1 Introduction
The geological complexity of the Alps range generates particular deep flow systems leading to ascending thermal waters. Examples showing the relation between thermal water and Alpine tectonic contacts have been described by many authors (Arthaud and Dazy, 1989; Perello et al., 2001; Sonney and Vuataz, 2009; Vuataz, 1982; Zuppi et al., 2004). In thrust faults related to Triassic formations, shallow and deep flow systems occur producing a Ca-SO4 and low-Cl water from the gypsum dissolution. Locally, water with high Na-Cl contents can emerge from a deep flow system in the crystalline domain, especially along the external crystalline massifs. Its fingerprint was described by Arthaud and Dazy (1989) assuming a remobilization of deep Na-Cl waters by regional flow systems until the water meets the thrust faults of the External Crystalline Massifs. The Saint-Gervais-les-Bains (SGLB) hydrothermal system is also related to this process and the objective of this paper is to provide evidence of the Na-Cl thermal component coming from the Aiguilles Rouges crystalline basement. The second objective is to present chemical processes occurring at shallow depth, important for the long-term exploitation of the thermal waters. Mostly, ascending thermal water drags circulating cold groundwater into the fractured zone to a shallow depth named the decompressed zone (Cruchet, 1985; Lhomme et al., 1996; Maréchal, 1999; Sonney and Vuataz, 2009) before mixing with more superficial groundwater in the Quaternary deposits. Over-exploitation of thermal water at SGLB has generated important decreases of temperature and mineralization in the past and poses problems for a sustainable long-term thermal water production. Geological, geochemical and isotopic similarities of thermal waters at SGLB and Lavey-les-Bains have been a point of great interest, which has been reviewed in detail in the SGLB hydrothermal system (Sonney, PhD thesis in progress). Old and new data are discussed in this paper, aimed at understanding the origin of the deep flow system of SGLB and the chemical processes between the ascending thermal water and the shallow groundwater.
2 Location and exploitation of thermal waters
The SGLB hydrothermal is a well-recognized therapeutic area located at the end of the narrow gorges of the Bon Nant mountain stream at the foot of the Mont Blanc Massif, in the Haute-Savoie French Department (Fig. 1). Currently, the spa continuously pumps the uprising thermal waters in three wells: Lépinay (101.5 m, 13 m3/h) and De Mey Est (196 m, 5.5 m3/h) are used as production wells, respectively for swimming pools and cosmetics, whereas De Mey Ouest water (207 m, 3.5 m3/h) is unused but guarantees a stable exploitation of the De Mey Est well. The old Gontard well and the F99 exploration well (99 m) are not exploited, but Gontard is used as an observation well. The maintenance of the natural overflow in Gontard prevents the over-exploitation of the resource and ensures that the pressure of the deep thermal aquifer remains higher than the pressure of the shallow groundwater. Finally, the cold springs named Sulfureuse, Ferrugineuse, Magnésienne and Cold Spring discharge water directly into the Bon Nant River. Downstream from the spa, the Ferrugineuse and Magnésienne springs emerge in the Bon Nant bed at the bottom of dolomitic and gypseous outcrops. These are two cold springs with iron oxide deposits, which have a stable temperature, conductivity and discharge in summer as well as in winter.
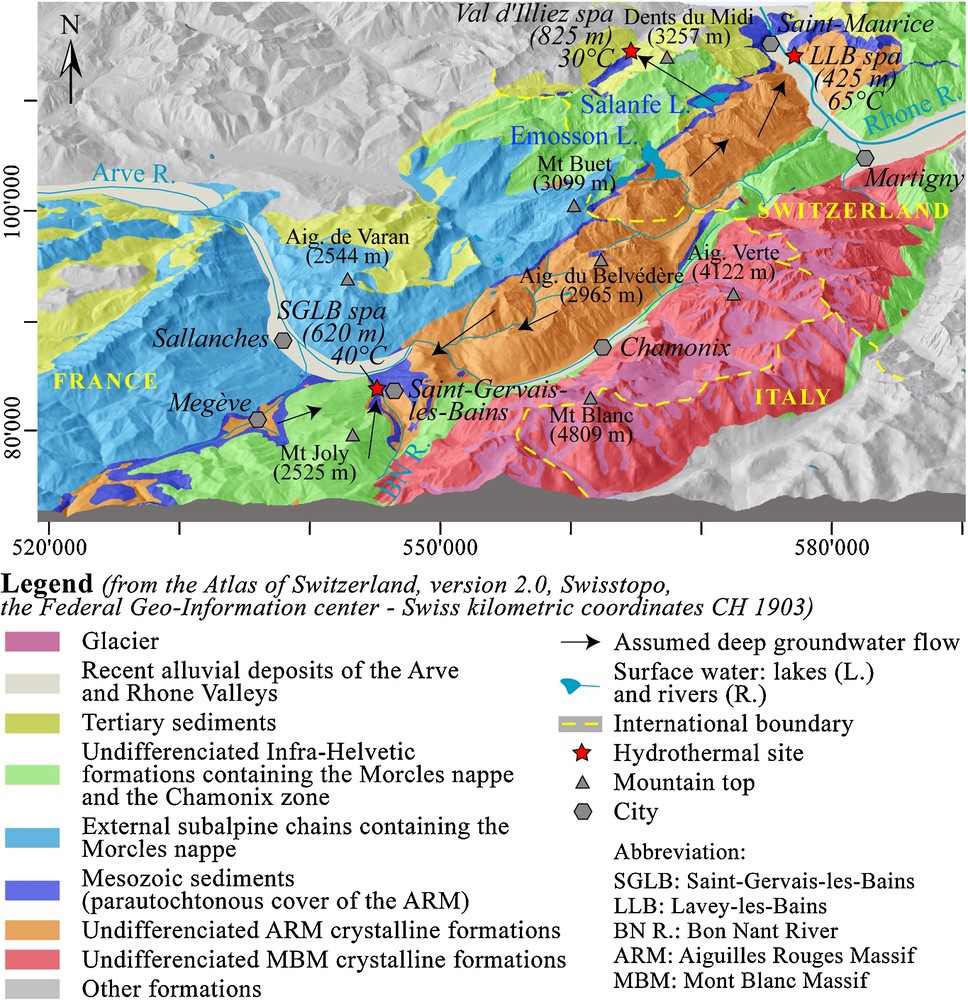
Regional geological setting of Saint-Gervais-les-Bains.
