1 Introduction
Since the discovery of the first microporous zinc phosphates (ZnPOs) by Gier and Stucky [1] in 1991, much effort has been devoted to preparing novel structures within the zinc-phosphate system. Hydro- or solvothermal synthesis conditions are often employed using organic amines as the structure directing agents or templates and these are usually incorporated, in their protonated forms, into pores or cavities within anionic zinc-phosphate frameworks. Over 100 templated ZnPOs have now been characterised, making this the fastest growing class of open-framework phosphates, and they exhibit great structural diversity with ZnO4 tetrahedra linked via PO4 units to generate 0-D (monomer), 1-D (chain, ladder), 2-D (layer) and 3-D architectures. As well as direct synthesis, a number of ZnPOs of higher dimensionalities have been prepared from low dimensional phases. For example, monomer [2], chain [3] and layered [4] ZnPOs transform to 3-D structures on heating under modest conditions, especially in the presence of additional amine. These transformations have thrown some light on the assembly mechanisms of 3-D ZnPO phases as structural units present in low dimensional frameworks are retained during the condensation processes [5,6]. Interestingly, some of these reactions are reversible, with 3-D frameworks forming 1-D ladders under acidic conditions [7].
Of the many 3-D zinc phosphates reported, particularly eye catching examples include [TH2][Zn3(PO4)2(HPO4)]·2H2O (T = 1,2-diaminocyclohexane) (ND-1) [8], containing large-pore 1-D channels bounded by 24-membered rings (24 MR) of alternating ZnO4 and PO4 units and [TH2][Zn4(HPO4)2]·3H2O (T = 1,6-diaminohexane), containing isolated trimers of ZnO4 groups together with 5- and 20-MR [9]. The range of stoichiometries found in the 3-D frameworks is now quite wide with examples known having P:Zn ratios of 3:4, 4:5, 5:6, 6:7, 1:1, 5:4, 4:3, 3:2 and 2:1. Pertinent to the present work are 3-D materials with P:Zn ratio of 3:2, of which six examples containing organic amine cations have been reported to date; namely, [TH]2[Zn2(HPO4)3 (T = ethylamine) [10], [TH2][Zn2(HPO4)3]·xH2O (x = 0, T = ethylenediamine [11], 1,2-diaminopropane [12] and piperazine [13]; x = 1, T = 1,4-diazacycloheptane (homopiperazine) [14]), and [TH2][Zn2(PO4)(H1.5PO4)2] (T = piperazine [13,15]). Here we report the synthesis and characterisation of a new zinc phosphate, [C5N2H14][Zn2(HPO4)3], with P:Zn ratio 3:2 and containing the cyclic amine, 1-methylpiperazine. The relationship of this structure to two 3-D ZnPOs with the same stoichiometry [12,14] is discussed.
2 Synthesis
Single crystals of the title compound, [C5N2H14][Zn2(HPO4)3], were synthesised under predominantly non-aqueous solvothermal conditions. ZnO (0.36 g) was dispersed in 6.00 cm3 of ethylene glycol by stirring. 1-Methylpiperazine (0.42 cm3) and orthophosphoric acid (0.70 cm3, 85 wt.%) were then added with further stirring. Finally, hydrochloric acid (0.20 cm3) was added to form a thick gel with the overall composition ZnO: 24.32 HO(CH2)2OH: 0.86 C 5N2H12: 2.31 H3PO4(aq): 0.55 HCl(aq). The gel was sealed in a 23 cm3 Teflon-lined stainless steel autoclave and placed in an oven at 433 K for 7 days. The solid product obtained from this synthesis consisted of a very hard dark glass-type material of unknown composition with a few large colourless blocks of the title product. The crystals could be recovered manually by adding deionised water to the glassy material. This operation transformed the glass into a brittle unidentified phase that could be removed from the crystals by applying light pressure. The crystals were then further washed with water and left to dry overnight at room temperature. Repeating the synthesis in the absence of HCl, but with the other component compositions remaining unchanged, also produced single crystals of the title compound embedded in a sticky gel. The addition of deionised water to the solid product transformed the sticky gel to a brittle solid from which the crystals could easily be removed.
Thermogravimetric analysis, performed under a flow of nitrogen using a Stanton Redcroft STA 1000 thermal analyser at a heating rate of 10 K min–1, showed a steady weight loss over the range 533–823 K of 20.45%, which corresponds well with the calculated value of 19.19% for the loss of 1-methylpiperazine. IR studies of individual single crystals of the product, recorded using a Brucker Scan2scope IR microscope, further confirmed the presence of amine in the framework.
