1 Introduction
Melatonin is known to be clearly implicated in the control of the circadian system in mammals 〚1〛 and its role has been demonstrated in lower vertebrates 〚2〛, and particularly in some birds 〚3, 4〛. In Japanese quail under photoperiodic conditions, like in many species, plasma melatonin levels are high during the scotophase, which corresponds to the rest phase for this diurnal bird. Furthermore, the duration of nocturnal secretion of melatonin depends on night duration 〚5〛. However, as at least two main potential sources of melatonin production exist in quail – the eye and the pineal gland 〚6〛 – , the experimental protocols are difficult when we want to cancel the hormonal signal. For example, no instances of arrhythmia for locomotor activity follow pinealectomy in quail in constant darkness 〚7〛, whereas blinding disrupts circadian rhythm of locomotor activity 〚8〛. Therefore, different effects on free running rhythm could follow, leaving only one of the two main sources. In the same way, trying to cancel the diurnal variation of melatonin with continuous administration could reveal the sedative effect of this hormone 〚9〛.
On the other hand, exogenous cyclic melatonin treatments seem to demonstrate implication of melatonin in the control of the circadian system in quail. Cyclic melatonin administration in drinking water synchronised the body temperature and activity rhythms in five birds reared in total darkness 〚10〛. However, this last study concerned sexually developed females and we know that steroid hormones can influence circadian rhythms in quail, at least for feeding activity and thermoregulation 〚11, 12〛. For example, testosterone filled capsule implantation leads to a lengthening of the free-running period of feeding activity in rhythmic castrated male quails 〚13〛. In addition, an identical experimental protocol, applied to arrhythmic birds, induces circadian rhythmicity 〚14〛.
Following this, we tested the influence of melatonin on an overt rhythm by sending a clear-cut diurnal signal to free running birds, these birds being formerly castrated in order to avoid any effect of steroid hormones.
2 Material and methods
2.1 Birds and housing
We used ten male domesticated Japanese quail, Coturnix c. japonica. They were issued from five crosses of the first generation of a line selected for the very clear circadian rhythm of feeding activity in constant darkness 〚15〛. The chicks were reared in communal boxes (98 × 60 × 16 cm) at 37 °C under artificial lighting (12.5 L, 11.5 D with natural twilight), until they were 3.5 weeks old. Then, they were placed in a soundproof chamber in individual boxes until the end of the experiment. The ambient temperature was maintained constant at 20 ± 1 °C. We provided food and water ad libitum and replaced this three times a week, each time at a different time of the day.
2.2 Experimental protocol
We castrated the males when they were three weeks old, just before sexual development in our rearing conditions. From the age of four weeks, we maintained them in constant darkness for a fortnight to record the circadian feeding activity, following our standardised criteria used in former experiments 〚15〛. Then, we transferred the birds to green constant dim light (2 lux) until the end of experiment. We divided the experiment into five successive phases, where liquid availability per 24-h cycle was different:
- • the W1 phase; during five weeks, the birds received water ad libitum; this phase was necessary to check the stability of the circadian activity;
- • the M phase; at the age of 11.5 weeks, the birds had access to melatonin (25 ml) for 9 h (from 8:30 a.m. to 17:30 p.m.) for 10 consecutive days and then to water for 15 h (40 ml); we know that administration of exogenous melatonin orally or by catheter is effective for the synchronisation of free running activity; we provided melatonin in drink because it is a non-invasive technique, the stress linked to this method being minimal;
- • the W2 phase; for the 10 next days, the birds had access to water ad libitum;
- • the C phase; for the following 10 days, we replaced the melatonin solution by a control solution, composed of only the alcoholic solvent of melatonin;
- • the W3 phase; the birds had access to water ad libitum again.
To monitor liquid intake, we measured the remained liquid when the solution was changed. Moreover, we collected four blood samples during the experiment. Two samples were taken during the M phase, the first one in the middle of melatonin solution availability phase, e.g. 4.5 h after the beginning of melatonin availability, and the second one 4.5 h after the beginning of water availability. We collected two other blood samples when birds were free running, one during the active period of the bird and one during the inactive period, after the W3 phase, to avoid the disruption of birds.
