1 Introduction
Titanium phosphates are attractive solids for many applications such as ion conductivity, ion exchange and non-linear optics [1–5]. However, if most research has been focused on the discovery of new metallophosphates based on Zn, Ni, Co, Al, Ga, Fe, Zr, V, etc. [6] open framework titanium porous solids characterised to date are still scarce with most titanophosphate solids reported being dense, [7–9] mostly due to some cristallisation issues. A few three-dimensional titanium phosphates with an open structure were however discovered in the last decade [10]. More recently, several templated mixed valence three-dimensional titanium phosphates were evidenced using titanium metal as precursor, [11–12] while a series of templated titanium phosphates synthesised from nonaqueous media have also been reported [13].
Our group meanwhile initiated a systematic study of the titanium system with the report of several new titanium phosphates, either with layered structures [14], or with an open structure such as TiIIITiIVF(PO4)2·2H2O or MIL-15 [15], and Ti6O3(H2O)3(PO4)7·(H3O)3·H2O or MIL-18 [16].
We present here the synthesis, the structure determination and a 1H, 13C, 19F 31P solid state NMR characterisation of a new templated one dimensional titanium(III/IV) phosphate: MIL-131 or TiIIITiIV(OH)F4(HPO4) (PO4)·(N((CH2)2NH3)3).
2 Experimental section
Hydrous titanium dioxide (TiO2.H2O) was prepared from the reaction of strongly acidic solutions of TiCl4 (Aldrich, 99%) in HCl (Prolabo, 36%) with ammonia (Prolabo, 20%) at room temperature. The precipitate was washed with demineralised water and dried at 373 K.
The title compound MIL-131 was hydrothermally synthesised from hydrous titanium dioxide, H3PO4 (Prolabo Normapur 85%), HF (Prolabo Normapur 40%), tris ethylenetriamine (Tren) (Aldrich 99%) and H2O in the molar ratio 1:1:1:1:80. The mixture was placed without stirring in a Teflon-lined steel autoclave under autogeneous pressure for 4 days at 473 K. The final synthesis pH is close to 4. The resulting deep blue powder was washed with demineralised water and dried at room temperature.
Elementary analysis gave ratios P/Ti, F/Ti, C/Ti, H/Ti and N/Ti of 0.99, 2.05, 3.19, 10.8 and 1.95, respectively close to the theoretical values (1, 2, 3, 12.5 and 2).
TGA experiment, operated with a Texas instrument TA 2050 apparatus under oxygen flow, was performed and showed several weight losses between 30 and 900 °C (Fig. S1). The first loss (26.15%), between 250 and 300 °C, corresponds to the departure of the protonated triamine in agreement with the calculated values (27.3%). The departure of the fluorine is observed between 600 and 700 °C with a corresponding weigth loss of 15.5% to produce finally titanium(IV) dioxide anatase and TiP2O7 (theoretical loss of 14.3%).
The density measurement, performed on a Micromeretics apparatus Accupyc 1330, was 2.12 g cm−3 (theoretical value: 1.96 g cm−3).
1H, 31P and 19F NMR spectra were recorded on a Bruker Avance-500 spectrometer operating at a magnetic field of 11.74 T and rotation frequency of 30 kHz with the use of 2.5 mm zirconium oxide rotors. The experimental conditions were as following: 1H NMR MAS spectra were obtained at 500.133 MHz, using frequency range 100 kHz, pulse duration 1 μs (π/4), time delay between pulses 4 s, and number of scans 128. Chemical shifts were measured with respect to external TMS. 31P NMR MAS spectra were recorded at 202.457 MHz in the frequency range 100 kHz, pulse duration 1 μs (π/4), pulse repetition time 2 s, and number of scans 1024. The chemical shifts were measured with respect to 85% H3PO4 as the external reference. 19F NMR MAS spectra were measured using a Hahn echo sequence, with respect to the external CCl3F, at frequency 470.628 MHz in the range 500 kHz, pulse length 2 μs and pulse repetition time 14 s. The number of scans was 128.