Contexte géologique régional de Saint-Gervais-les-Bains.
3 Geological setting
3.1 Regional setting
The SGLB hydrothermal site is positioned at the SW low elevated point of the Aiguilles Rouges Massif belonging to the External Crystalline Massifs as the Mont-Blanc Massif (Fig. 1). These two massifs consist of different types of crystalline rocks affected by Hercynian and Alpine stresses (Von Raumer and Bussy, 2004). At depth, the Mont Blanc Massif overlaps the Aiguilles Rouges Massif, this last one supposedly lies on another external Crystalline Massif at depth named Infra-Aiguilles Rouges (Pfiffner et al., 1997). Mont Blanc and Aiguilles Rouges are separated by a complex sedimentary syncline named the Chamonix zone, which is the root of the reversed limb of the Morcles nappe (Von Raumer and Bussy, 2004). The Aiguilles Rouges basement does not directly outcrop in the studied hydrothermal area, but is certainly present at shallow depth, 300–500 m below its autochthonous sedimentary cover, which consists of Permian-Triassic formations (Fig. 2). This cover is overlapped by the Morcles nappe formations consisting of the Mont Joly Massif. The Morcles nappe formations mainly consist of Mesozoic limestones and marls (Epard, 1989) drawing recumbent folds (Paréjas, 1925). In the area of Megève, this cover was eroded during Quaternary glaciation phases and some outcrops of the basement and of its autochthonous cover are visible. This area and another zone at the south of SGLB were previously considered as the recharge zone of the deep flow system.
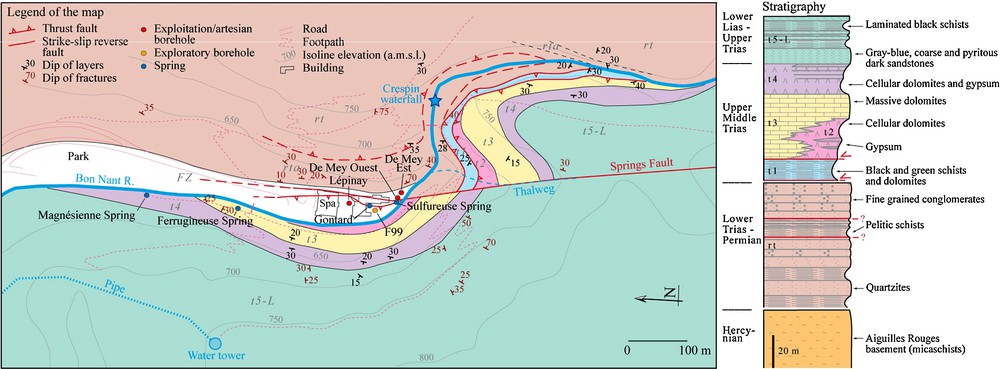
Local geological setting of the hydrothermal area of Saint-Gervais-les-Bains (modified from an unpublished report of the BRGM public institution). The sedimentary series in Fig. 2 covering the Aiguilles Rouges basement corresponds entirely to the geological unit named Mesozoic sediments in Fig. 1 in dark blue color.
Contexte géologique local de la zone hydrothermale de Saint-Gervais-les-Bains (modifié d’après un rapport non publié de l’institution publique du BRGM). La série sédimentaire de la Fig. 2 reposant sur le socle des Aiguilles Rouges correspond à l’unité géologique appelée « Mesozoic sediments » sur la Fig. 1 de couleur bleu foncé.
Vertical strike-slip systems or subvertical reverse faults from north-south to WSW–ENE are present and low-dip thrusts at the origin of the nappes occur. In the SGLB area, a major north-south faulted zone crosses all formations following the Bon Nant Valley, and certainly allows the uprising of the deep fluid from the potential hydrothermal reservoir to the surface.
3.2 Local setting
The autochthonous cover of the Aiguilles Rouges basement was intensely deformed by Alpine stresses. Geological investigations carried out by the BRGM during the 1980s, as well as after drilling of the boreholes Lépinay and F99 in 1999, highlighted the presence of a thrust fault below the Bon Nant filling. This thrust fault puts in contact a normal series of Upper-Middle Trias with black and green schists at the bottom on the Lower Triassic-Permian detrital deposits (Fig. 2). In detail, movements of the thrust fault near the contact generated a complex zone of imbricated structures with alternations of quartzites, dolomites and schists, as was observed in the boreholes Lépinay and F99. Lépinay crossed the Upper-Middle Trias down to the black and green schists (t1) whereas F99 penetrated in the Lower Triassic-Permian detrital deposits after having crossed these same schists.
Concerning De Mey Est and De Mey Ouest, only the Lower Triassic-Permian quartzites were drilled below the Quaternary filling, and therefore, these two boreholes are located on the other side of the thrust fault. Between them, it can be assumed that the two thermal springs (Gontard and Sulfureuse) emerge along a complex fault system including the Springs Fault, which is a dextral strike-slip and reverse fault shifting the thrust fault. It dips steeply towards the east and was probably intersected by the two De Mey wells.
4 Hydraulic connectivity
The hydraulic connectivity of the different springs and wells was demonstrated via pumping tests. This connectivity is clear for the two De Mey wells, however a variation of the flow rate in De Mey has no influence on Lépinay, Gontard, F99 and Sulfureuse, and vice versa. This process is explained by the presence of two separated aquifers below the Quaternary filling on each side of the thrust fault occurring in different geological settings (Fig. 5).
