1. Introduction
Estuaries are complex, dynamic and very productive areas [Day et al., 2012]. Despite their low diversity, estuarine habitats host high abundances of macro- and meiofauna, because they provide shelter [Elliott and McLusky, 2002] and are storehouses of nutrients and organic matter [Elliott and Quintino, 2007]. Organisms living in estuarine intertidal mudflats must endure intense environmental stress, as a result of daily (tidal cycles) to monthly (precipitation and river runoff) changes in marine and freshwater inputs [Elliott and McLusky, 2002, Jorissen et al., 2022]. Besides those stresses, coastal environments are increasingly threatened by ocean acidification [Cai et al., 2021, Cooley et al., 2022, Wootton et al., 2008]. This phenomenon jeopardises benthic organisms producing calcareous shells such as foraminifera.
Benthic foraminifera are widely present in estuarine areas [Debenay et al., 2000b]. These unicellular protists have the particularity to build a shell (called test), using different materials for its construction. Some species produce an organic test, others aggregate sediment particles, while a large group of species produce a test of calcium carbonate [de Nooijer et al., 2009, Debenay et al., 2000a]. Numerous authors have studied the distribution of foraminifera in estuarine mudflats [e.g., Moreno et al., 2005, Debenay et al., 2006, Mojtahid et al., 2016, Francescangeli et al., 2020, Thibault de Chanvalon et al., 2022]. The benthic foraminifera communities in the Auray estuary (French Atlantic coast) were investigated 30 years ago by Redois [1996] and recently in 2019 by Fouet et al. [2022] and 2020 by Daviray et al. [2024]. In 1996, Redois did not mention any marks of dissolution of calcareous foraminifera, whereas Fouet et al. [2022] and Daviray et al. [2024] noted at several stations numerous specimens of calcareous foraminifera with partly or completely dissolved test.
Numerous authors have described dissolution of calcareous tests of living foraminifera in coastal environments and various hypotheses have been put forward to explain the causes of the dissolution phenomenon. For instance, abiotic factors such as trace metal pollution [Buzas-Stephens et al., 2018], re-oxidation of reduced dissolved or particulate sulphidic compounds [Cesbron et al., 2016] or freshwater intrusion [Charrieau et al., 2018, Haynert et al., 2012] have been suggested to reduce the pH of pore water. Biotic factors which can reduce the saturation state of calcite have also been proposed such as intense microbial respiration of organic matter [Schönfeld and Mendes, 2022] or cable bacteria activity [Daviray et al., 2024].
Cable bacteria (CB) were discovered a decade ago in coastal marine sediments [Nielsen et al., 2010, Pfeffer et al., 2012]. Their activity (CBA) produces an electric gradient leading to ion migrations and a very particular geochemical signature in the sediment: a pH maximum in the oxic zone just below the sediment surface, followed by a strong pH decrease in the few centimetres below [Meysman et al., 2015, Nielsen et al., 2010, Pfeffer et al., 2012, Risgaard-Petersen et al., 2015, 2012]. CB have been described in various aquatic environments (e.g. open sea [e.g. Baltic Sea, Hermans et al., 2019]; Black Sea, Hermans et al., 2020], marine lakes [e.g. Grevelingen, Malkin et al., 2014], intertidal salt marshes [e.g. New England, Larsen et al., 2014], sandy intertidal flats [e.g. Wadden Sea, Malkin et al., 2017] and also in freshwater soils [e.g. Giber Å, Denmark, Risgaard-Petersen et al., 2015]), all around the world, see Burdorf et al. [2017] for a more extensive review of CBA occurrence worldwide. In French marine environments, CB were also observed especially in Mediterranean Lagoons such as Berre [Dam et al., 2021], Thau [Burdorf et al., 2017] in the Gulf of Lion and Urbino in Corsica [Burdorf et al., 2017]. Cable Bacteria were also observed in French Atlantic intertidal mudflats such as Arcachon bay and Auray estuary [Daviray et al., 2024].
Here, the new dataset of living foraminiferal communities sampled in 2020 in the Auray estuary is accompanied by oxygen and pH microprofiling to better understand the biogeochemical processes behind the dissolution of foraminiferal tests. To better constrain the significance of these observations, the samples from the same area collected in 1995–1996 [Redois, 1996] and in 2019 [Fouet et al., 2022] were re-examined. In addition, we re-investigated samples from other estuaries located along the French Atlantic coast [Fouet, 2022]. This short article signals the emergence of CBA in the sediments along the French Atlantic coast, and the repercussions in terms of environmental chemistry, ecology, and taphonomic processes.
2. Material and methods
The Auray estuary (Brittany, France; Figure 1) is a typical ria (drowned river valley), connected to the Morbihan Gulf (an enclosed marine bay), located along the north French Atlantic coast. This zone is subjected to a mesotidal to low macrotidal regime with a tidal range of about 4 m. More details about the studied site can be found in Fouet et al. [2022]. Living foraminiferal samples were collected in September 2020. All 10 stations were sampled along the estuary at low tide (Figure 1B). Stations 6B, 4B, 2C of this study corresponds to stations 1, 2, 3 respectively in Daviray et al. [2024], who also investigated the presence of CB by qPCR at stations 1 (6B) and 2 (4B).