In addition to the direct polarization spectra, 31P{1H} and 31P{19F} CPMAS spectra were measured to locate the spatial proximities of proton and fluorine with respect to phosphate groups. For such experiments, contact times of 2 ms or 4 ms and recycle delays of 3 s were used, with high-power proton or fluorine decoupling, respectively. The 2D 31P{1H} heteronuclear correlation MAS NMR experiments were recorded in the States mode with a 3-s repetition time and 64 to 254 individual experiments with 32 acquisitions.
High resolution powder diffraction data was collected on the SNBL at ESRF B-station. A channelcut monochromator delivers an unfocused parallel beam on a 4 × 1 mm spot. The wavelength was set to 0.6996 Angstroms. The powder sample was placed in a capillary and spun for averaging. The diffractometer consists of a 6 detector-si111-analyzer array. Data was collected in continuous scan mode between 0.3 and 40° 2θ. The raw data from the six channels were normalized to the synchrotron current and to the relative channel to channel efficiencies and finally summed with the corresponding angular offsets. A binsize of 0.0025° was chosen to get the enough points over the FWHM of the diffraction peaks.
The X-Ray thermodiffractometry, performed in the furnace of a Siemens D-5000 diffractometer in the θ–θ mode, showed several steps in the decomposition. This point will be discussed further in this paper.
Finally, these analyses confirmed the formula deduced from the structure determination for MIL-131: TiIIITiIV(OH)F4(HPO4) (PO4)·(N((CH2)2NH3)3).
3 Structure determination
No single crystal could be obtained even with increasing synthesis times. A high resolution X-Ray powder diffraction pattern was thus recorded at the Swiss Norwegian Beamline at ESRF. The pattern of MIL-131 was indexed with the Dicvolgv program [17]. Solutions with adequate figures of merit were found (M(25) = 75/425 (0.0014, 41)). Systematic absences were consistent with the space groups P-1 (no. 2) or P1 (no. 1).
The pattern matching was performed first with Fullprof, [18] and its graphical interface Winplotr [19], to confirm the cell parameters and the space group deduced from Dicvolgv. In a second step, the profile parameters deduced from Fullprof and three independent titanium TiX6 octahedral (X = O, OH, F), two PO4 tetrahedra and one Tren molecules were defined within the Fox software which uses simulated annealing Monte Carlo calculation methods [20]. Their positions were changed using anti-bump criteria such as minimum O-O, O-C, C-C and C-N distances (= 2.8 Å) to prevent interpenetration of the different motifs. The calculation finally converged to an acceptable solution. Atomic coordinates deduced from Fox were finally refined using Fullprof. Soft distances constraints, an overall thermal parameter and a preferred orientation correction parameter, were applied during the refinement. Details of the structure determination are summarized in Table 1.
Crystal data and structure refinement parameters for MIL-131 or TiIII(OH)TiVIF4(HPO4) (PO4).(N((CH2)2NH3)3).
| Chemical formula | Ti2P2O9N4C6H23 |
| Molar weight (g mol−1) | 1057.6 |
| Calculated density (g cm−3) | 2.09 |
| Crystal system | triclinic |
| Space group | P-1 (no. 2) |
| a (Å) | 14.109 (1) |
| b (Å) | 8.462 (3) |
| c (Å) | 7.179 (1) |
| α (°) | 93.772 (1) |
| β (°) | 96.566 (2) |
| γ (°) | 98.004 (1) |
| V (Å3) | 840.36 (2) |
| Z | 2 |
| Figures of merit M25/F25 | 75/424 (0.0014, 41) |
| Radiation λ | 0.6996 |
| Temperature (K) | 296 |
| 2θ range (°) | 0.33–40.4875 |
| Step | 0.0025 |
| No. reflections | 1641 |
| No. independent atoms | 28 |
| No. intensity parameters | 75 |
| No. profile parameters | 13 |
| No. soft distance/angle constraints | 30/10 |
| Chi2 | 4.1 |
| Rp | 7.4% |
| RWP | 9.16% |
| RBragg | 8.5% |
| Isotropic thermal factor (Å2) | 1.5(1) |
| Profile function | Pseudo-Voigt |
| Background | Experimental (55 points) |
| Number of asymmetry parameters | 2 |
| Preferred orientation vector | 001 |
The final agreement factors are satisfactory: Rp = 7.4%, Rwp = 9.16% and RBragg = 8.5%.
The final Rietveld plot is reported on Fig. 1.