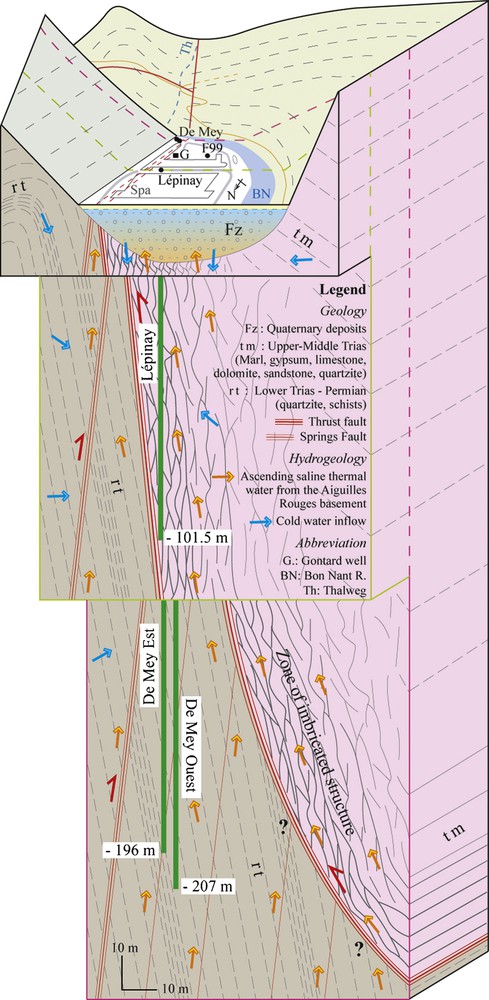
Conceptual model of mixing processes of the Saint-Gervais-les-Bains hydrothermal system.
Modèle conceptuel des processus de mélange du système hydrothermal de Saint-Gervais-les-Bains.
5 Water chemistry
It was possible to collect the existing data, many from unpublished reports, and to make a new sampling campaign in 2008 (Table 1). Old and new data are discussed in this paper, aimed at understanding the origin of the deep flow system and of the chemical processes of the uprising thermal fluids mixed with shallow groundwater. On the basis of the chemical analyses and diagrams in Fig. 3, the sampled waters can be subdivided into three groups. The first group includes Na-SO4 water rich in chloride with high electrical conductivity (above 5 mS/cm) and temperature (above 35 °C). This group represents the ascending thermal end-member. In detail, water from Lépinay is slightly different, with less chloride and more sulphate compared to De Mey Est and De Mey Ouest. Lépinay is closer to the diluted water of Gontard. The second group refers to Ca-SO4 waters with medium salinity (≈2.5 mS/cm) and low temperature (8–12 °C), represented by the cold water springs, Ferrugineuse and Magnésienne. This group with low-Cl contents is representative of waters circulating inside gypseous formations. The last group is characterized by the superficial groundwater of the Bon Nant Quaternary filling. Temperatures vary in function of the seasons (4–12 °C) and the salinity is lower than 0.5 mS/cm. Before the exploitation of Lépinay, the Gontard well and Sulfureuse spring had a higher temperature and mineralization close to the current thermal component.
Caractéristiques chimiques et isotopiques des eaux thermales et froides de Saint-Gervais-les-Bains. Les données des eaux les plus chaudes des sites de Lavey-les-Bains et de Val d’Illiez en Suisse ont été ajoutées afin de comparer leurs caractéristiques chimiques et isotopiques par rapport aux eaux de Saint-Gervais-les-Bains. Les eaux thermales de Lavey-les-Bains et de Val d’Illiez proviennent respectivement d’écoulement profond dans la massif cristallin des Aiguilles Rouges et dans la couverture sédimentaire autochtone. Pour ces deux sites, les données sont documentées dans Sonney (2010), thèse de doctorat en préparation. Les concentrations des espèces chimiques dissoutes sont données en mg/L.
| Site name | ||||||||||||
| Saint-Gervais-les-Bains | – | – | – | – | – | – | – | – | – | Lavey-les-Bains | Val d’Illiez | |
| Latitude | 6°42′22″ | – | – | – | – | – | – | – | – | – | 7°01′06″ | 6°53′60″ |
| Longitude | 45°53′48″ | – | – | – | – | – | – | – | – | – | 46°12′14″ | 46°12′27″ |
| Elevation (a.m.s.l.) | 620 | – | – | – | – | – | – | – | – | – | 417 | 790 |
| Sampling point | Lépinay | De Mey Est | De Mey Ouest | F99 | Gontard | Sulfureuse | Ferrugineuse | Magnésienne | Cold spring | Bon Nant | P600 | F3 |
| Type of sampling point | Well | Well | Well | Well | Well | Spring | Spring | Spring | Spring | River | Well | Well |
| Depth (m) | 101.5 | 196 | 207 | 99 | 7.5 | 595 | 120 | |||||
| Flow rate (L/s) | 3.6 | 1.5 | 0.8 | 0.3 | <0.2 | <0.02 | 1 | 2 | <0.1 | >100 | 5 | 19.5 |
| Sampling date | 16.07.2008 | 17.07.2008 | – | – | – | – | 16.07.2008 | – | 17.07.2008 | – | 12.09.2006 | 15.06.2009 |
| Meas. temperature (°C) | 39.0 | 35.8 | 27.4 | 11.7 | 20.1 | 9.8 | 10.5 | 10.5 | 10.8 | 12.0 | 65.2 | 27.9 |