3 Structure determination
A crystal of the title compound in the form of a large colourless block was mounted on a glass fibre using cyanoacrylate adhesive. X-ray intensity data were collected at room temperature using a Nonius KappaCCD diffractometer (graphite-monochromated Mo Kα radiation). Data sets were processed using DENZO [16] and SCALEPACK [17]. Full crystallographic details are given in Table 1.
Crystallographic data for [C5N2H14][Zn2(PO3(OH))3]
| Formula | [C5N2H14][Zn2P3O12H3] |
| Mr | 520.90 |
| Crystal size (mm) | 0.16 × 0.28 × 0.40 |
| Crystal habit | Colourless plate |
| Crystal system | Orthorhombic, P212121 |
| a (Å) | 10.0517(2) |
| b (Å) | 10.4293(2) |
| c (Å) | 14.9050(5) |
| Cell volume (Å3) | 1562.52 |
| Z | 4 |
| Temperature (K) | 293(2) |
| ρcalc (mg m–3), μ(Mo Kα) (mm–1) | 2.214, 3.442 |
| Radiation, wavelength (Å) | Mo Kα, 0.71073 |
| Unique data, observed data (I > 3σ(I)) | 3490, 3238 |
| Rint | 0.01 |
| Residual electron density (min, max) (e Å–3) | –0.86, 0.70 |
| Number of parameters refined | 227 |
| R(F), wR(F) | 0.0260, 0.0293 |
The structure was solved in the orthorhombic space group P 21 21 21 [18] by direct methods using the program SIR92 [19] and all nonhydrogen atoms of the framework and template located. Subsequent Fourier calculations and least-squares refinements on F were carried out using the CRYSTALS suite of programs [20]. All the framework and template hydrogen atoms were located in difference Fourier maps, but during the refinement, the template hydrogen atoms were placed geometrically after every cycle and the positional parameters of the framework ones were refined with the O–H distances restrained to be 1.00(1) Å and U(iso) fixed at 0.05 Å2. The Flack parameter was refined to a value of 0.01(1) indicating that the absolute structure is as given in the results. In the final cycle, 227 parameters, including anisotropic thermal parameters for all nonhydrogen framework and template atoms, were refined. A three-term Chebyshev polynomial was applied as a weighting scheme [21] and the refinement converged to give R(F) = 0.0260 and wR(F) = 0.0293.
Fractional atomic coordinates and isotropic thermal parameters are given in Table 2 and selected bond distances and angles in Table 3. The local coordination of the framework and template atoms is shown in Fig. 1.
Fractional atomic coordinates and isotropic thermal parameters (Å2) for [C5N2H14][Zn2(PO3(OH))3]
| Atom | x | y | z | U(iso) |
| Zn(1) | 0.86651(4) | –0.19428(3) | 0.67659(2) | 0.0163 |
| Zn(2) | 0.62939(4) | 0.14276(3) | 0.76325(2) | 0.0194 |
| P(1) | 0.59850(7) | –0.15920(7) | 0.77818(5) | 0.0169 |
| P(2) | 0.93664(8) | 0.10943(7) | 0.70409(5) | 0.0172 |
| P(3) | 0.92528(8) | –0.14857(8) | 0.46899(5) | 0.0198 |
| O(1) | 0.8844(3) | –0.2291(2) | 0.54962(15) | 0.0273 |
| O(2) | 0.6786(2) | –0.2204(3) | 0.70245(17) | 0.0301 |
| O(3) | 0.9411(2) | –0.0353(2) | 0.72007(16) | 0.0245 |
| O(4) | 0.9659(3) | –0.3286(3) | 0.73428(19) | 0.0433 |
| O(5) | 0.5535(3) | –0.0256(2) | 0.75495(19) | 0.0341 |
| O(6) | 0.8001(3) | 0.1633(3) | 0.7084(3) | 0.0542 |