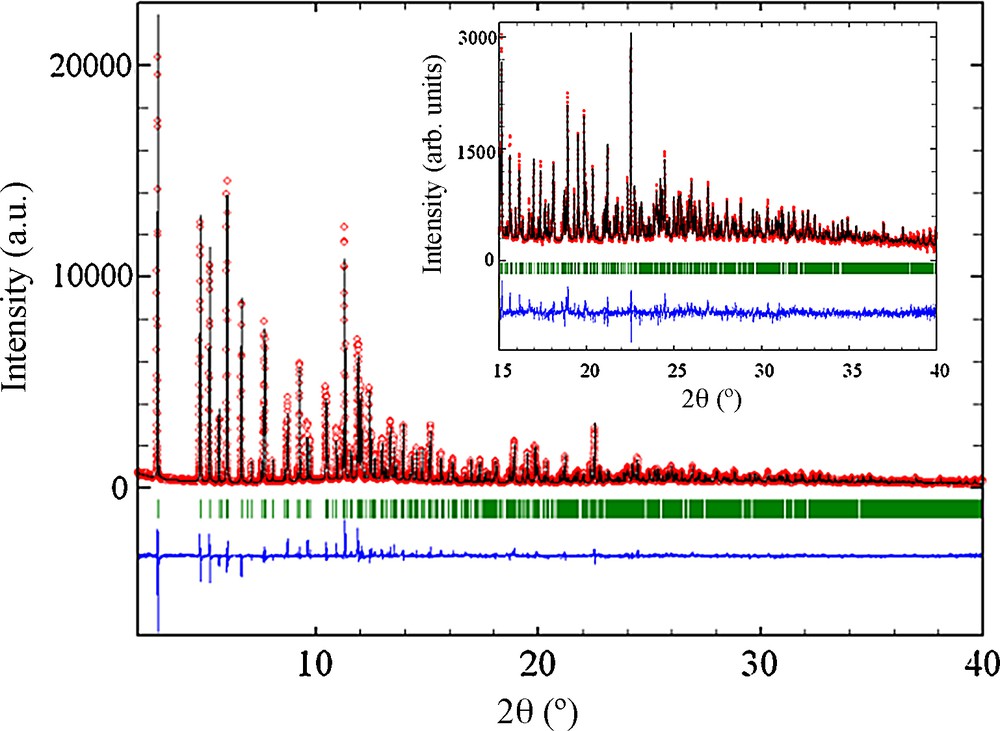
Final Rietveld plot of MIL-131. The corresponding reflections are pointed out using two different sets of vertical bars.
4 Discussion
MIL-131 exhibits a one-dimensional structure (Fig. 2) with an inorganic sub-network built up from inorganic chains made of TiO5X octahedral, single TiO2X4 octahedra (X = OH, F) and PO4 or HPO4 tetrahedra. These units are related together to produce trans-linked corner sharing Ti(2)O5X and Ti(3)O5X octahedral chains (X = OH, F) on which phosphate groups are connected. These units are then connected to Ti(1)X 4O2 single octahedra. The organic structuring agents are located within the small cavities delimited by the single octahedral motifs and chains. This participates to the cohesion of the inorganic motifs via strong hydrogen bondings.

View of the structure of MIL-131. Titanium and phosphate polyhedra are in grey while carbon and nitrogen atoms are in black and white, respectively.