| TDS (mg/L) | 4245 | 4821 | 3101 | 1778 | 1621 | 329 | 2369 | 2470 | 736 | 287 | 1428 | 1764 |
| pH | 6.93 | 6.94 | 7.06 | 7.06 | 7.12 | 7.6 | 6.91 | 7.01 | 7.32 | 8.03 | 7.70 | 7.2 |
| Li | 6.7 | 8.7 | 4.6 | 0.28 | 1.4 | 0.01 | 0.028 | 0.022 | 0.046 | 0.001 | 3.7 | 0.10 |
| Na | 992 | 1157 | 609 | 49 | 212 | 4.1 | 11.3 | 12.4 | 10.1 | 3.4 | 376 | 20.3 |
| K | 29.9 | 32.9 | 17.8 | 3.0 | 8.6 | 1.9 | 1.8 | 1.8 | 2.1 | 1.1 | 11.5 | 1.32 |
| Mg | 29.4 | 31.6 | 40.7 | 63.0 | 35.7 | 10.1 | 68.7 | 66.5 | 27.3 | 7.2 | 1.5 | 76.7 |
| Ca | 268 | 302 | 268 | 341 | 210 | 65.7 | 612 | 629 | 147 | 61 | 56.7 | 394.9 |
| Sr | 10.9 | 13.4 | 10.4 | 10.0 | 7.1 | 2.1 | <0.5 | <0.5 | 4.1 | <0.5 | 2.2 | 14.4 |
| F | 3.02 | 3.65 | 2.83 | <0.1 | 1.14 | 0.1 | 0.21 | 0.30 | 0.20 | 0.04 | 6.1 | 1.73 |
| Cl | 585 | 1167 | 584 | 48.2 | 129 | 4.4 | 16.8 | 18.3 | 10.2 | 4.0 | 242 | 4.0 |
| Br | 4.1 | 16.6 | 6.5 | 0.102 | 0.68 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 1.3 | <0.5 |
| SO4 | 2000 | 1787 | 1283 | 993 | 776 | 95.2 | 1352 | 1428 | 324 | 99.3 | 577 | 1111 |
| HCO3 | 272 | 235 | 251 | 257 | 221 | 141 | 301 | 306 | 204 | 106 | 87.4 | 127 |
| SiO2 | 39.9 | 44.5 | 20.5 | 12.3 | 16.9 | 5.1 | 5.9 | 6.8 | 7.5 | 4.2 | 65.7 | 12.8 |
| Ionic balance (%) | -1.5 | -2.9 | -3.2 | -3.1 | -0.5 | -0.4 | 4.5 | 3.0 | -1.0 | -1.1 | 1.2 | 2.9 |
| D H2O (‰) | −90.1 | −93.4 | −88.6 | −84.1 | −84.6 | −80.5 | −81.6 | −82.0 | −80.1 | −82.0 | −97.1 | −95.7 |
| 18O H2O (‰) | −12.51 | −13.01 | −12.28 | −11.53 | −11.80 | −11.42 | −11.31 | −11.17 | −11.33 | −11.27 | −13.15 | −13.19 |
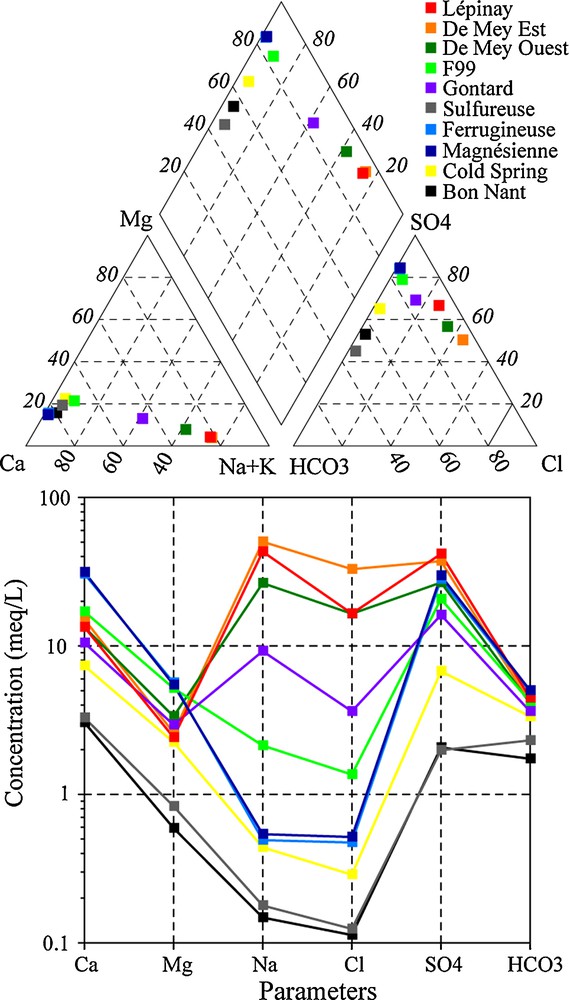
Piper and modified Shoeller diagrams for waters in the Saint-Gervais-les-Bains hydrothermal area according to data collected during the new sampling campaign in 2008. Locations of the samples are shown in Fig. 2 except for the Cold Spring, which is located close to the Gontard well.
Diagrammes de Piper et de Shoeller modifiés pour les eaux dans de la zone hydrothermale de Saint-Gervais-les-Bains d’après les données issues de la nouvelle campagne d’analyse en 2008. La localisation des captages est donnée sur la Fig. 2 sauf pour la Source Froide qui est localisée à proximité du puits Gontard.
6 Mixing processes
The interpretation of the correlations of the physico-chemical parameters allowed the identification of the mixing processes between three end-member components, but all the plots correlated using chloride and calcium are represented in Fig. 4. Mixing processes are different for the two deep aquifers. For the Permian aquifer crossed by the De Mey wells, thermal waters are slightly diluted by the Bon Nant groundwater but considerably by a Ca-SO4 and low-Cl water circulating in the Permian quartzites, which is similar to that of the Ferrugineuse and Magnésienne springs. The Ca/SO4 ratio of this cold water is aligned on the line of gypsum dissolution. For this mixing system, the dilution of the saline thermal end-member is accompanied by a significant decrease of temperature, TDS, alkaline ions and chloride (Fig. 4). On the contrary, calcium and magnesium considerably increase whereas sulphate slightly decreases because these two components have high sulphate concentrations. Thereby, this process indicates mixing between a Na-SO4 and high-Cl thermal water from the Aiguilles Rouges basement with a cold Ca-SO4 and low-Cl water circulating in the autochthonous sedimentary cover (Fig. 5).