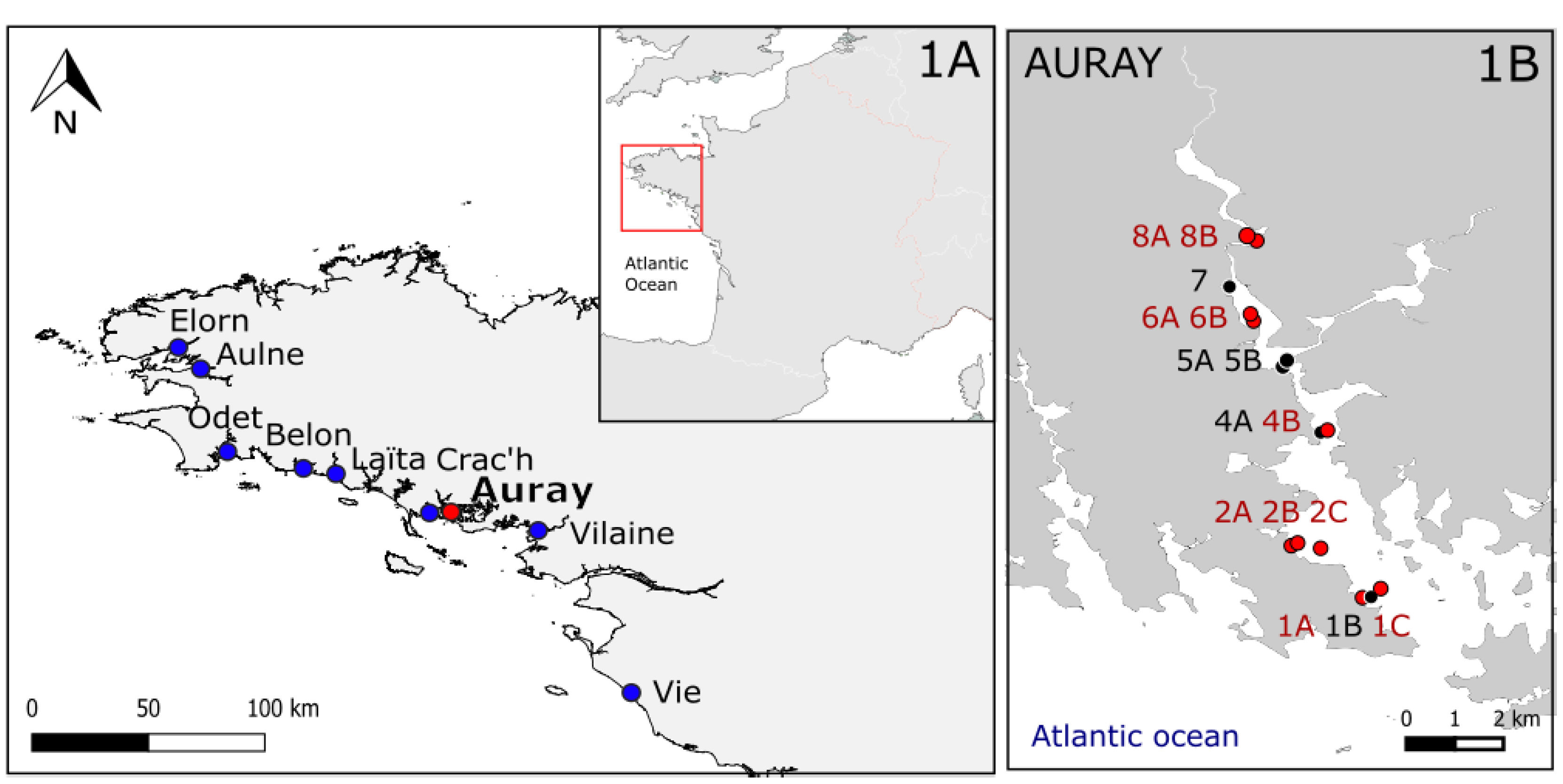
1A: Location of the studied estuaries (in red: Auray estuary, in blue: eight other estuaries). 1B: Location of the stations in the Auray estuary sampled in 2019 and 2020 (oxygen and pH profiles were measured in 2020 for stations indicated in red). Modified from Fouet [2022].
At each station, the first two 0.5 cm of the sediment were collected by hand using tubes with an internal diameter of 9.6 cm. Samples were stained with 2 g/L Rose Bengal and preserved in 96% ethanol. In the laboratory, samples were sieved over a 125 μm mesh. All stained foraminifera were picked in water, using a Leica MZ16 stereomicroscope. Based on stereomicroscope observations of calcareous tests contained in each sample (examples of individuals showing dissolution marks are shown in Figure 2), three types of samples were distinguished, characterised by different type of Foraminiferal Test Dissolution (FTD):
- Type 1 (no visible test dissolution)—Most individuals have a transparent, glassy test; less than 10% of the test is whitish and opaque; this is considered as the first sign of dissolution.
- Type 2 (moderate dissolution)—More than 10% of the individuals have a whitish, matt test, while some specimens show more advanced stages of test dissolution.
- Type 3 (advanced to severe test dissolution)—Most specimens show holes and cracks in the test with organic lining sometimes becoming partially or completely visible. Some glassy specimens may still be present in the assemblage.
The 0–0.5 and 0.5–1 cm levels were studied separately, and the overall assessment of FTD type of each sample was based on the layer which showed the most severe dissolution (nearly always the 0.5–1 cm level). To investigate whether dissolution caused the loss of foraminiferal tests in slightly deeper sediment layers, we calculated a Calcareous Test Preservation Ratio (CTPR) that was defined as the ratio between the density of tests of living calcareous foraminifera in the 0–0.5 cm level and their total density in the 0–1 cm layer. When CTPR was below 0.6, high densities of calcareous specimens were recorded in the subsurface layer (0.5–1 cm); when CTPR was between 0.6 and 0.90, their densities were higher in the surface layer, and when CTPR was above 0.90, the population living in the subsurface layer had largely disappeared.