| O(7) | 0.8593(3) | –0.2015(2) | 0.38640(14) | 0.0278 |
| O(8) | 0.4825(3) | –0.2424(3) | 0.80479(16) | 0.0291 |
| O(9) | 0.6963(2) | –0.1510(2) | 0.86162(17) | 0.0276 |
| O(10) | 0.9885(3) | 0.1388(2) | 0.60599(16) | 0.0262 |
| O(11) | 0.9010(3) | –0.0069(2) | 0.48404(16) | 0.0320 |
| O(12) | 1.0814(3) | –0.1670(3) | 0.4569(2) | 0.0408 |
| N(1) | 0.3296(3) | –0.0179(3) | 0.5438(2) | 0.0248 |
| N(2) | 0.4635(4) | 0.0873(3) | 0.3889(2) | 0.0363 |
| C(1) | 0.4755(4) | 0.0122(4) | 0.5464(2) | 0.0301 |
| C(2) | 0.5358(4) | 0.0036(4) | 0.4534(3) | 0.0321 |
| C(3) | 0.3185(5) | 0.0613(4) | 0.3880(3) | 0.0351 |
| C(4) | 0.2603(4) | 0.0733(4) | 0.4815(3) | 0.0318 |
| C(5) | 0.2701(4) | –0.0128(4) | 0.6361(3) | 0.0363 |
| H(1) | 0.658(5) | –0.092(4) | 0.908(3) | 0.0500 |
| H(2) | 0.943(5) | 0.083(4) | 0.561(3) | 0.0500 |
| H(3) | 1.106(5) | –0.213(4) | 0.401(2) | 0.0500 |
Selected bond lengths (Å) and angles (°) for [C5N2H14][Zn2(PO3(OH))3]
| Zn(1)–O(1) | 1.935(2) | P(3)–O(1) | 1.523(2) |
| Zn(1)–O(2) | 1.947(2) | P(3)–O(7) | 1.503(2) |
| Zn(1)–O(3) | 1.932(2) | P(3)–O(11) | 1.515(3) |
| Zn(1)–O(4) | 1.924(2) | P(3)–O(12) | 1.591(3) |
| Zn(2)–O(5) | 1.919(2) | ||
| Zn(2)–O(6) | 1.913(3) | O(9)–H(1) | 1.00(1) |
| Zn(2)–O(7)(a) | 1.939(2) | O(10)–H(2) | 1.00(1) |
| Zn(2)–O(8)(b) | 1.931(2) | O(12)–H(3) | 1.00(1) |
| P(1)–O(2) | 1.526(2) | ||
| P(1)–O(5) | 1.505(2) | N(1)–C(1) | 1.500(5) |
| P(1)–O(8) | 1.507(2) | N(1)–C(4) | 1.500(5) |
| P(1)–O(9) | 1.588(2) | N(1)–C(5) | 1.502(5) |
| P(2)–O(3) | 1.528(2) | N(2)–C(2) | 1.488(5) |
| P(2)–O(4)(c) | 1.490(3) | N(2)–C(3) | 1.482(6) |
| P(2)–O(6) | 1.485(3) | C(1)–C(2) | 1.514(5) |
| P(2)–O(10) | 1.582(2) | C(3)–C(4) | 1.516(5) |
| O(1)–Zn(1)–O(2) | 104.92(11) | O(4)(c)–P(2)–O(10) | 105.60(16) |
| O(1)–Zn(1)–O(3) | 116.9(1) | O(6)–P(2)–O(10) | 105.73(17) |
| O(2)–Zn(1)–O(3) | 115.5(1) | ||
| O(1)–Zn(1)–O(4) | 104.60(11) | O(1)–P(3)–O(7) | 108.94(14) |
| O(2)–Zn(1)–O(4) | 108.28(14) | O(1)–P(3)–O(11) | 112.20(14) |
| O(3)–Zn(1)–O(4) | 105.88(12) | O(7)–P(3)–O(11) | 114.11(14) |
| O(5)–Zn(2)–O(6) | 115.58(15) | O(1)–P(3)–O(12) | 106.81(17) |
| O(5)–Zn(2)–O(7)(a) | 111.94(11) | O(7)–P(3)–O(12) | 107.32(16) |
| O(6)–Zn(2)–O(7)(a) | 108.48(15) | O(11)–P(3)–O(12) | 107.08(17) |
| O(5)–Zn(2)–O(8)(b) | 107.59(11) | ||
| O(6)–Zn(2)–O(8)(b) | 103.19(12) | Zn(1)–O(1)–P(3) | 133.89(14) |
| O(7)(a)–Zn(2)–O(8)(b) | 109.59(11) | Zn(1)–O(2)–P(1) | 126.82(14) |
| O(2)–P(1)–O(5) | 112.07(17) | Zn(1)–O(3)–P(2) | 141.64(15) |
| O(2)–P(1)–O(8) | 111.21(14) | Zn(1)–O(4)–P(2)(d) | 158.9(2) |
| O(5)–P(1)–O(8) | 111.16(16) | Zn(2)–O(5)–P(1) | 135.46(17) |
| O(2)–P(1)–O(9) | 105.98(14) | Zn(2)–O(6)–P(2) | 143.6(2) |
| O(5)–P(1)–O(9) | 108.44(14) | Zn(2)(e)–O(7)–P(3) | 129.29(15) |
| O(8)–P(1)–O(9) | 107.71(14) | Zn(2)(f)–O(8)–P(1) | 132.05(15) |
| O(3)–P(2)–O(4)(c) | 108.25(15) | ||
| O(3)–P(2)–O(6) | 113.20(17) | P(1)–O(9)–H(1) | 109.6(31) |
| O(4)(c)–P(2)–O(6) | 114.6(2) | P(2)–O(10)–H(2) | 110.5(30) |
| O(3)–P(2)–O(10) | 109.00(14) | P(3)–O(12)–H(3) | 113.7(30) |

Local coordination of the framework and template atoms in [C5N2H14][Zn2(HPO4)3] (drawing package ATOMS [22]).