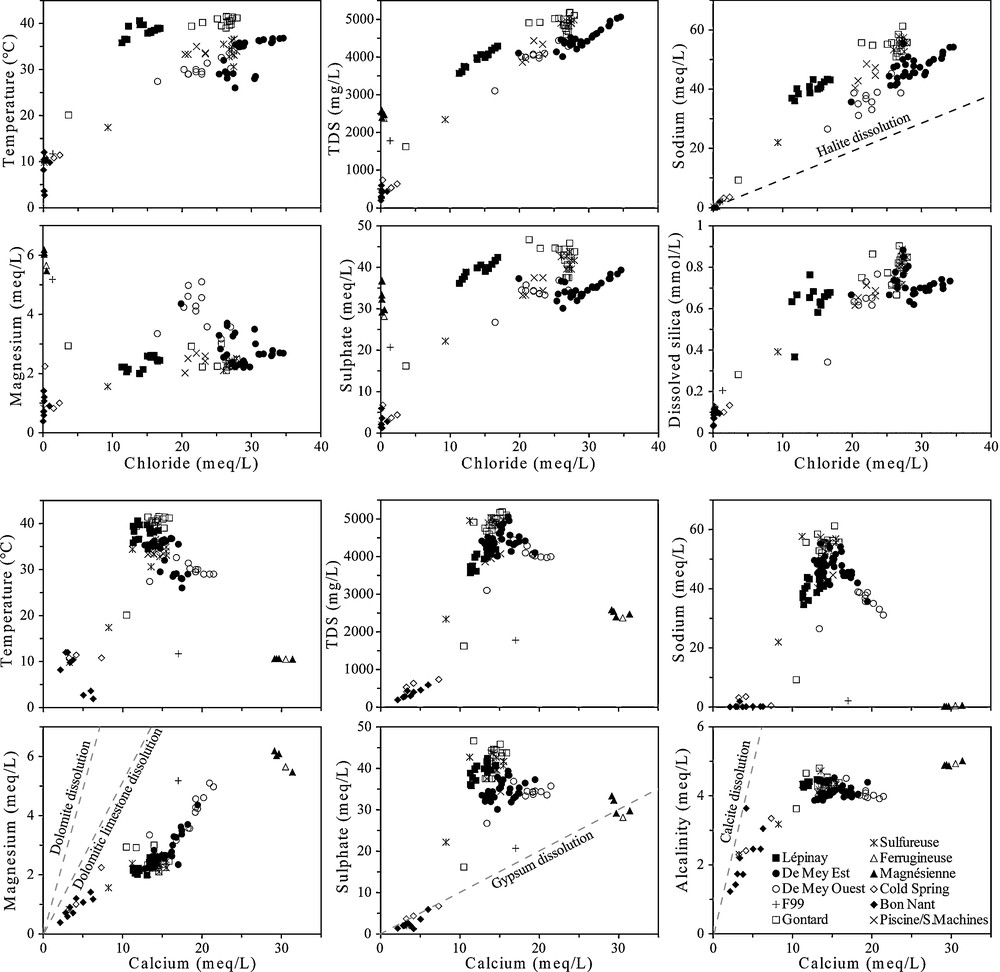
Plots of physico-chemical parameters versus chloride and calcium content in waters for all existing data. The Piscine/Salle des Machines sampling points were not found on the site during the sampling campaign in 2008, but their historical data were taken into account.
Graphiques des paramètres physicochimiques, avec les teneurs en chlorure et le calcium dans les eaux pour toutes les données existantes. Les points d’échantillonnage Piscine et Salle des Machines n’ont pas été retrouvés en 2008, mais leurs données historiques ont été prises en compte.
Concerning the aquifer in the zone of imbricated structures (Upper-Middle Trias) crossed by Lépinay and partially by F99 (Fig. 5), chemical processes seem to be more complicated because pumped waters have half the concentration in chloride with temperatures around 39 °C. The plots correlated against chloride show that Lépinay seems to be a mixture between Gontard and a low-Cl water, which would have a temperature around 35–40 °C. This assumption calls upon an additional thermal end-member rich in sulphate and poor in chloride resembling to a Triassic water type. Lépinay and Gontard waters are also diluted with the Bon Nant groundwater. Currently, the Lépinay production rate generates a decrease of the ascending thermal pressure in Gontard, supporting Bon Nant groundwater inflow in the Gontard well (20 °C and 1.6 g/L). The only analysis available from the unused borehole F99 shows a mixing between the Bon Nant groundwater, the Ca-SO4 and low-Cl cold water with an almost negligible proportion of thermal water.
Correlations in the water between stable isotopes versus chloride and temperature were tested but not illustrated in this paper. Results highlighted the impact of seasonal climatic variations in the Bon Nant groundwater. In summer, the snow-melt brings surface water from higher elevation whereas in winter, precipitations higher than 1500 m are mostly stored. Generally, the two cold components have an infiltration area lower than the thermal end-member.
7 Water–rock interactions
7.1 Chemical equilibrium
Fluid-mineral equilibria for Lépinay, De Mey Est and Magnésienne were computed with PHREEQC (Parkhurst and Appelo, 1999). Generally, negative SI values were found for feldspars and chlorite with tested aluminium contents lower than 10 μg/L. Lépinay, De Mey Est and Magnésienne are strongly over-saturated with respect to K-mica and kaolinite. Waters are clearly in equilibrium with calcite and aragonite, which are controlled by their solubility in relation to temperature and slightly undersaturated with respect to dolomite. They are also undersaturated in gypsum and anhydrite, except for Magnésienne, which is in quasi-equilibrium. Calculated SIs of quartz are positive between 0.2 and 0.7 for waters whereas SIs of chalcedony for the two thermal waters are closer to equilibrium.
7.2 Origin of the Na-Cl components
Cl/Br ratios are useful indicators to define if the salinity comes from seawater brines in crystalline domain (Aquilina et al., 1997; Bottomley et al., 1994; Louvat et al., 1999; Pauwels et al., 1993; Richard, 2000; Stober and Bucher, 1999a, b) or from dissolution of halite and gypsum rich in halite. For the seawater, the Cl/Br molar ratio is about 655 whereas it increases (>1000) for groundwater leaching halite-rich formations. Residual seawater brines after the precipitation of halite have a Cl/Br molar ratio lower than 655 and down to 80, and shallow groundwaters of low-TDS have a ratio around 100–300 (Alcalá and Custodio, 2008). In Figs. 6 and 7, two groups of Alpine thermal waters were shown and compared to SGLB: first, the waters circulating mostly in acid crystalline rocks and second the waters circulating in Triassic formations, sometimes heavily influenced by dissolution of evaporites. SGLB waters belong to the crystalline environment (Fig. 6) with Cl/Br molar ratios lower than the seawater value. On the contrary, Alpine thermal waters in Triassic environment are influenced by the dissolution of halite with ratios up to 1000, sometimes reaching 6000. This strongly suggests the origin of Na-Cl components from fluid inclusions in the Aiguilles Rouges basement with a chemical composition close to the present-day seawater and diluted in variable proportions by meteoric water. Moreover, Na-Cl does not originate from the autochthonous Triassic cover because the Triassic evaporite contains little halite at SGLB (low-Cl content in cold water) and also at Val d’Illiez in Switzerland (Bianchetti et al., 1992). According to Arthaud and Dazy (1989), evidence of halite is very scarce in evaporitic sequences of Triassic age in the Alps. They also concluded that salt springs in the Western Alps resulted from mixing, to various degrees, of recent meteoric water and old deep-seated brines. For the external crystalline massifs, Arthaud and Dazy (1989) evoked that infiltration of meteoric water in fractures and faults from crystalline reliefs reach great depths (4 to 5 km) where they enter into the brine reservoirs and thrust faults. The uprising of the mixture towards the low-elevated points of crystalline massifs is supported by strike-slip fault systems such as the ones observed for SGLB.
