1 Introductıon
Industrial and municipal wastewater, agricultural activities and atmospheric inputs are the main routes by which chemical contaminants enter the marine environment (Gherras Touahri et al., 2016). Organisms in aquatic environments are generally exposed to a complex mixture of chemicals, including the parent compounds and their transformation products that cause multiple damages to organisms, populations and ecosystems (Bolognesi and Cirillo, 2014). Therefore, monitoring approaches should have an integrative character combining chemical and biological evaluations with abiotic and biotic parameters (Schettino et al., 2012). Bivalves belong to the first-choice species as bioindicators for environmental and chemical stress. They are sentinel benthic organisms living as filter-feeders and exposed to different environmental compartments (Helmholz et al., 2016). Due to their wide distribution, resistance to variable environmental conditions, which predispose them to the direct absorption and accumulation of a wide spectrum of waterborne chemicals, they are prolific tools for the biomonitoring of chemical pollution (Boillot et al., 2015). Moreover, the possibility to measure several biochemical, cellular and physiological biomarkers makes bivalves suitable organisms for investigating the effects of chemical pollutants (Regoli et al., 2014). Biomarkers include a variety of molecular, cellular, or physiological alterations measurable in organisms in response to pollutants or other environmental stress factors (Moore et al., 2006). The glutathione S-transferase (GSTs) are a multiple-enzyme family involved in phase-II detoxification processes and are used as biomarkers of exposure to several groups of pollutants, including organochlorine pesticides, PCBs and petrochemical products in invertebrates (Hoarau et al., 2001; Lima et al., 2007). They have been recently identified as a suitable biomarker for monitoring chemical pollution in highly productive marine coastal ecosystems (Vidal-Liňán, 2010). Acetylcholinesterase (AChE) is involved in the hydrolysis of the neurotransmitter acetylcholine. The measurement of AChE inhibition in marine organisms has been widely used as an indicator of environmental contamination by organophosphate and carbamate pesticides (Blaise et al., 2016; Magni et al., 2006). Metallothioneins are low-molecular-weight, cysteine-rich cytosolic proteins able to bind to many metals, such as Ag, Cd, Co, Cu, Hg, Ni, Pb, Pd and Zn (Frank et al., 2008; Ng et al., 2007). In general, MTs are involved in different biological processes, i.e. Homeostasis of essential metals, detoxification of toxic metals and cell protection against oxidative stress (Geffard et al., 2005; Ng et al., 2007).
Donax trunculus L. is an edible mollusk species found in high densities in the sand beaches of the Gulf of Annaba in Algeria (Hafsaoui et al., 2016) and widely used as bioindicator species for the assessment of marine pollution through the measurement of several biomarkers (Amira et al., 2011; Sifi et al., 2013; Soltani et al., 2012; Tlili et al., 2013). Their habitats are exposed to several pollutants from different sources (Abdennour et al., 2000, 2004; Beldi et al., 2006; Merad and Soltani, 2017). Two rivers (the Seybouse River and the Mafrag River) drain into the Gulf of Annaba and receive agricultural water discharges from cereal farming, market gardening, and arboriculture, as well as domestic releases from important conurbations (Khelifi-Touhami et al., 2006) and untreated sewage (Abdennour et al., 2000). Metallic pollution was major, and cadmium one of the most common pollutants in our area (Belabed et al., 2013; Beldi et al., 2006; Larba and Soltani, 2014; Merad et al., 2016).
Sediments are an important sink for metals and other pollutants (Belhadj et al., 2017); they have been described as a non-point source of metals and sediment-bound metals that can be released into overlying waters and adversely affect aquatic organisms (Wang et al., 2004). Benthic invertebrates are known to take up and accumulate metals, both essential and nonessential, from water and sediment as well as from their food supply (Buzzi and Marcovecchio, 2016; Wang and Fisher, 1999).