4 Structural description
The structure consists of a 3-D framework of stoichiometry [Zn2(HPO4)3]2–, charge balanced by 1-methylpiperazinium cations residing as guest species in the pore network.
There are two crystallographically distinct Zn atoms, both of which are tetrahedrally coordinated to oxygen atoms at distances typical of those observed in other ZnPOs (Zn–Oav = 1.930 Å). Both the ZnO4 tetrahedra share their vertices with phosphorus-based tetrahedra. There are three crystallographically distinct PO4 units, two of which, based on P(1) and P(2), share three vertices with ZnO4 units and possess one terminal P–O linkage, whilst the third, based on P(3), shares two vertices and has two terminal P–O bonds. Of the terminal P–O bonds, three were found to be elongated (P(1)–O(9), 1.588(2) Å; P(2)–O(10), 1.582(2) Å and P(3)–O(12), 1.591(3) Å) compared to the bridging P–O bonds (P–Oav = 1.506 Å). The location of hydrogen atoms in difference Fourier maps at ~1 Å from O(9), O(10) and O(12) suggests that these constitute P–OH groups. The remaining terminal bond is shorter than the other three (P(3)–O(11), 1.515(3) Å), and is assumed to have some degree of multiple-bond character. These assignments are confirmed by bond-valence calculations [23].
The ZnO4 and PO3(OH) units are linked in an alternating manner to generate the three dimensional framework. The structure can be described in two parts. The Zn(1)O4, Zn(2)O4, HP(1)O4 and HP(2)O4 tetrahedra link to form layers lying in the ab plane containing circular 4- and elliptical 8-membered rings of alternating Zn- and P-based polyhedra with cross ring dimensions of 3.932(4) × 4.185(5) Å (O(3)…O(5) and O(2)…O(6)), and 6.268(4) × 8.274(4) Å (O(4)…O(8) and O(4)′…O(8)′), respectively (Fig. 2). These layers stack in an ABAB sequence and are in turn connected by linking of the Zn(1)O4 and Zn(2)O4 tetrahedra in adjacent sheets through P(3)O3(OH) units via O(1) and O(7), respectively, to generate the three dimensional structure (Fig. 3). The hydroxo groups of the P(1)O3(OH) and P(2)O3(OH) units are directed into the interlayer space and involved in strong hydrogen bonding interactions to O(11), one of the terminal framework oxygen atoms of the bridging P(3)O3(OH) unit (O(9)…O(11), 2.645(4) Å and O(10)…O(11), 2.527(3) Å). The remaining hydroxo group, attached to P(3), hydrogen bonds to the layer oxygen atom O(2) (O(12)…O(2), 2.824 Å).

View along the c-axis showing one [Zn2(HPO4)3]2– layer containing 4 and 8 MR of alternating ZnO4 and PO3(OH) tetrahedra (the (4.8) net).

View along the a axis of the zinc phosphate layers in the ab plane linked via PO3(OH) units to generate the 3-D structure. Hydrogen atoms have been omitted for clarity. O...O distances across the 12 MR ring are O(10)...O(10)′, 6.373(3), O(9)...O(9)′, 6.822(3) and O(11)...O(11)′, 7.362(4) Å.