2.3 Melatonin and control solutions
We obtained the main melatonin solution (15 mg ml–1) by diluting 150 mg crystalline melatonin (97%, Sigma) in 10 ml ethyl alcohol (95%). According to the protocol administration used by Zeman et al. 〚9〛 for three times lighter birds compared to our quail strain, we diluted this solution again in distilled water to obtain a concentration of 15 μg ml–1. This may seem rather a large value, but we wanted to cancel any endogenous variation of the hormone. We realised the control solution (10 μg ml–1 of alcohol) by diluting 1 ml 95% alcohol in one litre of distilled water.
We measured melatonin by RIA, using a specific antibody (R19540) provided by INRA, Nouzilly, Tours at a first dilution of 1/200 000 and 2 iodomelatonin I125 as the label. We extracted melatonin from the plasma using dichloromethan (DCM) according to the method described by Brown et al. 〚16〛.
2.4 Data collection
We continuously recorded the feeding activity of each bird using infrared detectors. The method has been previously detailed 〚11〛. As the food and water troughs were placed at the front of the cage, we were able to record pellet and water intake at the same time.
2.5 Data analysis
Circadian feeding activity was analysed throughout the experimental phases of 10 cycles of 24 h for each, by spectral analysis, using programs developed in our laboratory by J.-P. Richard and D. Leray on a Silicon graphics computer. We detected the presence or absence of a periodic event and, when the activity was rhythmic, we determined the circadian period. With spectral analysis, a peak was taken into account, and then the bird considered as rhythmic when the height of the peak was more than 0.05 of the spectrum power. We used one index to quantify the clarity of circadian activity, corresponding to the area of the spectrum circadian peak. When the activity was arrhythmic, no peak appeared, and we attributed the value zero as clarity index to the bird activity 〚14〛. We measured the quantity of activity per cycle when the bird was rhythmic, per 24 h when it was arrhythmic. We analysed the results by non-parametric tests (Statview 4.0).
3 Results
3.1 Liquid intake
Liquid intake per 24-h cycle was higher during the M phase (21.9 ± 1.1 ml, N = 10) than during the C phase (19.8 ± 1.1 ml, N = 10) (Wilcoxon, p = 0.013, N = 10).