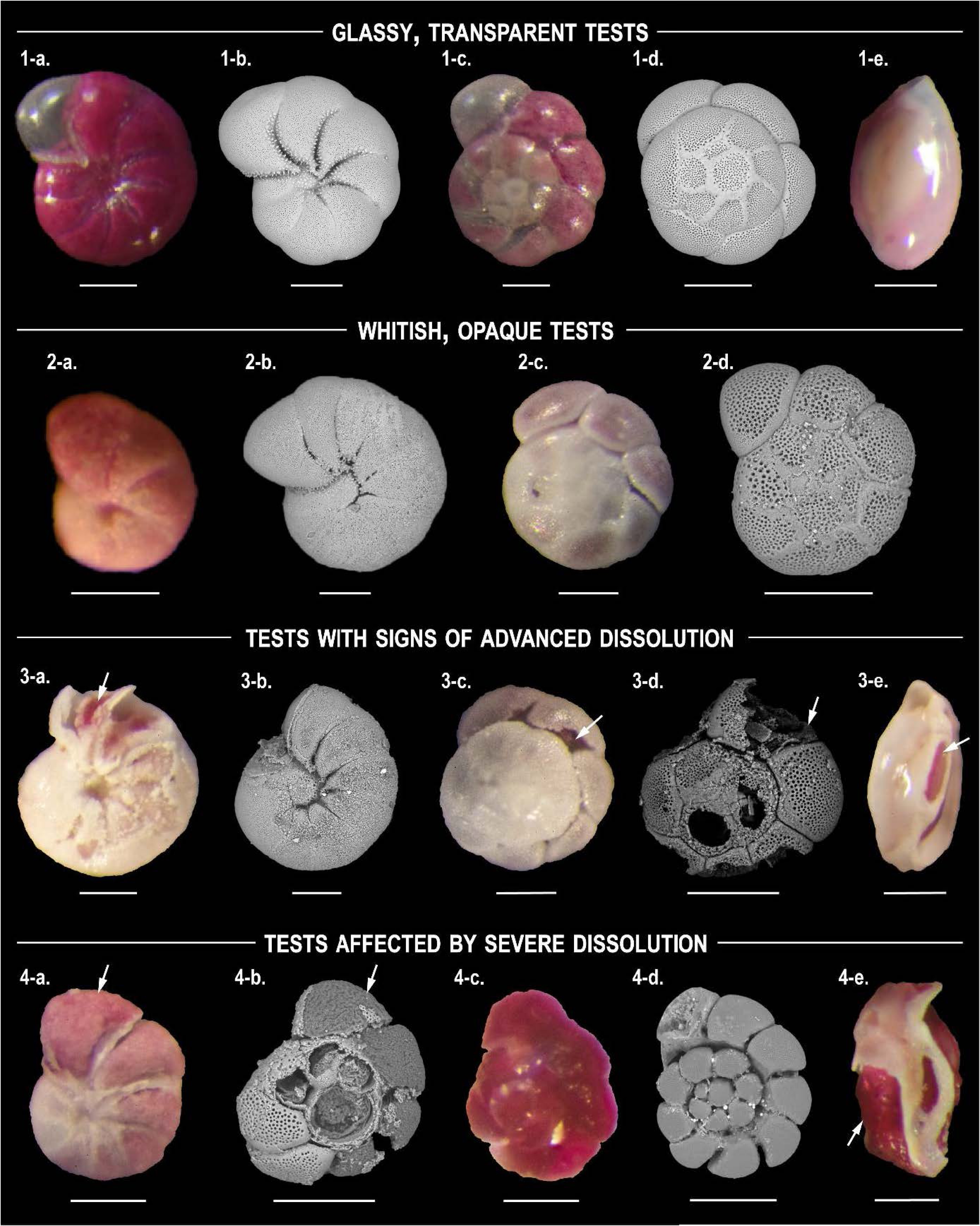
Examples of gradual foraminiferal test dissolution. (1) Glassy, transparent tests, (2) Whitish, matt tests, (3) Tests with signs of advanced dissolution, (4) Tests affected by severe dissolution, for Haynesina germanica (1-a, 1-b, 2-a, 2-b, 3-a, 3-b, 4-a), Ammonia spp. (1-c, 1-d, 2-c, 2-d, 3-c, 3-d, 4-b, 4-c, 4-d) and Quinqueloculina oblonga (1-e, 3-e, 4-e). For each dissolution stage, figures a, c and e have been produced with an optical microscope, figures b and d with a Scanning Electronic Microscope. White arrows indicate the organic lining; scale bars: 100 μm. Masquer
Examples of gradual foraminiferal test dissolution. (1) Glassy, transparent tests, (2) Whitish, matt tests, (3) Tests with signs of advanced dissolution, (4) Tests affected by severe dissolution, for Haynesina germanica (1-a, 1-b, 2-a, 2-b, 3-a, 3-b, 4-a), Ammonia ... Lire la suite
To investigate both the spatial and historical extent of dissolution processes, additional samples were re-examined using a stereomicroscope to define the FTD. Concerning the spatial extent, samples from Fouet [2022] were used to describe the FTD of samples coming from eight other estuaries (133 samples) (Figure 1A). Concerning the historical aspect, Auray estuary samples collected 25 years ago [Redois, 1996] and in 2019 [Fouet et al., 2022] were also inspected for FTD and CTPR values. Samples collected by Fouet and co-workers in 2019 used the same methodology as presented here. However, their study performed triplicates. The methodology used by Redois [1996] was slightly different. He studied the whole 0–1 cm level, collected with a spoon on a surface of 100 cm3, stained with Rose Bengal and preserved in ethanol, washed on a 50 μm mesh size sieve and dried the samples for binocular observation. Therefore, CTPR could not be calculated for 1995–1996 samples, and these samples were only investigated for FTD.
Oxygen and pH microprofiling were performed at each station. After retrieval in the field, cores were immerged in an aquarium with estuarine water at in situ temperature, where oxygen saturation was maintained by air-bubbling. Two Unisense© profiling systems were used simultaneously. One consisted of two Clark-type oxygen microsensors with a 50 μm tip [Revsbech, 1989, Revsbech and Jørgensen, 1986], and the other of a pH sensor with a 500 μm tip diameter (PH500, Unisense©). Both microsensors were mounted on a motorised micromanipulator linked to a computer, and connected to a MultiMeter S/N. We used a 50-μm increment for oxygen, whilst for pH we chose a 100-μm increment around the sediment-water interface, which was further adapted in real-time according to the evolution of the observed pH profile, down to 4–5 cm depth. To calibrate the oxygen microsensor, two points were used, the water column for 100% saturation and pore waters in the anoxic sediment for 0%. For pH calibration, three NBS buffers were used (values 4.0, 7.0 and 9.2).
3. Results and discussion
3.1. Sediment geochemistry
Figure 3 shows oxygen and pH profiles at and below the Sediment-Water Interface (SWI) for 10 stations in the Auray estuary taken in September 2020. All stations showed typical pore-water oxygen profiles with a strong decrease in the topmost sediment. The oxygen penetration depth (OPD) ranged from 0.7 to 1.5 mm depth. Based on the pH profiles, which showed greater variability, three groups of stations could be distinguished:
- Group I (stations 2A, 6A, 8A, 8B): the pH strongly decreased from ∼7.8 at the SWI to ∼7.0 at 2–4 mm depth, to remain relatively stable in deeper sediment layers.