There are several series of channels generated within the structure, which run parallel to the three cell axes. In addition to the channels bounded by 8- and 4-membered rings lying parallel to the c axis produced by alignment of the layers as described above, there are rectangular-shaped channels bounded by 12-membered rings and more irregular ones bounded by 8-membered rings running parallel to the a and b axes, respectively. In the latter cases, P–OH and P=O groups project into the cavities reducing their effective pore diameters (Fig. 3). The channels intersect to form a 3-D network in which the 1-methylpiperazinium cations are located (Fig. 4). Both nitrogen atoms of the diaminocation are within hydrogen bonding distance of a number of framework oxygen atoms (N(2)…O(3), 2.747(4) Å; N(2)…O(10), 2.869(4) Å and N(1)…O(1), 3.034(4) Å).
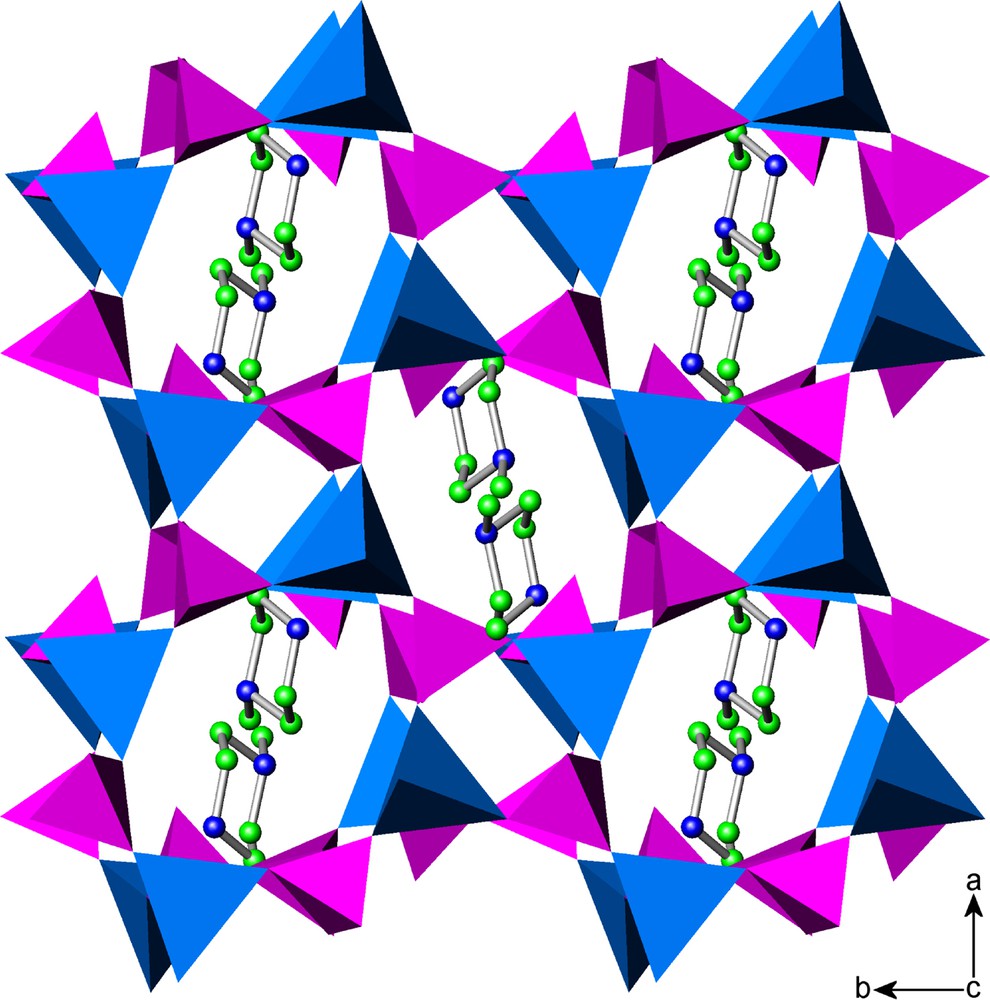
Polyhedral representation of [C5N2H14][Zn2(HPO4)3] along the c-axis showing the location of the 1-methylpiperazine cations. Key: ZnO4 tetrahedra, blue; PO3(OH) tetrahedra, pink.
The openness of a structure can be defined in terms of the framework density (FD), which is equal to the number of tetrahedral atoms per 1000 Å3 [24]. In [C5N2H14][Zn2(PO3(OH))3], the FD is 12.8, a value comparable to those determined for many aluminosilicate zeolites and aluminophosphates with large-pore volumes.