Fig. 1 describes the mean drink consumption per hour and per bird during the M and C phases. During the M phase, water consumption (1.1 ± 0.1 ml h–1) was higher than melatonin solution consumption (0.7 ± 0.2 ml h–1) (Wilcoxon, p = 0.0051, N = 10). During the C phase, the consumption of control solution (0.8 ± 0.1 ml h–1) and of water (0.8 ± 0.1 ml h–1) did not differ significantly (p = 0.45). The birds drank significantly more water during the M phase than during the C phase (p = 0.0071). In parallel, the consumption of melatonin solution was significantly lower than that of control solution (p = 0.0051).
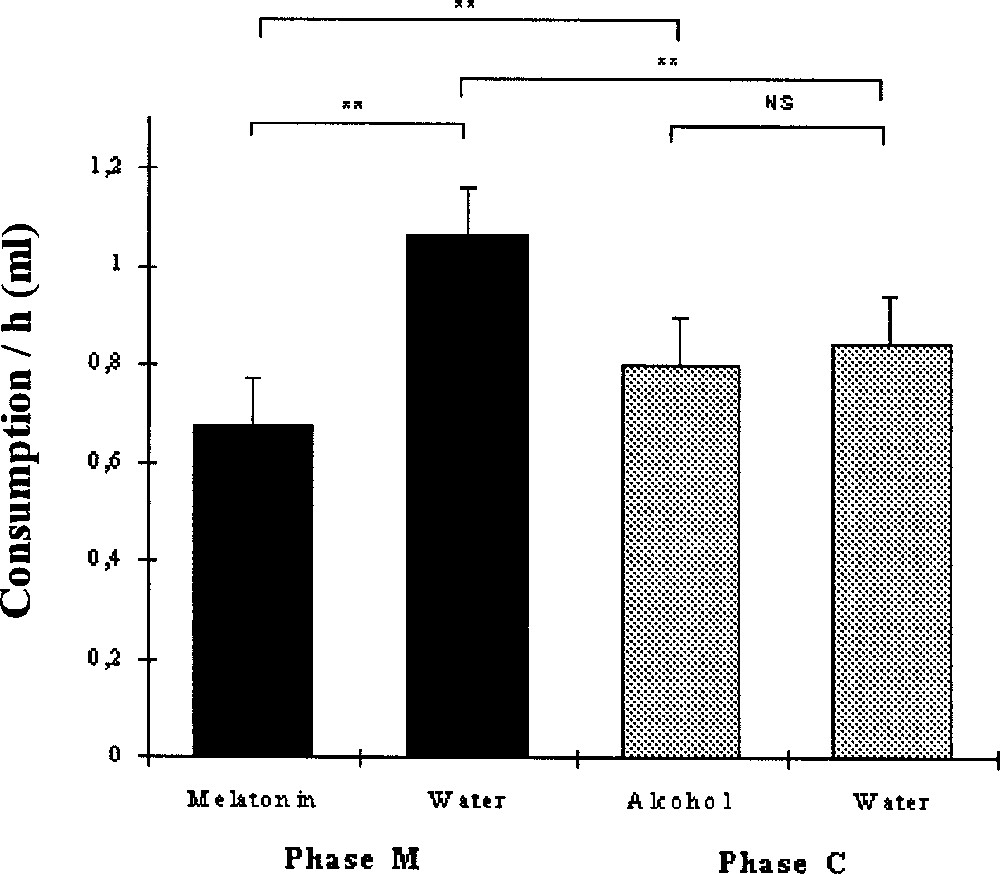
Mean liquid consumption per hour and per bird (+SE) of melatonin solution and water during the M phase, and of alcoholic solution and water during the C phase. Wilcoxon: **, p < 0.01.
3.2 Plasma concentration of melatonin
In constant darkness, before the W1 phase, eight quails showed a rhythmic circadian activity, while the two other birds presented an arrhythmic circadian activity (see next paragraph). Their rhythmic phenotypes were identical at the end of the experiment. Then, for the arrhythmic birds, the melatonin levels of the two daily blood samples were pooled. One rhythmic bird died at the end of the M phase. During the M phase, the assays of the nine treated birds were regrouped, independently of their endogenous rhythmicity.
At the end of the experiment, the plasma melatonin levels of the seven remaining rhythmic birds during the active phase (16.1 ± 4.4 pg ml–1, N = 7) were lower than those during the inactive phase (37.5 ± 5.2 pg ml–1; Wilcoxon, p = 0.018). The melatonin levels of the two arrhythmic birds (19.8 ± 3.9 pg ml–1, N = 4 data) were not significantly different from those of the rhythmic birds during the inactive (Mann-Whitney, p = 0.058, N = 11) and active phases (p = 0.233, N = 11).
During the M phase, for all the birds, the melatonin levels measured during the melatonin availability phase (1 399.8 ± 245.4 pg ml–1, N = 9) were significantly higher than those measured during the water availability phase (77.8 ± 22.5 pg ml–1, N = 9; Wilcoxon, p = 0.008). The average plasma level was elevated around four times the normal peak night-time levels (around 400 pg ml–1) in Japanese quail exposed to LD 〚8, 17〛, and around 17 times the normal levels (around 100 pg ml–1) in quail maintained under constant dim light 〚18〛. Moreover, these levels were higher than those measured when the birds were free running whatever the active (Mann-Whitney, respectively: p = 0.008 and p = 0.008) or rest (p = 0.008 and p = 0.028) phases.
3.3 Circadian feeding activity
Actograms of feeding activity of rhythmic birds are illustrated in Fig. 2.