In this context, the main objective of this work was to study the in situ seasonal responses of some biochemical biomarkers (GST, AChE, MTs) in D. trunculus, a locally prevalent edible mollusk sampled at two sites in the Gulf of Annaba: a polluted site (Sidi Salem) and a relatively clean site (El Battah). In addition, to evaluate the metallic pollution reported previously (Abdennour et al., 2000, 2004; Beldi et al., 2006; Larba and Soltani, 2014; Merad et al., 2016), the concentrations of heavy metals (Cd, Cu, Pb, Zn, Mn and Fe) were determined in sediments originating from these two sites.
2 Materials and methods
2.1 Study area
The Gulf of Annaba is located in the Northeast of Algeria. It is limited by the Rosa Cap (8°15′E and 36°38′N) to the east and by the Gard Cap (7°16′E and 36°68′N) to the west. The El Battah site (36°50′N–8°50′E), is located about 30 km to the east of Annaba far from any human activities and is considered as a relatively clean site. Sidi Salem site (36°50′N–7°47′E), located about 1 km to the east of Annaba city, receives industrial and domestic wastewater, and is considered as the polluted area (Fig. 1).
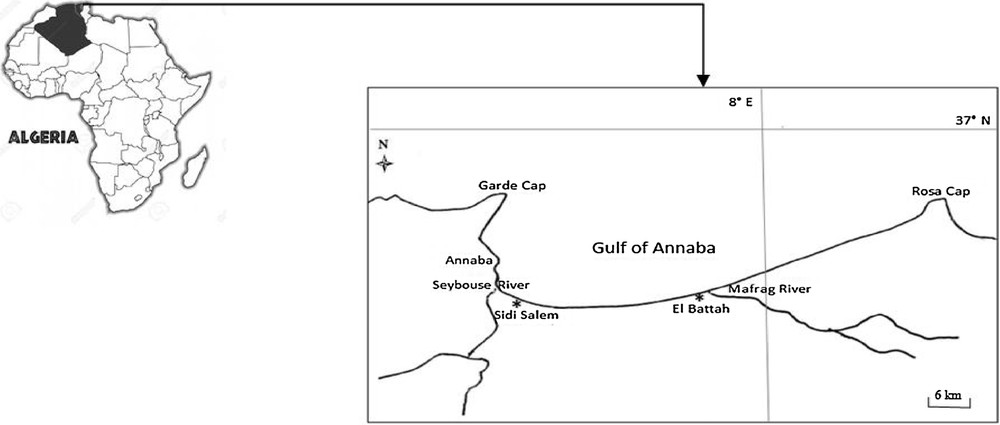
Location of the two sampling sites in the Gulf of Annaba: Sidi Salem and El Battah.
2.2 Samples collection
Mollusk bivalves (D. trunculus) with the same range shell length (25 ± 1 mm) were collected seasonally in the of autumn of 2014 (September), and in the winter (January), the spring (April), and the summer (July) of 2015 from two sampling sites (El Battah and Sidi Salem) and transferred to the laboratory.
Sediment samples were also collected at the two sampling sites in winter 2015 (January) for metal analysis. They were taken manually with a grab near the bivalves collecting points over a depth not exceeding 0.5 m (5–10 cm). In the laboratory, they were put to dry at room temperature until constant weight.
2.3 Biomarkers analysis
The mantle of six bivalves was dissected and samples were prepared for biomarker analyses. GST activity was measured according to Habig et al., 1974, using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. The activity rate was expressed as μmol/min/mg protein. AChE activity was determined following the procedure of Ellman et al., 1961, with the use of acetylthiocholine (ASCh) as substrate. The activity was expressed as μmol/min/mg protein. The protein content was evaluated according to Bradford (1976) using serum albumin as standard (BSA, Sigma). MTs were determined according to the method of Viarengo et al., 1997, previously described (Merad et al., 2016). The mantles samples were homogenized in three volumes of 20 mM Tris-sucrose buffer with 0.1% of β-mercapto ethanol and 0.5 mM of PMSF, followed by ethanol/chloroform extraction, after incubation with DTNB. The levels of MT (MT-SH) were calculated assuming the relationship: 1 mol MT-SH = 20 mol GSH and expressed as μg of MTs per mg of fresh weight (FW).