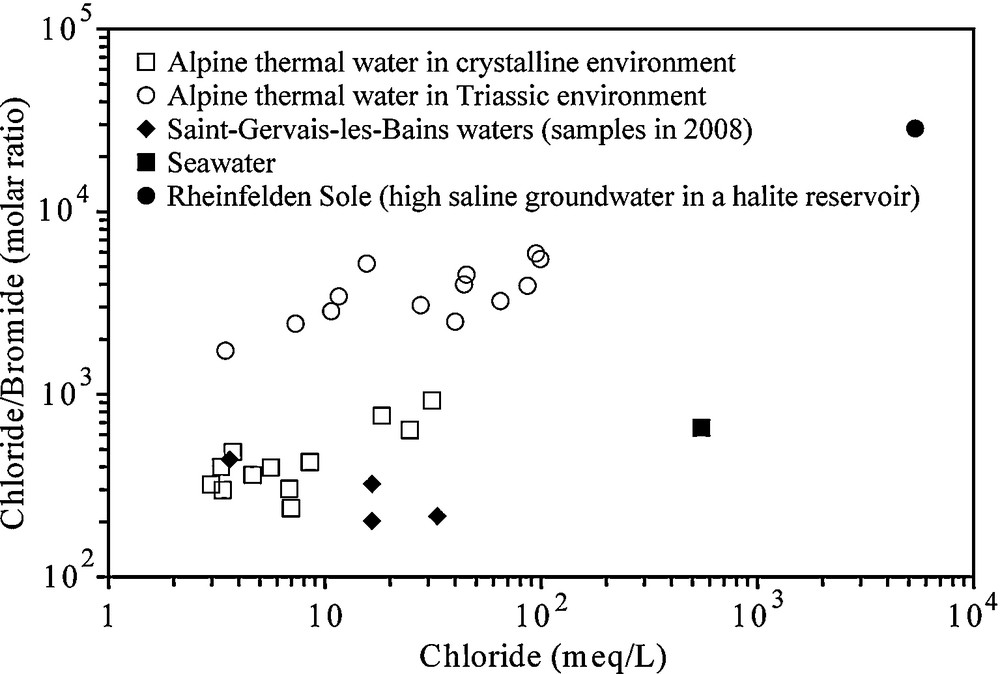
Plot of Cl content versus Cl/Br molar ratio in Alpine thermal waters in relation with their geological setting (Sonney and Vuataz, 2010). All selected waters have a chloride content higher than 100 mg/L. The Rheinfelden brine is a subthermal spring in the north of the Molasse Basin in Switzerland, which dissolves halite rocks in Keuper (Sonney and Vuataz, 2008).
Graphique des rapports Cl/Br en fonction de Cl pour les eaux thermales des Alpes, en relation avec leur contexte géologique (Sonney and Vuataz, 2010). Toutes les eaux sélectionnées ont des teneurs en Cl supérieures à 100 mg/L. La saumure de Rheinfelden est une source subthermale localisée au nord du bassin molassique suisse, qui dissout la halite du Keuper (Sonney and Vuataz, 2008).
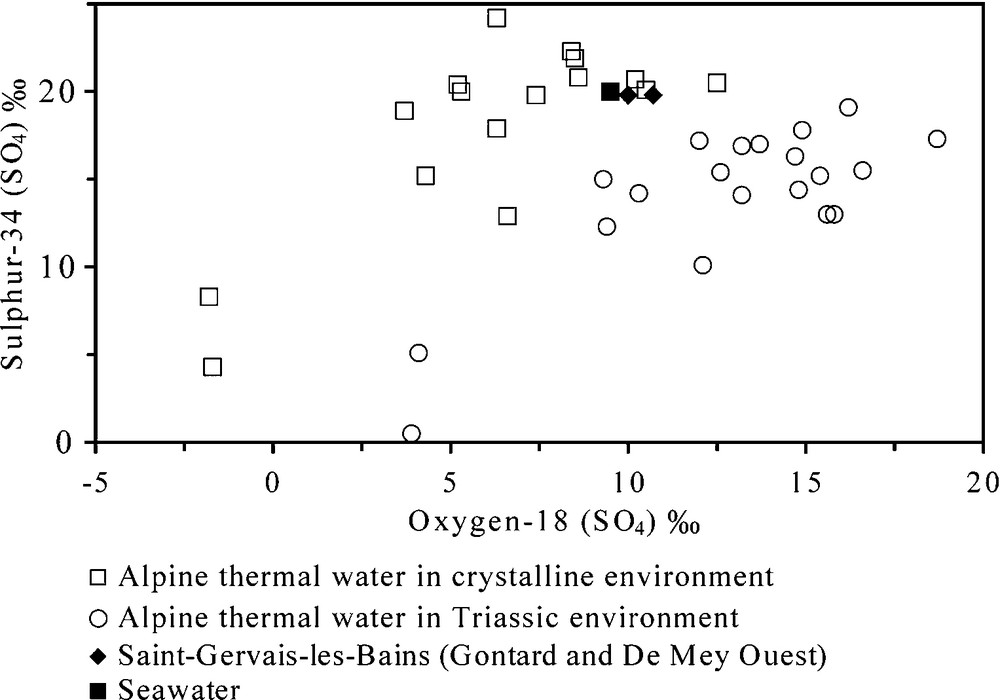
Plot of 18O (SO4) and 34S (SO4) in Alpine thermal waters in relation to their geological setting.