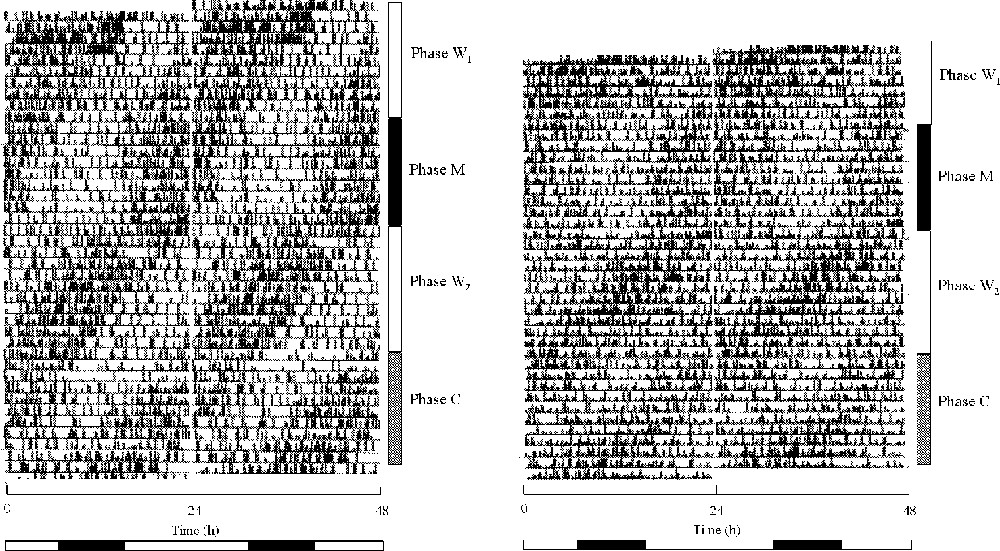
Double plotted actograms of feeding activity of two castrated male quails maintained in LLdim. W1 and W2 phases: phase of constant water as a drink; M phase: phase of melatonin and water administration; and C phase: phase of alcohol and water administration. At the bottom, the white horizontal bar represented the period of water availability, and the black bar the period of melatonin availability during the M phase and of alcohol availability during the C phase. The W3 phase is not illustrated. When we gave the cyclic melatonin, the rest phase corresponded to the period of melatonin availability. Observe on the left actogram that the activity offset progressively advanced.
3.3.1 Circadian period
During the W1 phase, the rhythmic individuals presented a mean circadian period of 22.9 ± 0.2 h (N = 8). During the M phase, the circadian period increased significantly to reach a mean value of 23.9 ± 0.2 h (Wilcoxon, p = 0.011, N = 8; Fig. 3). At this time, the active phase corresponded to the period of water availability and the inactive phase to that of the melatonin availability. During the W2 phase, the circadian periodicity decreased significantly (p = 0.0277, N = 7), and went back to a mean value of 22.9 ± 0.2 h (N = 7). The period remained stable during the C phase (22.8 ± 0.2 h, p = 0.18, N = 7) and during the W3 phase.
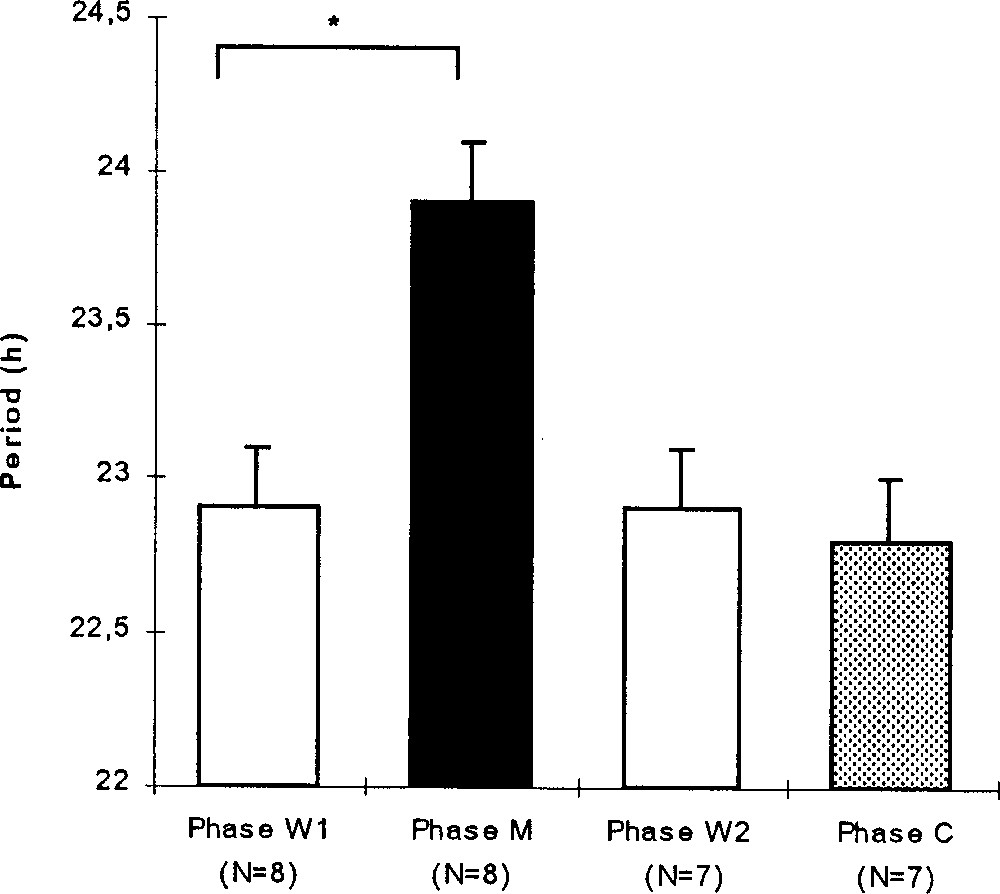
For the rhythmic birds, mean (+SE) circadian period (hour) during the experimental phases: before (W1), during (M) and after (W2) the melatonin treatment and during the control phase (C). Wilcoxon: *, p < 0.05 (one male died during the M phase).