- Group II (stations 1C and 6B): the stations showed pH profiles with slightly acidic pore waters in the suboxic zone. Station 1C showed a minor pH peak (7.9) just below the SWI, but deeper down, the pH remained stable around 6.6 until 4 cm depth. Station 6B showed a minor acidification in the suboxic zone (pH = 6.8). According to Daviray et al. [2024], DNA sequencing analysis at station 6B (station 1 in their article) showed low cable bacteria densities.
- Group III (stations 1A, 2B, 2C, 4B): the pH decreased to a minimum well below 7.0 between 0.5 and 1.0 cm depth, in the suboxic zone (i.e., without detectable oxygen). It increased again towards greater depth but remained below 7.0. In addition, the profiles of stations 2C and 4B showed a small but distinct pH peak just below the SWI. According to Daviray et al. [2024], DNA sequencing analysis at station 4B (station 2 in their article) showed high CB densities.
O2-pH profile groups can be discriminated according to the knowledge of early diagenesis processes and geochemical fingerprint of cable bacteria activity [CBA; Meysman et al., 2015, Nielsen et al., 2010, Pfeffer et al., 2012, Risgaard-Petersen et al., 2012, 2015]. Profiles of group I are considered as typical for marine environments without CBA. Conversely, the profiles of group III suggest acidification stems from CBA as shown in Nielsen et al. [2010]; Pfeffer et al. [2012] and Meysman et al. [2015]. Daviray et al. [2024] confirmed the presence of CB [Candidatus Electrothrix sp.; Trojan et al., 2016] at station 4B by DNA analysis and estimated a CB filament density of 74.4 ± 5.0 m⋅cm-3 per bulk sediment in the first sediment centimetre. Finally, in group II, O2-pH profiles show only a slight pH decrease and are not typical of intense CBA. Even if the pH profile do not show a clear pH decrease, Daviray et al. [2024] indicated the presence of genetical material of cable bacteria in station 6B in 2020 with a low CB filament density of 7.4 ± 0.4 m⋅cm-3 per bulk sediment. Therefore, these two profiles are interpreted as sustaining moderate CBA.
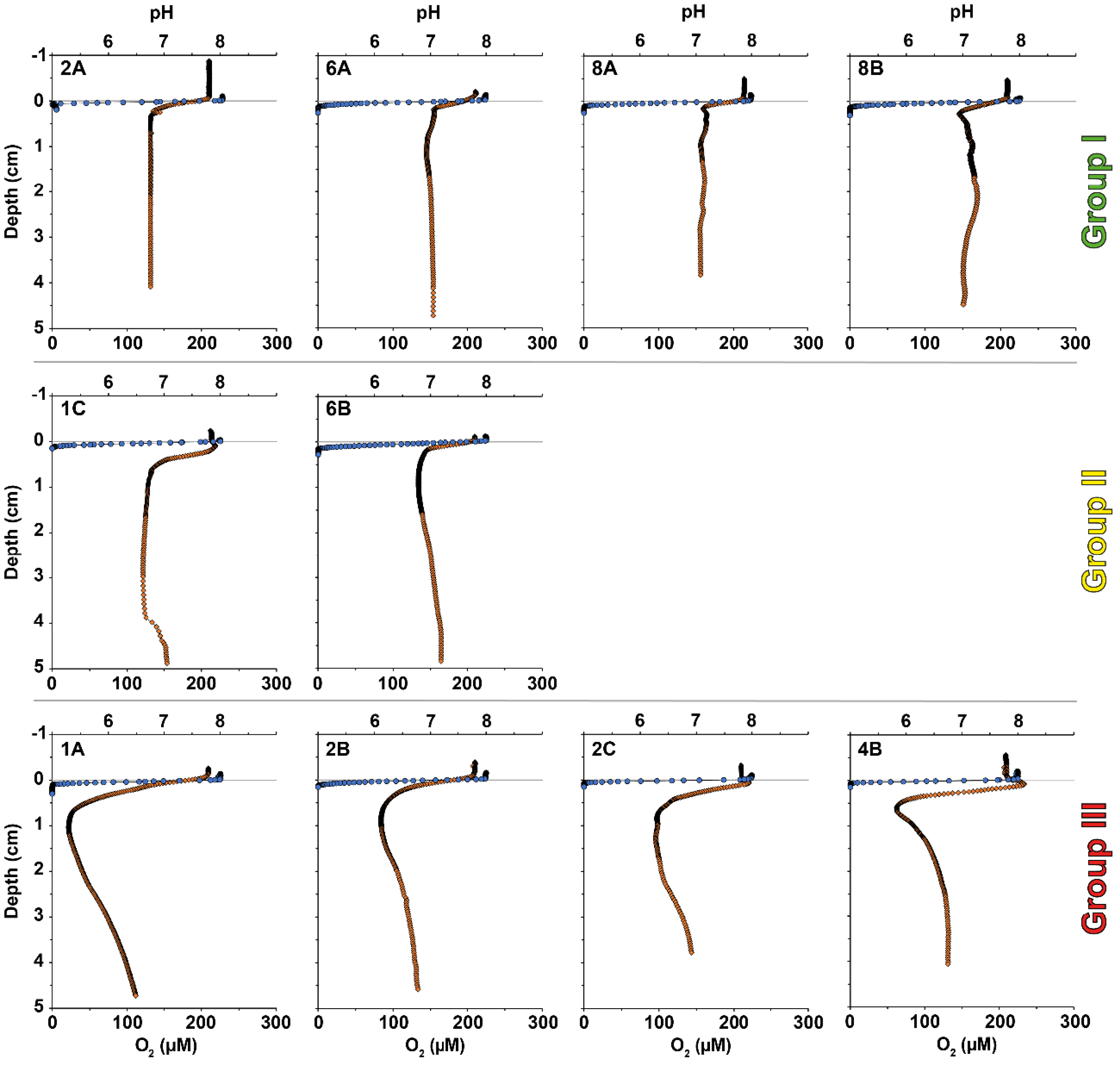
pH (orange) and oxygen (blue) profiles measured in September 2020 in the Auray estuary. Three groups were established according to the shape of the O2-pH profiles (group I: low acidification; group II: moderate acidification; group III: strong acidification). Profiles from stations 2C, 4B and 6B are also published in Daviray et al. [2024].