2.4 Metal analysis in sediments
The total metal content (Cd, Cu, Fe, Mn, Pb and Zn) in dry sediments were determined by atomic absorption spectrometry (AAS) after high-pressure microwave digestion with nitric acid following previously optimized methodology (Almeida et al., 2004). Depending on the metal levels, either AAS with flame atomization (PerkinElmer) or AAS with electrothermal atomization applying a Zeeman effect background correction system (ETAAS) (PerkinElmer4100 ZL, coupled with an AS-70 autosampler) was used. Aqueous standard solutions were used for external calibrations as no matrix effects were detected (checked by doping samples with known amounts of each metal and calculating recovery values). Blank solutions were prepared, following the respective sample treatment. Three independent replicates of each sample were prepared and analyzed, after blank subtraction, mean values and respective standard deviations were calculated. Concentration of each metal was related to sediment dry weight (DW).
2.5 Statistical analysis
Normality of data was verified using the Kolmogorov–Smirnov test and homogeneity of variances was checked by Levene's test. The results are presented as mean ± standard deviation (SD). The comparison of the mean values between sites was made by Student's t-test. The combined effects of seasons and sites were investigated using two-way analysis of variance (ANOVA). All statistical analyses were performed using MINITAB Software (Version 16, PA State College, USA). Differences were considered as significant when p < 0.05.
3 Results
3.1 Glutathione S-transferase activity
The results for seasonal variation in GST activity in the mantle of D. trunculus are presented in Fig. 2. GST activity increased significantly in winter (p < 0.01) at Sidi Salem compared to El-Battah. However, there was no significant difference (p > 0.05) during autumn and spring. Furthermore, the highest activity of GST was observed during autumn (7.44 ± 1.56 μmol/min/mg proteins) in El Battah and during spring (7.67 ± 0.72 μmol/min/mg proteins) at Sidi Salem. A two-way ANOVA revealed a significant effect of season (F1, 112 = 12.28, p < 0.001) and for sites (F3, 112 = 3.94, p < 0.05).
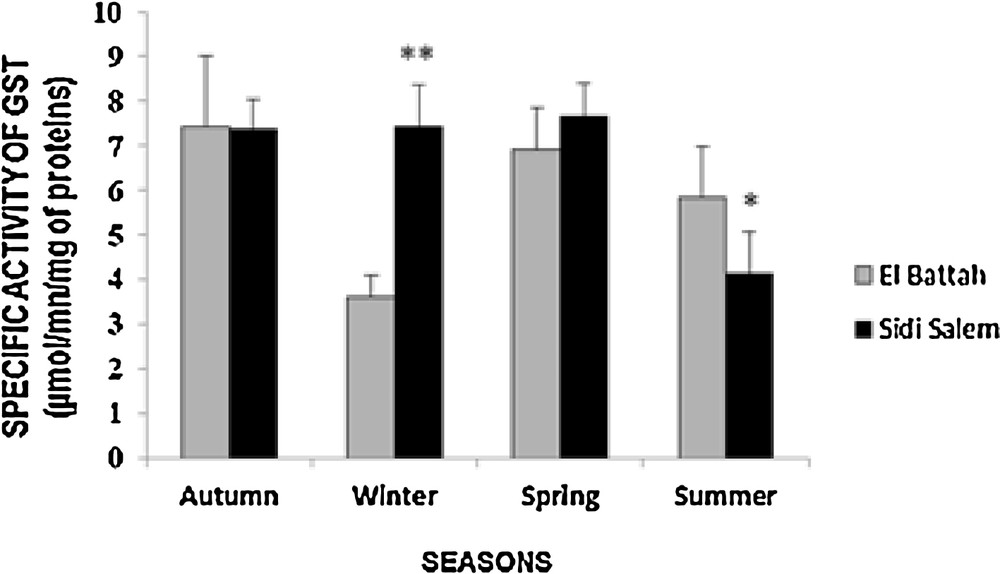
Seasonal variations of specific activity of glutathione S-transferase (GST) in D. trunculus from two sites in the Gulf of Annaba (autumn 2014, winter, spring and summer 2015) (mean ± SD; n = 6; *: significant difference at p < 0.05; **: significant difference at p < 0.01).
