reported that the oxidation stability of palm BDF, made by crude palm oil containing 644 ppm of vitamin E as the natural antioxidant, could meet the specification of the EN14214 standard [3,11]. However, the palm BDF derived from RBDPO possessed very poor oxidation stability in the range of 3–4 h, due to the fact that the Vitamin E and carotenes had already been removed by the refining process. To improve the oxidation stability of palm BDF to more than 6 h, i.e. the previous specification of the EN14214 standard, one promising solution is to add several tens or hundreds of ppm of natural antioxidants, such as vitamin E, and artificial antioxidants, such as butylated hydroxytoluene and tert-butyl hydroquinone. The other commercial palm BDFs with similar compositions and cold flow properties purchased from different Thai BDF manufacturers were carefully examined (Supplemental Table S1). All of them, in which the antioxidants are certainly added, have unusually high oxidation stability in the range of 13–19 h. We further doubt that the artificial antioxidants are added in RBDPO as oil feedstock for either production of palm BDF or the food chain. Since excess amounts of artificial antioxidants can be harmful to the biochemical system and living environment, the partial hydrogenation technology which provides a relatively safe, cost-effective and environmentally friendly way to improve the quality and stability of palm BDF is studied hereafter.
Partial hydrogenation of palm BDF into palm H-FAME over supported Pd catalysts.a
| palm BDF | palm H-FAME | ||||
| Partial hydrogenation | Catalysts | – | 0.5wt%Pd/SBA-15 | 0.5wt%Pd/Zr-SBA-15 | 0.5wt%Pd/γ-Al2O3 |
| WHSVBDF (h−1) | – | 111 | 111 | 37 | |
| Initial average rate (mmol h−1)b | – | 7.9 | 7.3 | 0.86 | |
| Polyunsaturated FAME conv. (%)c | – | 36.4 | 36.6 | 22.2 | |
| cis-mono-unsaturated FAME select. (%)c | – | 86.4 | 81.7 | 89.9 | |
| The composition and fuel properties of palm H-FAME | Saturated FAME (wt%) | 49.07 | 51.35 | 50.56 | 52.27 |
| Mono-unsaturated FAME (wt%) | 41.01 | 42.27 | 42.87 | 41.81 | |
| trans-Mono-unsaturated FAME (wt%) | 0.11 | 7.73 | 8.30 | 4.18 | |
| cis-Mono-unsaturated FAME (wt%) | 40.90 | 34.47 | 34.56 | 37.63 | |
| Di-unsaturated FAME (wt%) | 9.54 | 6.00 | 6.17 | 7.45 | |
| Tri-unsaturated FAME (wt%) | 0.19 | 0.07 | 0.05 | 0.10 | |
| Oxidative stability (h) | 19.4 | 27.9 | 26.0 | 23.7 | |
| Cloud point (°C) | 12.0 | 13.0 | 13.0 | 13.0 | |
| Pour point (°C) | 12.0 | 12.0 | 12.0 | 12.0 | |
| Metals or impuritiesd | Na+K (ppm) | 1.1 | 2.0 | 1.6 | 1.9 |
| Ca+Mg (ppm) | 0.046 | 0.047 | 0.031 | 0.034 | |
| P (ppm) | n.d. | n.d. | n.d. | n.d. | |
| Pd (ppm) | n.d. | n.d. | n.d. | n.d. | |
| Si (ppm) | 0.37 | 2.4 | 4.7 | 0.42 | |
| Al (ppm) | n.d. | n.d. | n.d. | 0.025 |
a Reaction conditions: 100 °C, 0.3 MPa of H2 (150 Ncc min−1), 0.37 g min−1 of Palm BDF, 0.2 or 0.6 g of catalysts.
b Determined at the first 0.5–2 h of TOS.
c Determined at 28 h of TOS.
d The ICP-OES data were obtained by the procedures of the prEN 14538 method.