3.2. Foraminiferal assemblages
The density of living foraminifera (excluding organic linings) in the first centimetre varied from 76 to 5120 individuals per 50 cm3 with 52–2164 individuals per 50 cm3 in the surface half centimetre and 22–3575 individuals per 50 cm3 in the subsurface (0.5–1 cm) layer (Figure 4). These values are comparable to those observed in 2019 [Fouet et al., 2022] and in other estuaries [e.g., Camacho et al., 2015, Debenay et al., 2006, Francescangeli et al., 2021, Mojtahid et al., 2016]. The foraminiferal density was generally higher in the superficial sediment layer (0–0.5 cm) than in the 0.5–1 cm layer, except for station 8B. For many stations (i.e., 1A, 4B), the densities were an order of magnitude lower in the second layer.
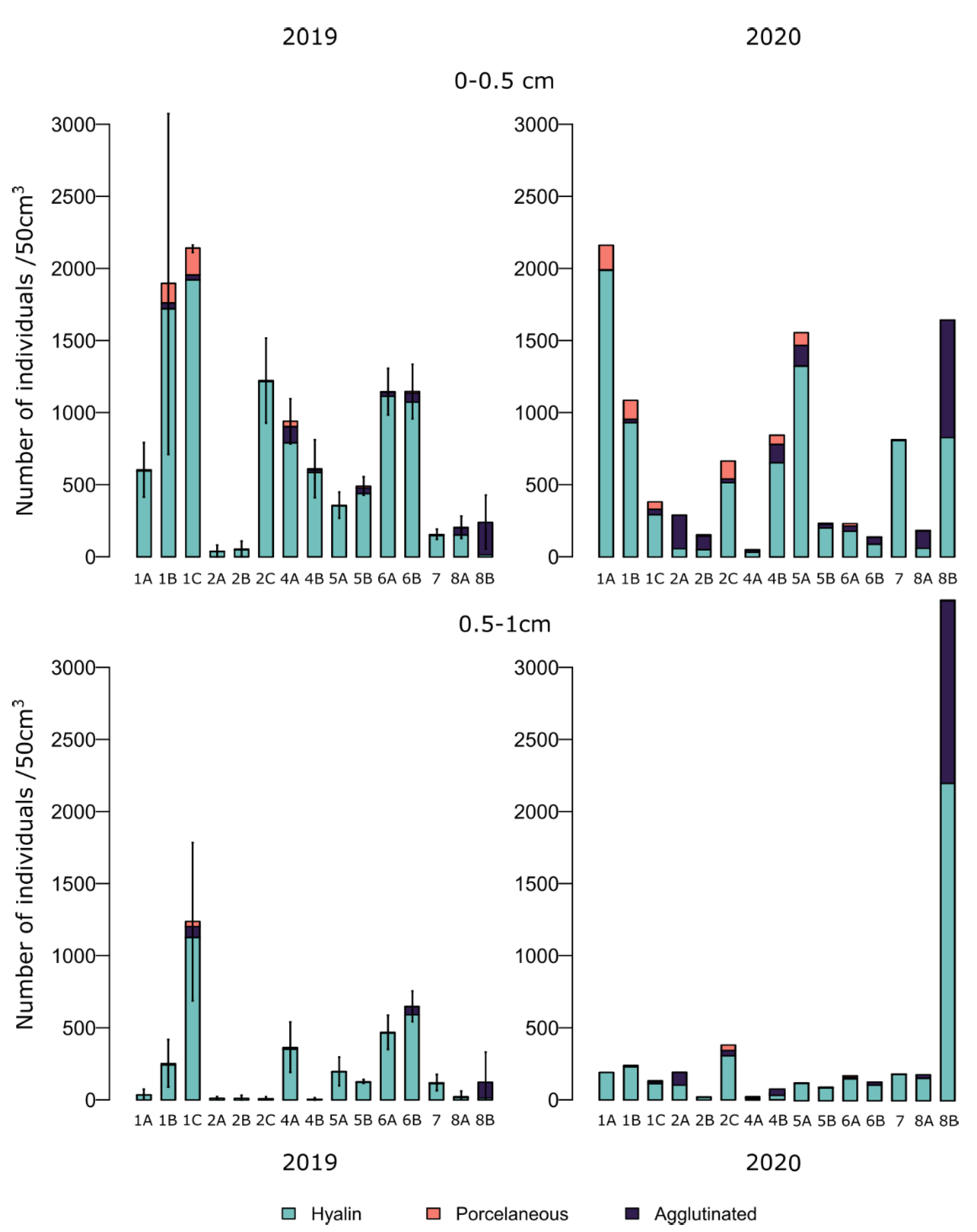
Foraminiferal density in the >125 μm fraction for the 0–0.5 and 0.5–1 cm levels, expressed in individuals per 50 cm3 (for samples collected in July 2019 by Fouet et al. [2022] and September 2020 (this study)). The three replicates of 2019 were averaged, and the standard deviation is presented overhead. The stations are ordered from the outer (left) to the inner part of the estuary (right).
The Calcareous Test Preservation Ratio (CTPR), which describes the density differences within the first centimetre, varied between 0.27 and 0.96 (Table 1). Five stations showed values of CTPR higher than 0.6 (1A, 1C, 2B, 2C and 4B) suggesting a low occurrence of calcareous living foraminifera in the subsurface layer (0.5–1 cm). At stations of acidification groups II and III, dissolution, as shown by FTD was much more intense in the 0.5–1 than in the 0–0.5 cm layer, and consequently, foraminiferal densities were much higher in the first layer (Figure 4), leading to a high CTPR (Table 1). This is not surprising in view of the pH profiles of group III (Figure 3), which show that the strong pH minimum (5.3 < pH < 6.3) was always positioned between 0.5 and 1.0 cm depth.