3.2 Acetylcholinesterase activity
AChE activity in the mantle of D. trunculus was significantly lower in Sidi Salem compared to El Battah in autumn (p < 0.001), winter (p > 0.05), (p < 0.001) and summer (p < 0.01) (Fig. 3). However, no significant difference was observed in the spring (p > 0.05). The lowest values were recorded in autumn for the two sites (36.25 ± 2.90 μmol/min/mg proteins at Sidi Salem and 59.86 ± 5.1 μmol/min/mg proteins at El Battah). A two-way ANOVA indicated significant main effects of sites (F1, 112 = 28.51; p = 0.001) and seasons (F3, 112 = 56.49; p = 0.001).

Comparison of seasonal variations of specific activity of acetylcholinesterase (AChE) in D. trunculus from two sites in the Gulf of Annaba (autumn 2014, winter, spring and summer 2015) (mean ± SD; n = 6; *: significant difference at p < 0.05; **: significant difference at p < 0.01;***: significant difference at p < 0.001).
3.3 Metallothionein levels
A significant (p < 0.001) increase in MTs levels was observed at Sidi Salem compared to El Battah during all four seasons (Fig. 4). A two-way ANOVA revealed significant (p < 0.001) effects of site (F1,32 = 715.17; p = 0.000) and season (F3,32 = 59.68; p = 0.000).

Comparison of seasonal variations of metallothionein levels (μM/min/mg of proteins) in D. trunculus from two sites in the Gulf of Annaba (autumn 2014, winter, spring and summer 2015) (mean ± SD; n = 6; *: significant difference at p < 0.05; **: significant difference at p < 0.01; ***: significant difference at p < 0.001).
3.4 Metal concentrations in sediments
Table 1 reports the metal levels in sediments at El Battah and Sidi Salem. The comparison between the two sites showed significantly higher concentrations of Cd, Cu, Pb and Zn at Sidi Salem than at El Battah. On the contrary, Mn was higher at El Battah than at Sidi Salem and no significant difference between the two sites was observed for Fe.
Metal concentrations of Cd, Cu, Pb, Zn and Mn (μg/g DW) and of Fe (mg/g DW) in sediment collected from two sites in the Gulf of Annaba in the autumn of 2015.
| Site | Fe (μg/g) | Mn (μg/g) | Zn (μg/g) | Pb (μg/g) | Cu (μg/g) | Cd (μg/g) |
| El Battah | 5.9 × 103 ± 0.4 | 132 ± 7 | 19 ± 2 | 2.7 ± 0.1 | 0.95 ± 0.22 | 0.083 ± 0.005 |
| Sidi Salem | 5.8 × 103 ± 0.6 | 86 ± 10** | 27 ± 2* | 6.6 ± 0.2** | 1.7 ± 0.26* | 0.188 ± 0.004*** |
* Significant at p < 0.05.
** Significant at p < 0.01.
*** Significant at p < 0.001, respectively.
4 Discussion
Environmental pollution can be assessed by using sentinel organisms as bioindicators. Several molecular parameters, known as biomarkers, respond early to environmental stressors and alert before severe and irreversible damage appears in ecosystems (Nieto et al., 2010). In this study, the higher activity of GST observed at El Battah in autumn is probably associated with the restoration of reserves, the period of sexual rest and to food availability in this season. In Sidi Salem, GST activity was lower in summer; this decrease might be related to the spawning period of D. trunculus. According to Hamdani and Soltani Mazouni, (2011), D. trunculus from the Gulf of Annaba presented a period of sexual rest in autumn and two spawning periods (spring and summer). Similarly, a recent study on the population dynamics and secondary production of D. trunculus in the Gulf of Annaba showed a major increase in the bivalve's condition index in March/April, June/July and October (Hafsaoui et al., 2016). This reflected two main periods of gonad development and an increase in the level of stored reserves at the end of the reproductive period. Giarratano et al. (2011) also related the variation of GST activity to the reproductive cycle, where the maximum activity coincides with the restoration period and the gametogenesis, while the lowest values occurred during the spawning period. In El Battah, the lowest GST activity is recorded in winter when the temperature is low. Indeed, Benali et al. (2015) have recorded minimal GST activity in winter in Mytilus galloprovincialis collected from an unpolluted site on the western coast of Algeria.