The partial hydrogenation of palm BDF into palm H-FAME over supported Pd catalysts was studied by a continuous fixed-bed reactor under the condition of 100 °C, 0.3 MPa and 150 Ncc mL−1 H2. The results are summarized in Table 2. The initial average rate was measured at a time-on-stream (TOS) of 0.5–2 h, where the deactivation of supported Pd catalysts in partial hydrogenation was insignificant (Supplemental Fig. S2). The polyunsaturated FAME conversion and cis-mono-unsaturated FAME selectivity were measured under steady state condition (TOS = 28 h). Table 2 shows that the initial average rate and polyunsaturated FAME conversion are decreased in the following manner: 0.5wt%Pd/SBA-15–0.5wt%Pd/Zr-SBA-15 > 0.5wt%Pd/γ-Al2O3. The 0.5wt%Pd/SBA-15 and 0.5wt%Pd/Zr-SBA-15 catalysts with short channeling pores are efficient in H-FAME synthesis, due to the fact that the ultra-small Pd nanoparticles with electron-deficient surfaces as the catalytically active sites for partial hydrogenation are highly accessible. The cis-mono-unsaturated FAME selectivity over the supported Pd catalysts is found to be significantly influenced by the pore structure and acidity. When the polyunsaturated FAME conversion is around 36%, the 0.5wt%Pd/SBA-15 catalyst shows high cis-monounsaturated FAME selectivity in comparison to the 0.5wt%Pd/Zr-SBA-15 catalyst (Table 2). For a wide variety of polyunsaturated FAME conversion, the 0.5wt%Pd/SBA-15 catalyst is also superior to the 0.5wt%Pd/γ-Al2O3 catalyst in cis-mono-unsaturated FAME selectivity (Fig. 4). When the partial hydrogenation of palm BDF is catalyzed by the supported Pd catalysts, the unsaturated FAMEs are diffused into the pores, adsorbed on the metallic Pd surfaces and then stepwise hydrogenated by the H atoms from dissociation of the H2 molecules on the metallic Pd surfaces. After hydrogenation, the partially hydrogenated intermediates are desorbed from the metallic Pd surfaces and then the catalytically active sites are ready for the next catalytic cycle [21]. However, the partially hydrogenated intermediates with electron-rich double bonds, such as cis-mono-unsaturated FAME, may tightly adsorb on the acidic supports, followed by isomerization and hydrogenation in the presence of spillover H atoms. In this case, the trans-mono-unsaturated FAME as an unwanted component for either fuel or food application is formed [10]. Among the supported Pd catalysts, the higher cis-mono-unsaturated FAME selectivity of the 0.5wt%Pd/SBA-15 catalyst is associated with the weakly acidic nature of the siliceous SBA-15 framework, which is inactive in adsorption of partially hydrogenated intermediates. In addition, the expelling of partially hydrogenated intermediates from the 0.5wt%Pd/SBA-15 catalyst with short channeling pores is faster than that of the commercial 0.5wt%Pd/γ-Al2O3 catalyst with disordered pores. The isomerization of cis- to trans-monounsaturated FAME over the 0.5wt%Pd/SBA-15 catalyst should be low. As a result, the 0.5wt%Pd/SBA-15 catalyst with higher Pd dispersion, weaker acidic framework and better molecular diffusion gives the highest activity and cis-mono-unsaturated FAME selectivity in H-FAME synthesis.
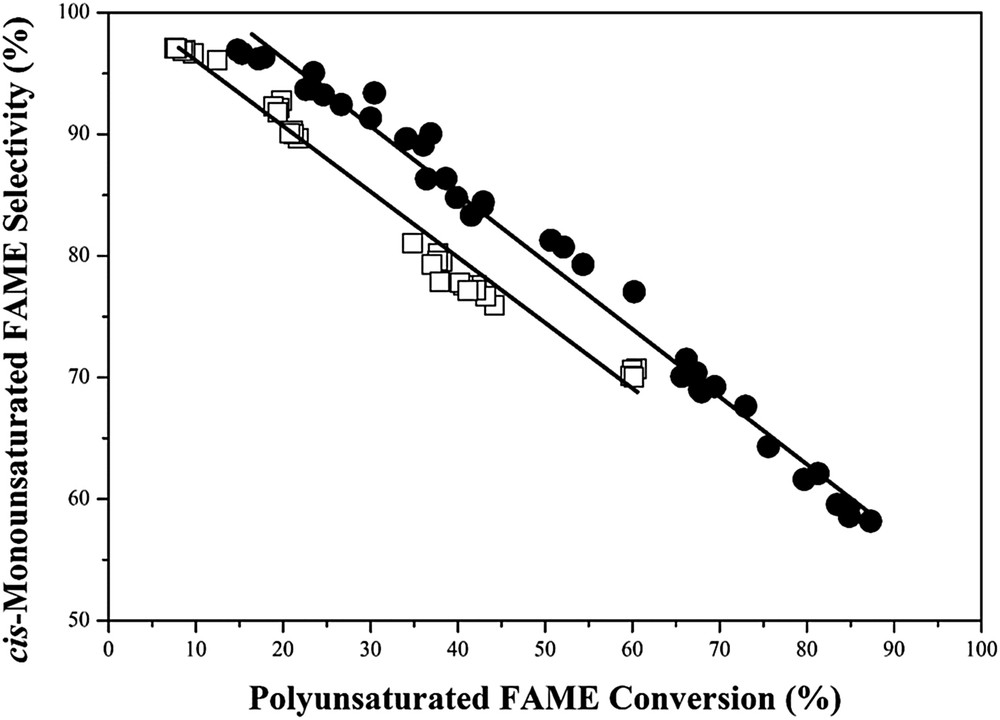
The cis-mono-unsaturated FAME selectivity as a function of polyunsaturated FAME conversion over (●) 0.5wt%Pd/SBA-15 and (□) commercial 0.5wt%Pd/γ-Al2O3 catalysts.