During the experimental phases, the circadian period showed significant variations (Friedman, p = 0.0023, N = 7). The circadian period during the W2 and C phases were similar to those expressed during the W1 phase (respectively: p = 0.414, p = 0.194).
The two non-rhythmic birds remained arrhythmic throughout the experiment, even if a hint of rhythmicity of a 24-h period appeared for one bird during the M phase.
3.3.2 Clarity of the circadian rhythm
For all the birds, the area of the spectrum peak varied significantly during the experiment (Friedman, p = 0.0191, N = 9, Fig. 4). The index of rhythm clarity during the W1 phase (3.8 ± 0.9, N = 10) did not change significantly during the M (3.3 ± 1.1, N = 10; Wilcoxon, p = 0.327) and W2 phases (3.5 ± 1.0, N = 9; p > 0.99). During the C phase, the clarity of the circadian rhythm decreased significantly (1.4 ± 0.5, N = 9; p = 0.0116).
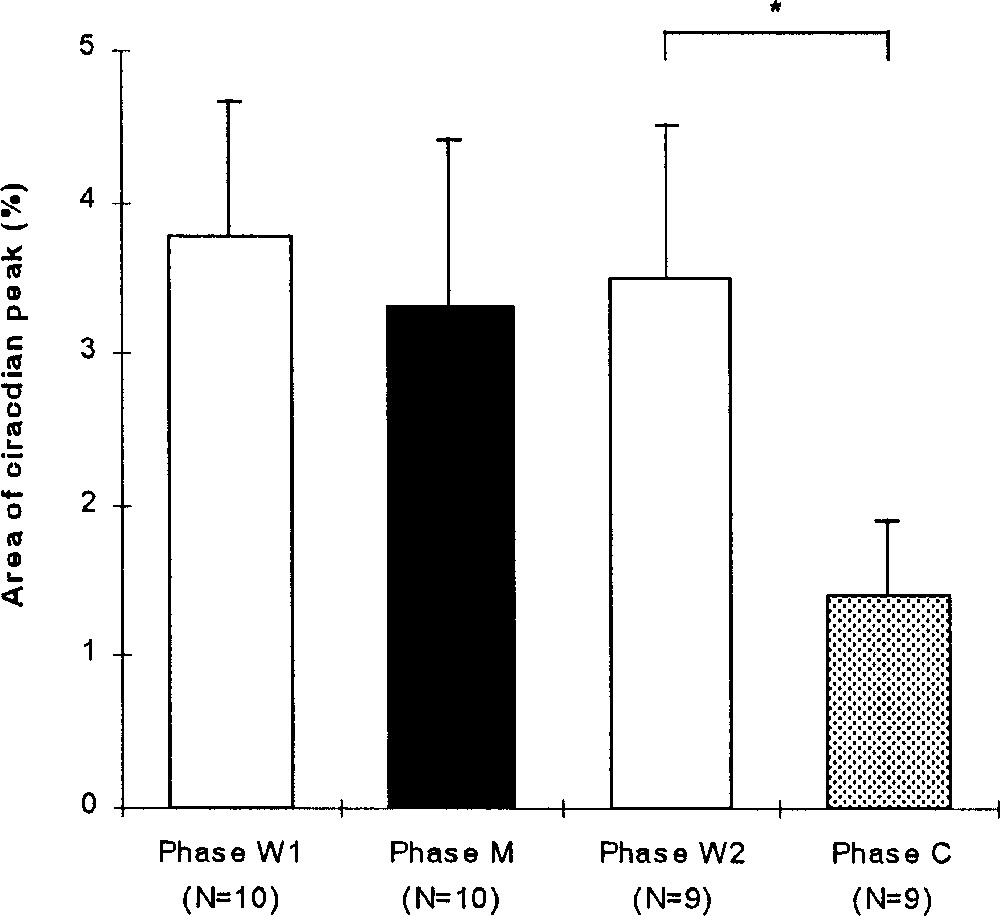
Mean (+SE) area of the spectrum peak (%) expressing the clarity of the circadian rhythm during the experimental phases: before (W1), during (M) and after (W2) the melatonin treatment and during the control phase (C). Wilcoxon: *, p < 0.05.