In the present study, significantly higher GST activities were recorded in bivalves from Sidi Salem in comparison with those of El Battah considered as the reference site. The site of Sidi Salem is exposed to the waste products from complex FERTIAL specialized in the production of fertilizers and also to domestic and agricultural waste transported by the Seybouse River (Belabed et al., 2013). Previous studies have determined the concentration of heavy metals (Zn, Cu, Pb and Cd) in tissue (Beldi et al., 2006) and investigated the impact of pollution in D. trunculus from the Gulf of Annaba (Amira et al., 2011; Bensouda and Soltani-Mazouni, 2014; Hamdani et al., 2014; Sifi et al., 2013; Soltani et al., 2012). The same results have been reported by Tlili et al. (2013) in the Gulf of Tunis (Tunisia) where they found a significant increase in GST activity in D. trunculus collected bi-monthly from three polluted sites relative to a reference site. A higher GST activity is recorded in M. galloprovincialis collected in polluted sites compared to a reference site on the Spanish coast (Vidal-Liňán, 2010).
The seasonal variation of AChE activity in the mantle of D. trunculus showed a decrease in autumn–winter compared to spring–summer at both sampling sites. The natural variability in the activity of AChE is not directly related to the age, sex, or reproductive period of the organism, but to the seasonal variation of temperature, as the decrease of AChE activity is positively correlated with the seawater temperature. According to Bocquené and Galgani (2004), water temperature is the most important regulating factor for AChE activity. Furthermore, Pfeifer et al. (2005) reported the highest AChE activity levels in summer and the lowest in winter in Mytilus collected from the Southwest of the Baltic Sea. In the present study, inhibition of AChE was detected in bivalves from Sidi Salem compared to those from El Battah. Recent studies have shown that other types of pollutants such as metals, detergents and algal toxins and PAHs may also inhibit AChE activity (Grintzalis et al., 2012; Vidal-Linan et al., 2015). The region of Annaba is the most important touristic and economic coastal zone in eastern Algeria. It is impacted by various contaminants from urban, agricultural, harbor, and industrial activities (Abdenour et al., 2000; Soltani et al., 2012). The absence of significant differences in AChE activity observed in spring between the two studied sites could be related to the marine currents, which could contribute to the dilution of contaminants in sea water.
The MT concentrations in D. trunculus collected from the Annaba Gulf varied between each season with higher concentrations in summer and lower values in spring. Basal levels of MT in organisms have been shown to alter with season, reproductive state, water temperature (George and Olsson, 1994), salinity and dissolved oxygen levels (Viarengo et al., 1999). Seasonal or otherwise temporal variability in MT concentrations in bivalves has been observed in Mytilus galloprovincialis (Romeo et al., 2003; Viarengo et al., 1997), Ruditapes decussatus (Serafim and Bebianno, 2001), Corbicula fluminea (Baudrimont et al., 1997). Metallothioneins have been reported to be induced in different tissues of aquatic mollusks following exposure to various metals (Chandurvelan et al., 2015; Gillis et al., 2014; Pytharopoulou et al., 2011). MT levels are considered to reflect the bioavailability and toxic impacts of metals (Le et al., 2016). Our data showed an increase of MT levels in D. trunculus from Sidi Salem compared to El Battah, with the highest values recorded in summer. MT induction is stimulated by the presence of free metal ions in the cytosol (Dondero et al., 2005; Ivankovic et al., 2010). The cytosolic free metal ion concentration is probably one of the most important factors triggering MT induction (Falfushynska et al., 2013; Ivankovic et al., 2010). The relationship between metal and MT concentrations in bivalves depends on the concentration and chemical speciation of metals accumulated in the bivalves.