5 Discussion
The compound [C5N2H14][Zn2(PO3(OH))3], possessing a new 3-D framework structure, has been synthesised under solvothermal conditions in the presence of 1-methylpiperazine as structure directing agent with or without the addition of a small quantity of HCl. Although a 3-D ZnPO [C5N2H14][Zn(HPO4)2(H2O)], has been prepared previously in the presence of 1-methylpiperazine [25], the P:Zn ratio in this case is 2:1 and not 3:2 as is observed here.
Natarajan et al. [26–28] have recently used hydrochloric acid as a mineraliser in the preparation of a number of zinc phosphates with a range of dimensionalities but, as is observed here, no Cl was incorporated into the final ZnPO products. However, chlorinated ZnPOs [TH][Zn(HPO4)Cl] (T = cyclohexylamine [29], piperidine [30] and 1,6-diaminohexane [31]) and [TH]2[Zn(HPO4)2Cl2] (T = cyclohexylamine [32]), have also been prepared in the presence of HCl. All have layered structures assembled from ZnO3Cl and PO4 tetrahedra. This behaviour parallels that of HF, which has also been used as a crystallising agent in the synthesis of many AlPOs and GaPOs. Although many non-fluorinated products have been produced, this approach can also lead to the incorporation of fluorine into the products, either directly bonded to framework metal atoms, for example as M-F or M-F-M units, as in a number of the ULM GaPO materials, or encapsulated in cavities, as in cloverite and the Mu materials Mu-2 [33], Mu-3 [34] and Mu-15 [35], where it stabilises double 4-ring (D4R) cages.
The vertex linking of tetrahedra to form layers containing 4 and 8 MR ((4.8) net sheets) observed in [C5N2H14][Zn2(HPO4)3] (Fig. 2) is a structural feature previously identified in a number of zeolite frameworks e.g. ABW, ACO, APC, APD, GIS, PAU, SBE, VNI, WEI, YUG [24]. Some of these structure types have been reported previously for zinc phosphates as well as aluminosilicates. For example, several alkali-metal ZnPOs are known with the ABW structure type (e.g. AZnO4, where A = Li+ with extraframework water molecules [36,37] and Na+, Rb+, Cs+ and NH4+ [38,39]), together with two organically templated ZnPOs with the GIS structure type, [TH2]0.5ZnPO4 (T = ethylenediamine [11] and 1,3-diaminopropane [40]).
Of the six previously known 3-D frameworks with P:Zn ratio 3:2, two, [TH2][Zn2(HPO4)3]·H2O (T = 1,4-diazacycloheptane [14]) (II) and [TH2][Zn2(HPO4)3] (T = 1,2-diaminopropane [12]) (III), also contain layers with the same topology as those in the title compound, [C5N2H14][Zn2(HPO4)3] (I), but the structures adopted are all subtly different. In compounds (I) and (II), the layers stack so that the zinc (and phosphorus) atoms lie directly on top of each other, whereas in (III), they stack in an alternating fashion with zinc lying directly above phosphorus (Fig. 5). Zinc atoms in adjacent layers are linked together through PO3(OH) pillars which lie either along the edges of the channels generated by the 4MR ((I) and (III)) or join across adjacent 4-membered rings ((II)). The resulting 3-D frameworks contain additional channels generated perpendicular to the layers and bounded by 8- and 12- ((I) and (III)) and 8- and 10-membered rings ((II)), respectively. Interestingly, isolated layers with the same (4.8) net are found in [TH][Zn(HPO4)Cl] (T = cyclohexylamine [29]) where termination of the layers by Zn–Cl groups prevents crosslinking to form a three dimensional structure.

Schematic diagram (with oxygen atoms omitted) showing the stacking of the Zn2P2 4MR in adjacent layers in [C5N2H14][Zn2(HPO4)3] (I), [TH2][Zn2(HPO4)3].H2O (T = 1,4-diazacycloheptane [14]) (II) and [TH2][Zn2(HPO4)3] (T = 1,2-diaminopropane [12]) (III) and the different crosslinking of the layers via P-based units.
6 Supplementary material available
A CIF file has been deposited at the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK with CCDC reference number 237011.
Acknowledgements
A.M.C. thanks the Leverhulme Trust for a Research Fellowship and FOMG thanks the Reading Endowment Trust Fund (RETF) for a Research Studentship. Dr. A.R. Cowley, Chemical Crystallography Laboratory, University of Oxford is thanked for assistance with X-ray data collection.