Graphique des 18O (SO4) et 34S (SO4) dans les eaux thermales des Alpes en relation avec leur contexte géologique.
The Na/Cl molar ratios for Lépinay and De Mey are, respectively, close to 2.6 and 1.6. In the two cases, the molality of sodium is higher than chloride and it excludes any contribution by hydrolysis of biotite (Edmunds et al., 1985). It suggests however a second origin of sodium from fluid-rock reactions. The excess of sodium probably comes from the ion exchange process between sodium and calcium with schists of the Upper-Middle Trias or with micaschists in the basement, which has already been hypothesized by Vuataz (1982). For the thermal component, the molality of sulphate is higher than calcium and therefore, part of the calcium from the dissolution of gypsum is exchanged for sodium. Moreover, part of sodium could also come from the plagioclase weathering in the basement due to the long-time flow path (Henley et al., 1984). The importance and significance of plagioclase dissolution for the evolution of groundwater in crystalline rocks have been demonstrated by field studies from other basement complexes (Gascoyne and Kamineni, 1994; Sonney and Vuataz, 2009).
7.3 Origin of the SO4 component
The origin of sulphate content in waters of SGLB is discussed using the sulphur-34 and oxygen-18 (in sulphate) data of Gontard and De Mey Ouest compared to other thermal waters in the Alps. For thermal waters leaching Triassic evaporites in the Alps, values are higher in oxygen-18 and lower in sulphur-34 than waters in the crystalline environment dominating (Fig. 7). The two measurements in 1990 in Gontard and De Mey Ouest are close to the values of the crystalline environment and to the present-day seawater. Consequently, it can be assumed that sulphates of Gontard and De Mey Ouest result from the dissolution of gypsum or anhydrite veins in the Aiguilles Rouges crystalline basement, coupled to a lesser extent with the leaching of residual brines and oxidation of sulphides in crystalline rocks. Therefore, part of the dissolved sulphate in Lépinay water seems to come from fluid-rock reactions with gypsum in the Upper-Middle Triassic. New analyses will be needed to confirm these assumptions.
8 Regional deep flow system
Arthaud and Dazy (1989) evoked the role of thrust faults of external crystalline massifs guiding deep fluids towards the low-elevated points of these massifs such as SGLB and Lavey-les-Bains for the Aiguilles Rouges Massif. Interpretations based on geological, hydrogeological and hydrochemical methods have enabled the building of a regional conceptual model of the different flow paths (Fig. 1). The two other hydrothermal sites of Lavey-les-Bains and Val d’Illiez were added to the model for comparison of their geochemical type in relation to rocks crossed by the flow path. Pumped waters at Lavey-les-Bains in the basement have similar characteristics than SGLB, Na-SO4 waters rich in chloride (Sonney and Vuataz, 2009). Waters at Lavey-les-Bains are hotter than SGLB (65 °C against 40 °C) but less mineralized (1.5 g/L against 4.8 g/L). This temperature difference probably results from deeper circulations with a deepening of the thrust fault of the Aiguilles Rouges basement on the Infra-Aiguilles Rouges towards the north-east. In the Aiguilles Rouges Massif, various rock alterations, quantity of residual seawater brines and residence times can explain the salinity differences.
The Ca-SO4 and low-Cl cold water at SGLB certainly has an infiltration area at the bottom of the Mont Joly Massif in Triassic formations belonging to the autochthonous cover. This cold end-member with gypsum dissolution has the same chemistry as the thermal water at Val d’Illiez in Switzerland (see location in Fig. 1). Thermal water at Val d’Illiez around 30 °C results in a flow path in the autochthonous cover of the Aiguilles Rouges basement in the Salanfe Lake area (Bianchetti et al., 1992).
The study of the elevation of the infiltration area is based on data of water stable isotopes and on the equation of Kullin and Schmassmann (1991). For De Mey Est isotope data, which are close to those of the Lavey-les-Bains thermal component, the calculation leads to an average elevation around 1700–2100 m, whereas results for the Ca-SO4 and low-Cl end-member water at SGLB indicate a lower elevation around 1100–1300, which coincides with infiltration areas from the Mont Joly Massif. Values for Lépinay lie between De Mey Est and the cold end-member due to important mixing. Finally, values of SGLB, Lavey-les-Bains and Val d’Illiez thermal waters are close to the World Meteoric Water Line, as all the Alpine thermal waters.
9 Reservoir temperature
Traditional and multi-component geothermometers have been applied to estimate the reservoir temperature. In order to use this method it has been assumed that there is a fluid-mineral equilibrium as well as an equilibration between mineral assemblages at depth in the reservoir, and stabilization of dissolved chemical elements with the uprising before mixing. The first geothermal assessments of the reservoir temperature at SGLB gave values of 70 to 100 °C based on chemical geothermometers (Vuataz, 1982). The isotope geothermometer, using the fractionation of oxygen-18 in water and sulphate (Lloyd, 1968; Mizutani and Rafter, 1969), indicated temperatures around 60 °C for Gontard and De Mey Ouest.
Based on the total dissolved silica analyzed in 2008, calculated temperatures with the chalcedony geothermometers (Arnórsson et al., 1983; Fournier, 1977) for Lépinay and De Mey Est are about 67 °C and 62 °C, respectively. For reservoir temperatures lower than 120–160 °C, the chalcedony geothermometer is usually more adapted than the quartz geothermometer (Arnórsson, 1983), due to aqueous phase equilibrium depending on reservoir temperature (Arnórsson, 1975; Nicholson, 1993). Results using quartz from relations by Fournier (1977) and Truesdell (1976) are not presented in this study. Moreover, the silica geothermometers are strongly affected by mixing processes with cold waters containing low silica concentrations and silica re-equilibration during the upflow. Consequently, calculations made on the two thermal waters provide an underestimation of the temperatures.
Cationic geothermometers were also tested and gave results largely overestimating the reservoir temperature (>100 °C). Application of these geothermometers is more suitable for high-enthalpy systems (>150 °C), containing waters reaching equilibrium with feldspars (Fournier, 1981). Therefore, cationic geothermometers were not used in this study.