The first row indicates the O2-pH profile groups from green (group I: low acidification) through yellow (group II: moderate acidification) to red (group III: strong acidification). The second row describes the CTPR, with <0.6: green, 0.6–0.90: yellow, >0.90: red. The last row summarises observations of foraminiferal test dissolution (FTD), ranging from “no visible dissolution” (type 1, green), via moderate (type 2, yellow) to “advanced to severe dissolution” (type 3, red)
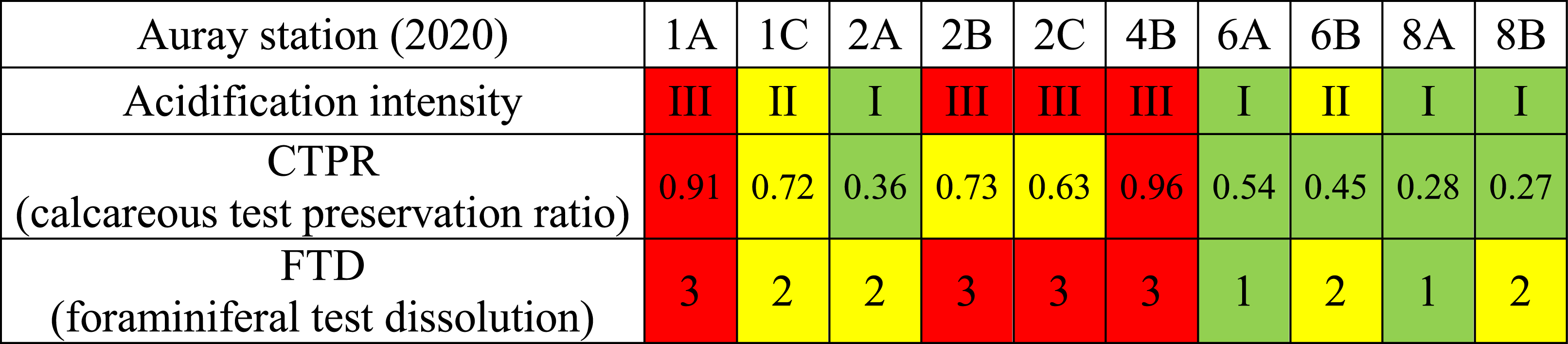 |
Table 1 shows the comparison of the results of the pH profiles, Foraminiferal Test Dissolution (FTD) and the Calcareous Test Preservation Ratio (CTPR). In many cases, scores yielded by these three methods converge. All three indicators suggest that there was intense sediment acidification at stations 1A and 4B, and to a lesser degree at stations 2B and 2C. At these stations, FTD was maximal, especially in the 0.5–1 cm layer, leading to high CTPR values (>0.6). Conversely, at stations where the pH profile did not indicate sediment acidification (i.e., 2A, 6A, 8A and 8B), the observed FTD was minimal and the CTPR was always below 0.6, indicating that calcareous individuals were well preserved in both sediment levels. Stations of pH group II (with atypical pH profiles) showed intermediate FTD and CTPR values, confirming our initial interpretation that these stations were slightly affected by acidification. However, there are some exceptions to this pattern. At stations 2A and 8B, where the pH profile suggested no acidification, moderate FTD was observed. Nevertheless, it appears that in the case of pH profiles indicative of strong acidification, the living foraminiferal assemblages show advanced to severe FTD, which is confirmed by an important loss of calcareous tests in the 0.5–1.0 cm layer, leading to a high CTPR.
These results show a good correspondence between observations of foraminiferal test dissolution and measurements of sediment acidification. In the literature, different causes of foraminiferal test dissolution have been proposed. First, global ocean acidification is one of the threats that could alter organisms that produce calcareous shells such as foraminifera [Guamán-Guevara et al., 2019, Kuroyanagi et al., 2021]. However, these changes are expected to impact all sites of the same ecosystem equally, so this explanation was not retained to explain the differences in sediment acidification observed between the different stations of this study. Therefore, the possibility of freshwater intrusion to explain the FTD [Charrieau et al., 2018, Haynert et al., 2012] is not retained either, because stations located in the inner part, and receiving more freshwater input (stations 8A and 8B), do not show stronger acidification than stations located in the outer part of the estuary (1A and 1C). Buzas-Stephens et al. [2018] proposed that trace metal pollution can explain FTD, but in our stations, measurement of trace metal did not show any sign of major pollution [Fouet et al., 2022].
Based on the similarities between our pH profiles showing sediment acidification associated with oxygen profiles (group III) and the representative one of Cable Bacteria Activity, as presented in Meysman et al. [2015], Nielsen et al. [2010] and Pfeffer et al. [2012], we hypothesised that CBA could be responsible of Foraminiferal Test Dissolution at these Auray estuary stations (1A, 2B, 2C, 4B). However, some discrepancies between the pH profiles and the observations of FTD and CTPR could be noted, for which two explanations can be advanced. First, as suggested by differences in FTD between stations collected in the same mudflat (i.e., station 1A versus 1C, and station 2A versus 2C and 2B or station 6A versus 6B), CBA could have a patchy distribution on the mudflat. The pH measurements are based on a few 1D punctual profiles (500 μm scale) across the depth of the sediment, whereas foraminiferal observations included a large sediment volume (72 cm3). The foraminiferal sampling zone may have been subject to scattered bacterial activity. Next, while pH measurements suggest acidification at the moment of sampling, the living foraminiferal community could reflect dissolution that has occurred over the last few weeks [Charrieau et al., 2018, 2002, Le Cadre et al., 2003]. Indeed, several authors have shown that CBA may vary considerably throughout the year [Seitaj et al., 2015, Sulu-Gambari et al., 2016].