It is noticed that the supported Pd catalysts are slightly deactivated during the production of H-FAME, especially if the WHSV value is reduced to 37 h−1 (Supplemental Fig. S2). Since the ICP study shows that all the H-FAME products have no Pd species, the slight deactivation of supported Pd catalysts caused by the loss of Pd species can be ruled out (Table 2). After the comprehensive study, the slight deactivation of supported Pd catalysts is mainly caused by adsorption of impurities, such as steryl glucoside derivatives or additives, such as antioxidants, on the Pd surfaces rather than the sintering or loss of Pd species. We have previously suggested that those impurities must be removed by the commercial adsorbents, such as aluminosilicate minerals or active clays, before the partial hydrogenation [22]. It is specially noticed that all of the palm oils and BDF in Thailand contain large amounts of antioxidants at a level of several hundred parts per million in order to enhance their oxidation stability. However, the partial hydrogenation of palm BDF is an alternative way to produce high-quality biodiesel fuel, where the addition of antioxidants is unnecessary. As to other undesirable species, little amounts of silica and alumina are found in the H-FAME product. It implies that the supporting materials of these Pd catalysts, such as mesoporous silica and commercial alumina, are slightly leached out under mild hydrogenation conditions, probably associated with their amorphous nature. This problem can be solved by coating a light carbon film on the surface of the catalysts based on our previous study [23].
For the fuel properties, the oxidation stability can be generally improved by conversion of polyunsaturated FAME to saturated counterparts whereas the cold flow properties give an opposite trend. In Table 2, the oxidation stability of palm H-FAME over the 0.5wt%Pd/SBA-15 catalyst is significantly improved from 19.4 to 27.9 h without compromising cold flow properties when the polyunsaturated FAME content is reduced from 9.5 to 6.0 wt% by partial hydrogenation. Fig. 5 shows an exponential growth of the oxidation stability of palm H-FAME as the polyunsaturated FAME conversion is increased. By contrast, a failure in the cold flow properties of palm H-FAME is found if the polyunsaturated FAME conversion is higher than 65%. Fig. 6 demonstrates that the decrease in the cold flow property of palm H-FAME by increasing the polyunsaturated FAME conversion is attributed to the formation of saturated FAME and trans-mono-unsaturated FAME.