10 Groundwater residence time
The only information allowing studying the residence time in SGLB is tritium data from Vuataz (1982) and several unpublished reports during the 1980s and 1990s. For Alpine hydrothermal systems in crystalline rocks, with relatively large residence times (older than nuclear weapons tests), tritium can be used to identify mixing with recent waters. Tritium data are in the range 0–5.3 TU in 1990 for De Mey (28–32 °C) and Gontard (39–41 °C) (Fig. 8). In 1990, the cold end-member for Gontard is represented by the Bon Nant groundwater according to the mixing trend passing by Sulfureuse, Cold spring and Bon Nant. The relation is different for De Mey because data in 1990 indicated preferential mixing with Ca-SO4 and low-Cl cold water from the Mont Joly Massif having probably low tritium content in 1990.
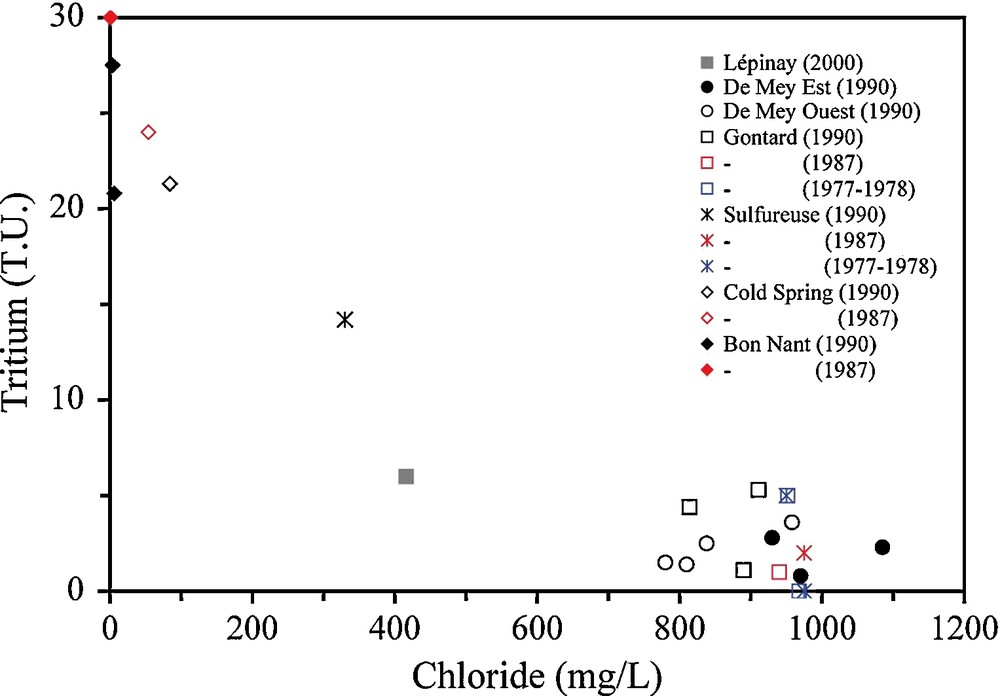
Chloride–tritium plot for SGLB waters. Each color of symbol represents one period data come from Vuataz (1982) and unpublished reports.
Graphique chlorure–tritium pour les eaux de Saint-Gervais-les-Bains. Chaque couleur représente une période. Les données proviennent de Vuataz (1982) et de rapports non publiés.
11 Conclusions
The combination of the geological, hydrogeological and geochemical studies made it possible to define chemical processes between an ascending Na-Cl thermal water and shallow groundwater at SGLB. This Na-Cl component is certainly related to the remobilization of trapped saline water by deep flow systems reaching the thrust fault of the Aiguilles Rouges Massif on the Infra-Aiguilles Rouges Massif. The origin of the deep flow system probably comes from infiltration and circulation within the fractures of the Aiguilles Rouges basement. Based on the above discussions, the presence of two aquifers with their own hydraulic connection and mixing process below the Quaternary deposits can be deciphered. Concerning the De Mey boreholes (27–36 °C), uprising Na-SO4 and high-Cl thermal water in the Lower Trias-Permian quartzites from the Aiguilles Rouges basement is mostly diluted by a Ca-SO4 and low-Cl end-member circulation in the autochthonous cover. The leaching of residual brines or fluid inclusions is responsible of the Na-Cl fingerprint due to Cl/Br molar ratios at SGLB lower than 655. The Na-Cl end-member does not result from halite dissolution in Triassic formations. Moreover, halite is not present in gypseous formations in SGLB because the Triassic cold water has a low-Cl concentration (< 20 mg/L).
Water–rock interactions occur during the upflow via north–south strike-slip faults in the basement and later on in the autochthonous cover. Data for De Mey Est borehole show that gypsum dissolution is occurring with cationic exchanges involving Na and low-temperature Mg dissolution from dolomite in the Triassic formations. For the imbricated structure zone crossed by the Lépinay well (39 °C), thermal waters are strongly mixed with a low-Cl water and gypsum dissolution also occurs.
Reservoir temperature for the thermal end-member was estimated up to 65 °C but not exceeding 100 °C. All groundwater end-members originate through circulation of meteoric waters but from different infiltration areas. The thermal end-member has the same infiltration area range (1700–2100 m) than the hydrothermal systems of Lavey-les-Bains and Val d’Illiez in the Aiguilles Rouges Massif. It is higher than the calculated infiltration area for the Ca-SO4 and low-Cl water at about 1100–1300 m, corresponding to flow paths from the Mont Joly area.
Acknowledgments
The authors want to thank Thierry Coffinet, director of the spa of Saint-Gervais-les-Bains, and Maud Durand, responsible of the Hygiene Service, for their agreement to collect and use the existing data. We also express our sincere gratitude to them for their reception and for the authorization to sample all the boreholes and springs. Our thanks are also addressed to François Iundt who helped us to collect the historical data, to Clémentine Schurmann who performed some structural fieldwork, and to Chantal Crisinel who enhanced the English syntax of the final version. We also thank three anonymous reviewers for their comments and suggestions to improve this paper.