The analysis of heavy metals (Cd, Pb, Cu and Zn) in sediments was performed only in winter 2015; the data obtained give information on the level of pollution in the gulf, complete previous reports and show that the Gulf of Annaba remains contaminated by metals (Abdennour et al., 2000, 2004; Beldi et al., 2006; Larba and Soltani, 2014; Merad et al., 2017). The concentrations registered were higher at Sidi Salem than at El Battah, with the exception of Mn, which is considered an essential metal. Metal analysis in sediments complete the biochemical analysis and the elemental concentrations in sediments are highly dependent on the grain size (Horowitz and Elrick, 1988; Howari and Banat, 2001). As grain size decreases, trace element concentrations increase, reflecting changes in physical and chemical factors that affect trace elements (Forstner and Wiitmann, 1981; Horowrrz, 1985; Jones and Bowser, 1978). This may explain the highest metal concentrations in Sidi Salem, which is constituted of fine sand compared to El Battah, which is characterized by medium sand having an average diameter of 0.26 mm. Furthermore, the concentration of metals depends on the sources of sediments, the chemical characteristics of elements, the physicochemical conditions, and on complex reactions such as adsorption, flocculation, and the redox condition of the sediments (Establier et al., 1984). Based on the present report, the site of Sidi Salem is located near several sources of pollution: harbor and many factories such as those producing phosphoric fertilizers and pesticides. Nevertheless, metals contents in sediments of both study sites herein were below the levels recognized as characteristic of polluted environments (Table 2). Thus, the metals analyzed in this study are rarely associated with adverse biological effects. So, despite the difference in metal levels between the two sites and the slightly higher metal levels at the most polluted site (Sidi Salem), the effects on D. trunculus were not severe.
Norwegian classification system for metals in sediments (SFT, 2007).
| I | II | III | IV | V | |
| Metals | Background | Good | Moderate | Bad | Very bad |
| Cadmium (mg Cd/kg) | < 0.25 | 0.25–2.6 | 2.6–15 | 15–140 | > 140 |
| Copper (mg Cu/kg) | < 35 | 35–51 | 51–55 | 55–220 | > 220 |
| Lead (mg Pb/kg) | < 30 | 30–83 | 83–100 | 100–720 | > 720 |
| Zinc (mg Zn/kg) | < 150 | 150–360 | 360–590 | 590–4500 | > 4500 |
| Manganese (mg Mn/kg) | n.d | n.d | n.d | n.d | n.d |
| Iron (mg Fe/kg) | n.d | n.d | n.d | n.d | n.d |
5 Conclusion
This paper reports data on some biochemical biomarkers (AChE, GST, MTs) measured in the mantle of D. trunculus, a useful sentinel organism for environmental monitoring. Moreover, the analysis of metals (Cd, Cu, Pb, Zn, Mn and Fe) in sediments confirm the metallic contamination of the Gulf of Annaba. The results will contribute to the development of an environmental monitoring program based on relevant biomarkers. The level of metallic pollution was found relatively high at Sidi Salem compared to El Battah.
Acknowledgements
This research was supported by the Algerian Fund for Scientific Research (Laboratory of Applied Animal Biology to Prof. N. Soltani), and by the Ministry of High Education and Scientific Research of Algeria (CNEPRU Project to Prof. N. Soltani) and by Centro Interdisciplinary de Investigação Marinha e Ambientol (CIIMAR), Portugal. Strategic Funding UID/Multi/04423/2013 through national funds provided by FCT–Foundation for Science and Technology and European Regional Development Fund (ERDF), in the framework of the program PT2020.



Vous devez vous connecter pour continuer.
S'authentifier