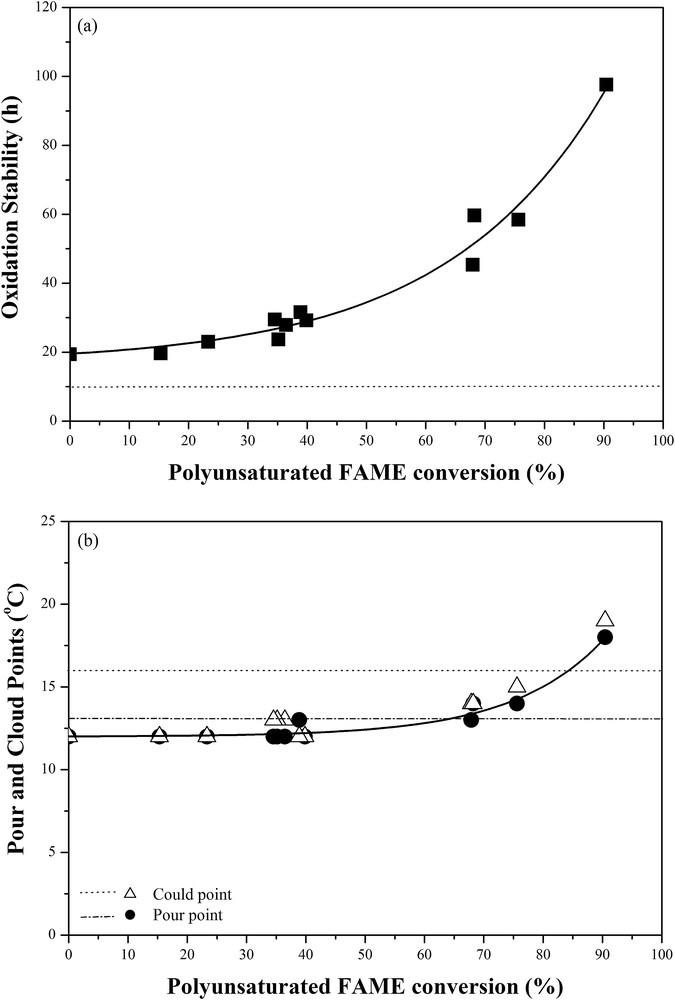
The oxidation stability and cold-flow property of palm H-FAME as a function of the polyunsaturated FAME conversion over the 0.5wt%Pd/SBA-15 catalyst. The dotted and dashed lines represent the specifications of the EAS-ERIA EEBS:2008 standard.

The composition of palm H-FAME as a function of the polyunsaturated FAME conversion over the 0.5wt%Pd/SBA-15 catalyst, where (□) is polyunsaturated FAME, (▵) is cis-mono-unsaturated FAME, (●) is trans-mono-unsaturated FAME and (▾) is saturated FAME.
In the East Asia Summit (EAS) region, most of the countries have formulated their national BDF standards by modifications of the US ASTM D6751 and EU EN14214 standards which use soybean and rapeseed oils as feedstocks [24]. However, these national BDF standards are slightly different from each other, making the BDF trade and utilization a difficult task [25]. The Economic Research Institute for ASEAN and East Asia (ERIA) has recently harmonized a regional benchmark EAS-ERIA BDF standard, in which the oxidation stability, cloud point and pour point of B100 are set at a minimum of 10 h, a maximum of 16 °C and a maximum of 13 °C, respectively (Supplemental Table S2). Although the oxidation stability of palm BDF in the Thai market can nearly meet with the specification of the EAS-ERIA standard, the unusually high oxidation stability is associated with large amounts of artificial antioxidants being originally added into RBDPO as oil feedstock for food or palm BDF production. To ensure food safety and fuel quality, this study has demonstrated that partial hydrogenation is an alternative way for upgrading of palm BDF into palm H-FAME with excellent oxidation stability and suitable cold flow properties. It also reported that H-FAME with lower polyunsaturated FAME content gives better fuel performance and lower NOx emission as compared with BDF [6]. Moreover, H-FAME is cheaper than HVO due to lower consumption of hydrogen and cost-effective equipment for the partial hydrogenation process [26]. It is therefore expected that high-quality and cost-effective H-FAME can be largely blended with petro-diesel to reach the goal of the AEDP 2012-21 project.
4 Conclusions
The hydrotreating technology for upgrading of palm BDF over supported Pd catalysts into palm H-FAME has been examined by using a continuous fixed-bed reactor under mild reaction conditions. The 0.5wt%Pd/SBA-15 catalyst with high Pd dispersion, weakly acidic framework and fast molecular diffusion gave the highest activity and cis-mono-unsaturated FAME selectivity in H-FAME synthesis. The high-quality H-FAME with excellent oxidation stability and suitable cold flow properties is expected to be a potential source for higher blend diesel without compromising the fuel properties and increasing the production cost, which can reach the goal of the AEDP 2012–21 project.
Acknowledgments
Financial support from JST-JICA's SATREPS project is gratefully acknowledged. Chen would like to thank Dr. A. Endo and Dr. A. Kawai of Research Institute for Chemical Process Technology, AIST, for XRD measurements, and Mr. Kaitsuka and Dr. M. Oguma of Research Institute for Energy Conversion, AIST, for the ICP-OES analysis.