The three octahedrally coordinated titanium atoms exhibit different environments (Fig. 3). Ti(2) and Ti(3) atoms are bonded to four oxygen (or fluorine) atoms shared with the two P(1)O4 and P(2)O3(OH) phosphate groups and two bridging hydroxyl groups (O(9)). Ti(1) is surrounded by four apical fluorine (or hydroxyl) atoms and two oxygen atoms from the phosphate groups. The two phosphorus atoms have three bridging oxygen atoms and one terminal oxo or hydroxo group (O(1) and O(6)). Due to powder data, the accurate nature of the bridging group of the titanium octahedral chains and the terminal groups bond to the phosphorus cannot be determined only using bond valence considerations deduced from inter-atomic distances. However, considering the acidity of the synthesis medium (pH = 4), amino groups would be better described as protonated. The mixed valence of MIL-131 can be deduced first qualitatively from the permanent blue color of the powder even after months of exposure to air atmosphere. Secondly, bond valence calculations using the O’Keefe and Brese equations [21] give values of 4.1, 3.1 and 3.2 for Ti(1), Ti(2) and Ti(3), respectively, which despite the discrepancy due to the use of high resolution synchrotron X-Ray diffraction powder data, clearly indicates that Ti(1) titanium atoms have different oxidation state (+IV) than Ti(2, 3) ones (+III). Furthermore, the absence of any 13C response from solid state NMR is in agreement with the presence of paramagnetic titanium(III) cations. The organics are much closer to Ti(1) titanium(IV) than Ti(2,3) titanium(III). Thus, to ensure the electroneutrality of the sample, MIL-131 could be described either with one oxo or one OH group, two HPO4 groups or one PO4 and one HPO4 motifs. This results in two possible formula:
- (1) [TiIIIX][TiIVX4](HPO4)2(N((CH2)2NH3)3) (X = O, F) or
- (2) [TiIIIX][TiIVX4](HPO4)(PO4)(N((CH2)2NH3)3) (X = OH, F).

Asymmetric unit of MIL-131. Titanium, phosphorus, oxygen, nitrogen and carbons atoms are in cyan, orange, red, blue and black, respectively.
Solid state NMR experiments (see further) will allow a full assignment of the different groups, and will be more consistent with the situation (2) rather than the one (1).
Interatomic distances are in good agreement with those usually observed for titanium phosphates Ti-O (or Ti-F) distances are between 1.79 and 2.07 Å. Within the titanium octahedral chains, Ti-O distances for oxygen (or fluorine) atoms bridging titanium atoms are of 1.94 and 1.96 Å, which is in agreement with the presence of one hydroxyl or one fluorine atom. P-O distances are between 1.48 and 1.55 Å. The triamine molecules exhibit typical N-C and C-C distances between 1.41 and 1.52 Å.
The three primary protonated amine groups (N(1), N(7) and N(10)) also interact differently with the inorganic framework. The first two amino groups (N(1) and N(10)) interact strongly with the terminal fluorine (or hydroxo) atoms bonded to Ti(1) (d(N(i)-F(j) within the 2.76-2.86 Å range; = 1,10 and j = 1–4) while the third primary amino group is more involved into hydrogen bondings with the terminal P = O or P-OH groups (see N(7)-O6) distance at 2.76 Å). As expected, the central tertiary amino group N(4) exhibits no significant interaction with the rest of the structure.
So far, hydroxyl, fluorophosphates or pure titanium(III or IV) phosphates made under hydro or solvothermal conditions exhibit either two-dimensional or three dimensional structures. MIL-131 is, to our knowledge, a scarce example of titanium (fluoro)phosphate with a 1D inorganic sub-network. Besides, liquid phase NMR studies of model titanium fluorophosphates systems revealed previously the presence of a large number of still unidentified titanium fluorophosphates species derivated from the phosphorylation of aqueous titanium fluorides complexes (TiOF(H2O)4+, TiF4(H2O)2, TiF5(H2O)− and TiF62−). [22] Meanwhile, in the case of the layered titanium(IV) fluorophosphate MIL-6 (Fig. 4), [14a] titanium octahedra exhibit two terminal fluorine atoms ruling out the possibility of a 3D inorganic sub-network through the connection of the inorganic layers. In MIL-28 (Fig. 4) [14b], another layered titanium fluorophosphate templated solid, the inorganic sheets are built up from corner sharing chains of titanium octahedra related together through single TiO4F2 octahedra and phosphate groups; apical fluorine atoms are present within the single octahedral and point both at the cavities, preventing from any connection between these octahedra along this direction. In the case of MIL-131 (Fig. 4), the fluorine content is as high as in MIL-6 (overall F/Ti ratio of 2) but with an inorganic sub-unit reminiscent from the one of MIL-28. Corner sharing chains of titanium TiIII(OH)O5 octahedra and single titanium TiIVO2F4 octahedra are connected together through phosphate groups. However, the single octahedra exhibit a higher fluorine content (F/Ti = 4) than in MIL-28 or MIL-6 (F/Ti = 1 or 2, respectively) ruling out any further connection not only through phosphate but also through titanium polyhedra. This explains why MIL-131 possesses a one-dimensional inorganic sub-network instead of two-dimensional ones for MIL-6 and MIL-28.