In order to explore whether FTD also occurs on individuals collected in other estuaries, samples from eight other estuaries [Figure 1; Fouet, 2022] were visually inspected for marks of dissolution. The results of these observations are presented in Table 2, using the same three types of sample classification, characterised by different stages of FTD, as in the Auray estuary. A few stations from Odet, Laïta and Crac’h estuaries showed moderate marks of test dissolution. Both Aulne and Belon estuaries had one station showing advanced to severe marks of test dissolution (Table 2). By analogy with Auray, we hypothesise that CBA could be present in these estuaries as well. This still remains to be confirmed by more distinctive methods such as pH profiling or molecular identification. Conversely, no FTD was observed in the Vie (two different field campaigns, in October 2018 and June 2020), Elorn and Vilaine estuaries. Also in the Loire estuary, re-inspection of foraminiferal assemblages described by Mojtahid et al. [2016] did not show any signs of FTD suggesting the absence of foraminiferal test dissolution at these locations (com. pers. Mojtahid). Moreover, concerning the geographical extent of this strong acidification phenomenon, advanced to severe test dissolution of living foraminifera have earlier been described in Arcachon Bay [Cesbron et al., 2016]. The authors explained this as the result of decreased pH due to bacterial activity around decomposing eelgrass roots. However, closer inspection of their pH profiles suggests a CBA signature. Together with the strong decrease of calcareous tests densities in the first centimetre of the sediment could also plead to intense seasonal cable bacteria activity. Summarising, in several intertidal mudflats of French transitional environments (estuaries and semi-enclosed bays), FTD has been observed that could be the result of CBA. It therefore appears that this acidification phenomenon could be widespread along the French coast.
Values of FTD (Foraminiferal Test Dissolution)
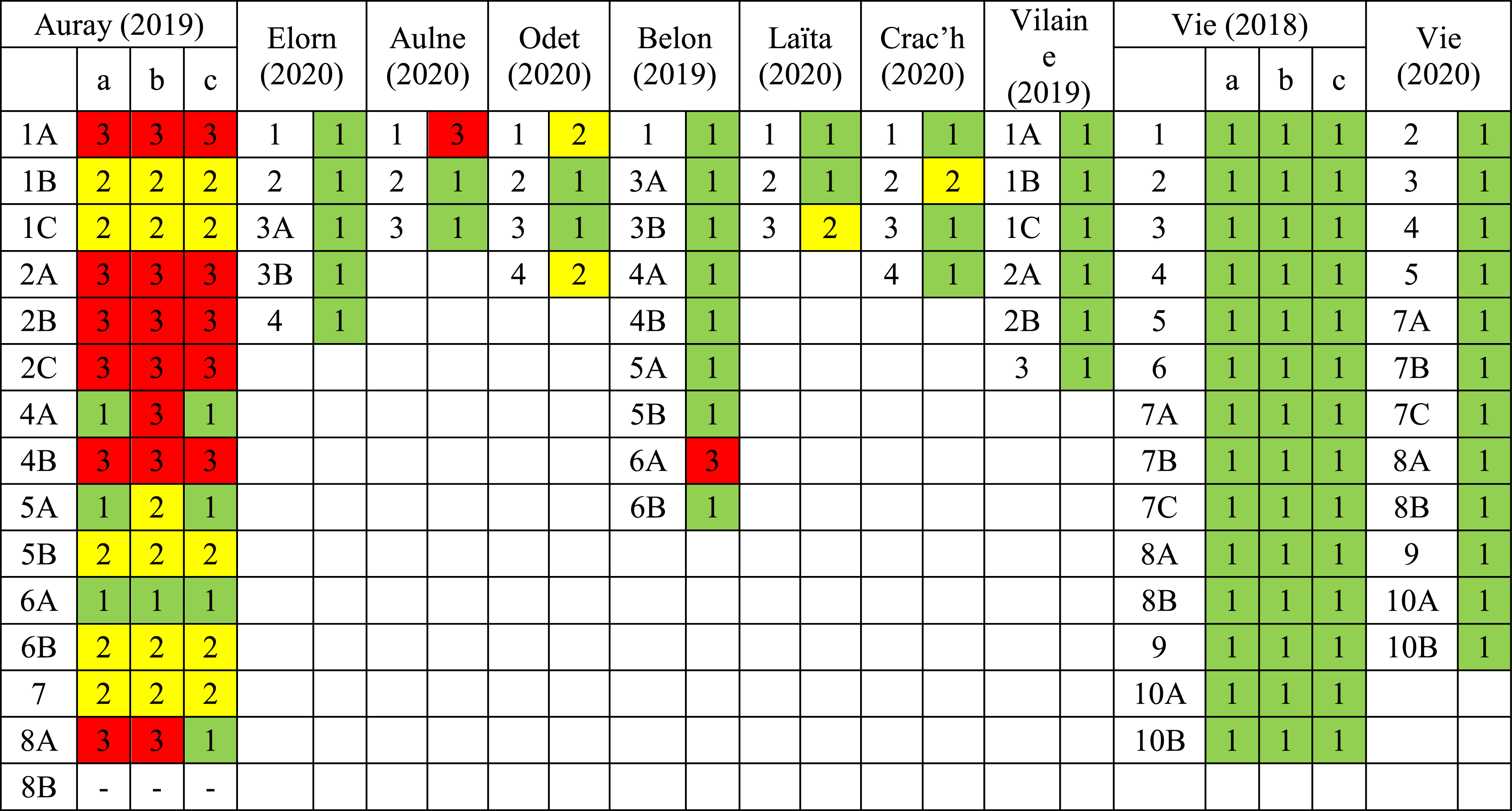 |
Stereomicroscope observations of foraminiferal test dissolution, ranging from “no visible test dissolution” (group 1, green) to “moderate” (group 2, yellow), “advanced to severe dissolution” (group 3, red) for samples collected in nine different estuaries studied in Fouet [2022]. The letters a, b, and c are the three replicates sampled in 2018 (Vie estuary) and 2019 (Auray estuary).