Crystal structures of various titanium fluorophosphates: from left to right: MIL-6, MIL-131 and MIL-28. For a better understanding, organic cations and free water molecules have been discarded from the figure. Titanium atoms (green) bounded to fluorine atoms are shown in ball and sticks mode (grey) while other titanium atoms are shown in polyhedral mode (white). Phosphate groups are in grey.
The thermal behaviour of MIL-131 has been investigated using X-Ray thermodiffractometry (Fig. 5). Below 200 °C, no change in the X-Ray powder pattern occurs. Above 250 °C, the structure of MIL-131 starts collapsing because of the departure of the organic template. Above 350 °C, an amorphous solid is formed and higher temperatures lead to the crystallization of a mixture of TiO2 anatase and the dense titanium phosphate TiP2O7.

X-Ray thermodiffractogram (λCo≈ 1.7906 Å) of MIL-131 under air atmosphere. Each color represents a different phase (black: MIL-131; blue: amorphous phase).
Two sets of solid state NMR experiments have been performed. First, 1H, 31P, 19F MAS experiments served to determine the nature of the different groups in MIL-131, and secondly CPMAS experiments were employed to determine the different interactions within the framework.
The 1H NMR spectrum shows the presence of four components (Fig. 6). The signal at 15.2 ppm is characteristic of acidic protons, and therefore is assigned to a P-OH group. The signals at 8.6 and 7.0 correspond to two types of NH3 groups in a 1:2 ratio, respectively. The 3.2 ppm is due to the CH2 aliphatic moieties of the amine. Based on these assignments, quantification is consistent with the general chemical formula of [TiIIIX][TiIVX4](HPO4)(PO4)(N((CH2)2NH3)3) (X = OH, F), because the NMR signal intensity ratio between 15.2 ppm and 3.2 ppm was found equal to 1:12, which correspond to (HPO4)/(N(CH2)2NH3)3) ratio of 1. For the other alternative formula [TiIIIX][TiIVF4](HPO4)2(N((CH2)2NH3)3) with a (HPO4)/(N((CH2)2NH3)3) ratio of 1, one would expect a NMR signal intensity ratio between 15.2 ppm and 3.2 ppm of 1:6. On the other hand, based on structural description, the signal at 8.6 ppm should correspond to NH3(N7) group, while the resonance at 7.0 ppm to NH3(N(1) and N(10)) group since the NMR signal intensity ratio between 8.6 and 7.0 ppm was found equal to 1:2.

Solid state 1H NMR spectra of MIL-131.
19F NMR experiments confirm the presence of at least three different fluorine environments of chemical shift of 82, 94 and 123 ppm, with a 2:1:1 ratio, in agreement with the presence of four equal population cristallographical sites for fluorine (Fig. 7). Thus, this clearly shows that bridging groups within the titanium octahedral chains are hydroxyl moieties and that apical atoms bonded to Ti(1) are fluorine atoms. This gives as the most suitable formula for MIL-131 [TiIIIOH][TiIVF4](HPO4)(PO4)(N((CH2)2NH3)3). Isotropic chemical shifts were determined after recording the same spectrum but with different rotation speeds. All these signals exhibit a strong CSA values (210–240 ppm) presumably due to the interaction with the paramagnetic titanium(III) [23]. As no clear relationship between the chemical shift of the species and the nature of the titanium fluorophosphates complex could be evidenced from previous systematic and fundamental studies of model titanium fluorophosphates [22], one cannot assign at this stage the 19F peaks to any crystallographical sites.

19F Hahn echo MAS experiment performed on MIL-131. As an inset, the spectral parameters of the different fluorines are shown.
31P NMR experiments show two signals at ca. −7.9 and −10.4 ppm (Fig. 8), in agreement with the crystal structure. The high field shift with respect to free phosphate is an indication of phosphate bonded to titanium as observed previously for phosphates groups in other titanium phosphates [24]. The direct polarisation spectrum (Fig. 8a) allows quantification confirming the equal population between −7.9 and −10.4 ppm signals (52:48). The presence of amorphous unreacted phosphates was also detected as impurity. 31P{1H} (Fig. 8b) and 31P{19F} (Fig. 8c) were also successfully recorded indicating spatial proximity of proton and fluorine to phosphate groups with a more efficient magnetisation transfer for proton than for fluorine.
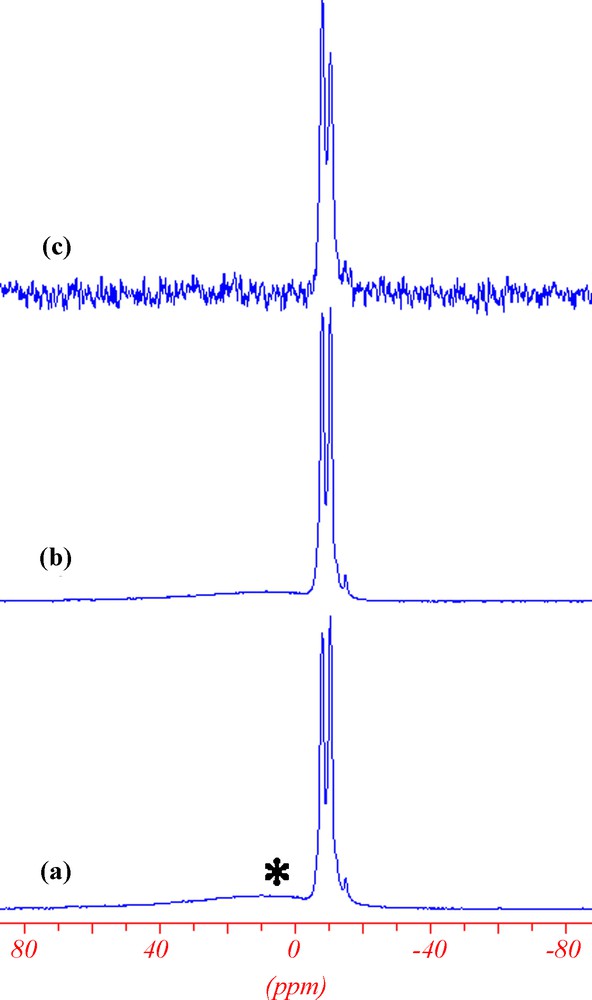
(a) 13P MAS, (b) 31P{1H} CPMAS, and (c) 31P{19F} CPMAS NMR experiment of MIL-131.
In a second step, two-dimensional solid state NMR experiments have been realised for a deeper insight of the local environments around phosphorus and protons. 31P{1H} HETCOR CPMAS enables to determine preferential environment for phosphate groups regarding organic moieties and OH groups in the structure. Using different contact times, from 2 ms down to 0.06 ms (Figs. S2, S3, S4), this is nevertheless possible to point out that the signal of POH at ca. 15 ppm correlates preferentially with the −7.9 ppm 31P signal. This allowed us to assign this latter to P(1)O3(OH), and consequently the signal at −10.4 ppm to P(2)O4 group. On the other hand, the signal of terminal protonated amino groups N(1) and N(10) at ca. 7 ppm is correlated preferentially with the P(1) signal, while the signal of ammonium N(7) with P(2). This is in total agreement with the crystal structure whereas N(1) and N(10) exhibit hydrogen bondings with oxygen atoms of the P(1)O3(OH) group while N(7) interacts only with the P(2)O4 group.
Finally, solid state NMR allowed us not only to confirm the formula deduced from the structure determination but also led to a careful analysis of the different hydrogen bondings interaction within MIL-131.
In conclusion, a new templated titanium hydroxyfluorophosphate, exhibiting a one-dimensional and mixed valence titanium(III/IV) inorganic sub-network built up from corner sharing chains of titanium (III) octahedra, phosphate groups and highly fluorinated titanium(IV) octahedra, has been characterised by X-Ray synchrotron powder diffraction data. 1H, 31P, 13C and 19F solid state NMR allowed a full assignment of the different functional groups (O, OH, F, P = O and P-OH) present within the new framework as well as a deeper analysis of the hydrogen bonding network within the framework. Further experiments are currently in progress and will hopefully lead to new porous titanium-based solids with interesting applications.
Acknowledgements
We gratefully acknowledge CNRS, RHODIA, université Versailles-St-Quentin in Yvelines for their financial support.