In addition, to look into the historical aspect of the presence of this phenomenon, we re-examined two foraminiferal datasets from Auray estuary: samples with three replicates per station collected in July 2019 [Fouet et al., 2022] and samples collected during a 10-month survey in 1995–1996 [Redois, 1996]. Samples were collected on the same mudflat as the present study (see method section). Concerning the samples collected in 2019 (Table 3), advanced to severe test dissolutions (FTD type 3) was observed at several stations and in several replicates that corresponds to similar FTD type found in the present study. It can be noted that CTPR values from calcareous foraminiferal communities collected in 2019 (Table 3) were in good agreement with those observed in 2020 for stations 1A, 1C, 2B, 4A, 5B, 7. CTPR values (Table 3) for the stations 2A, 2C, 6B and 8A show higher values in 2019 than in 2020 (>0.9 on average) while station 5A shows a higher CTPR value higher in 2020 (>0.95). Differences between samples could be attributed either to interannual variability (between 2019 and 2020) or to spatial heterogeneity (between 2019 replicates) of CBA. Concerning the samples collected in 1995–1996, although the sampling methodology and treatment were slightly different (0–1 cm layer sampled and samples dried so that organic linings were not preserved), visual inspection of the calcareous foraminiferal tests was still possible. The re-examination of the foraminiferal tests of these samples did not yield any marks of foraminiferal test dissolution, and all examined samples were classified as Type 1. This suggests that in the Auray estuary, sediment acidification is a recent phenomenon (present at least since 2019) that appeared in this area in the last 25 years.
Values of CTPR: ratio between the number of calcareous individuals in the 0–0.5 cm level and the total number of calcareous foraminifera in the 0–1 cm sample, for the three replicates (a, b, c) sampled in 2019 [after Fouet et al., 2022] and 2020 at all the fifteen stations along the Auray estuary
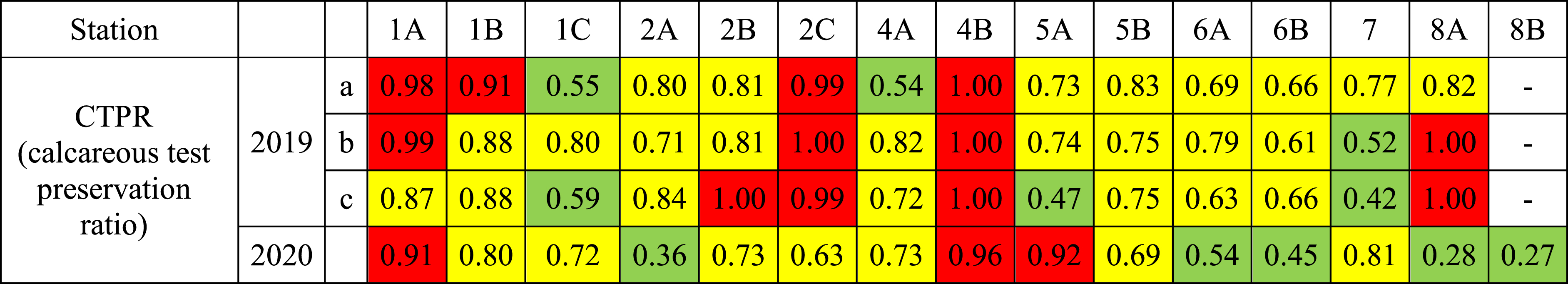 |
“-” indicates the sample where the density of calcareous species is <10 specimens.
To better assess the spatial and temporal dynamics of this phenomenon, the re-examination of foraminiferal tests collected in earlier studies is possible. Observation of archived samples may show FTD indicative of former acidic conditions at places where their presence had not been suspected in the past. This method represents a first, rapid and inexpensive approach for directing further analyses in order to characterise present and past dissolution processes using other approaches (elemental and isotopic analyses, sedDNA, etc.). We therefore recommend for future investigations studying foraminiferal living assemblages to use a resolution of 0.5 cm in the uppermost sediment to allow CTPR calculation. In addition, the ideal condition for observing FTD is a sediment that has been kept humid, in which foraminifera have been coloured (i.e., Rose Bengal or CTG) allowing inner organic linings observation. Thereby, the recognition of FTD in fossil assemblages preserved in sediment archives could yield information about the historical evolution of CBA, as shown by Richirt et al. [2022]. These authors observed in a sediment core from Grevelingen Lake (The Netherlands) very low densities of calcareous foraminifera at 20 cm depth, corresponding to a period of major change, the reoxygenation of bottom water due to the increase of water exchanges after the opening of the lake to the sea. They hypothesise that such changes could have promoted the enhancement of CBA since 2003, and therefore carbonate test dissolution. Such investigations could help to better understand the spread of these recently discovered bacteria, remaining poorly unknown, and their consequences for the ecology of the surrounding organisms. Species with a calcareous test could be more fragile, and others (such as agglutinated foraminifera or soft-walled organisms) more adapted to resist to dissolution, so this additional stress could lead to changes in the community structure [Daviray et al., 2024]. In addition, sediment acidification can result in a loss of sediment archives [Daviray et al., 2024], which could have implications for the interpretation of palaeontological data.
4. Conclusion
The foraminifera from sediment samples collected in September 2020 in the Auray estuary showed severe marks of dissolution. This process was not homogenous along this estuary. In addition, pH profiles showed acidification of the porewater at a few millimetres depth after the oxic zone in several stations. These profiles are typical of cable bacteria activity signature. We suggest that an important loss of calcareous foraminifera in the first half centimetre (high CTPR) coupled with foraminiferal test dissolution (FTD) can be considered as an indicator of a potential occurrence of porewater acidification, such as cable bacteria activity. It appears therefore that these metrics could be used as a rapid tool of sediment acidification, pending geochemical confirmation. FTD could also be used as a proxy of acidic conditions in historical records, where pH can no longer be measured. The study of FTD in samples collected at the Auray estuary in the 90s suggests that these acidic conditions have emerged over the last thirty years. Re-examination of calcareous foraminifera coming from other estuaries on the French Atlantic coast allows to propose that the corrosive phenomenon is or has become widespread over the last few decades.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
The authors received funding from the CNRS-INSU (program LEFE-CYBER, project CB-FOR), from the Angers University and the OFB (French Office of Biodiversity, grant number 3976-CT-RD-AMI-18-SURV-FORESTAT).
Acknowledgements
The authors are grateful to Nils Risgaard-Petersen for his advice during the early stages of the writing process, and Robin Fentimen for his proofreading and correction language of the text. The authors thank the participants in the field trips (2019 and 2020).





 CC-BY 4.0
CC-BY 4.0