Moreover, during the W3 phase, we found a significant positive correlation between the area of circadian peak and the ratio between the melatonin levels of the inactive and of the active phases (y = –1.01 + 1.85 x, r2 = 0.47, p = 0.043, N = 9; Fig. 5).
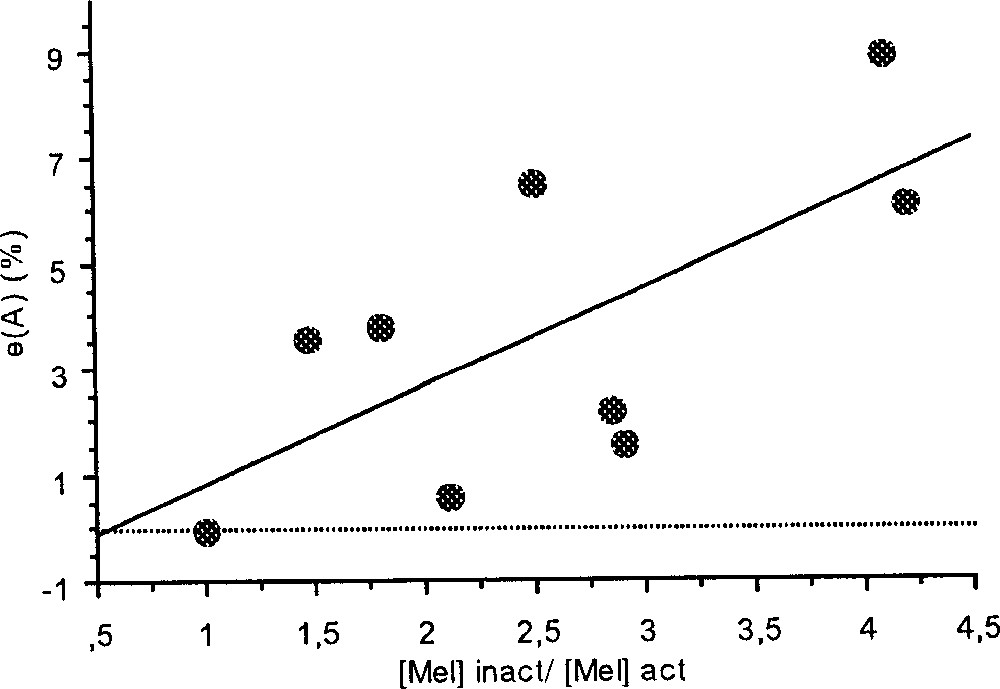
Correlation between the area of circadian peak and the ratio of plasma melatonin levels of the inactive phase and of the active phase measured on the W3 phase (y = –1.01 + 1.85 x, r2 = 0.47, p = 0.043, N = 9).
3.3.3 Level of activity per cycle
The amount of activity per cycle varied over the experimental phases (Friedman, p = 0.0075, N = 9; Fig. 6). The level of activity was equal to 778 ± 91 (N = 10) during the W1 phase and decreased significantly during the M phase (Wilcoxon, p = 0.0152, N = 10), and during the C phase (Wilcoxon, p = 0.0077, N = 9), and did not change during the W2 phase (Wilcoxon, p = 0.0858, N = 9).
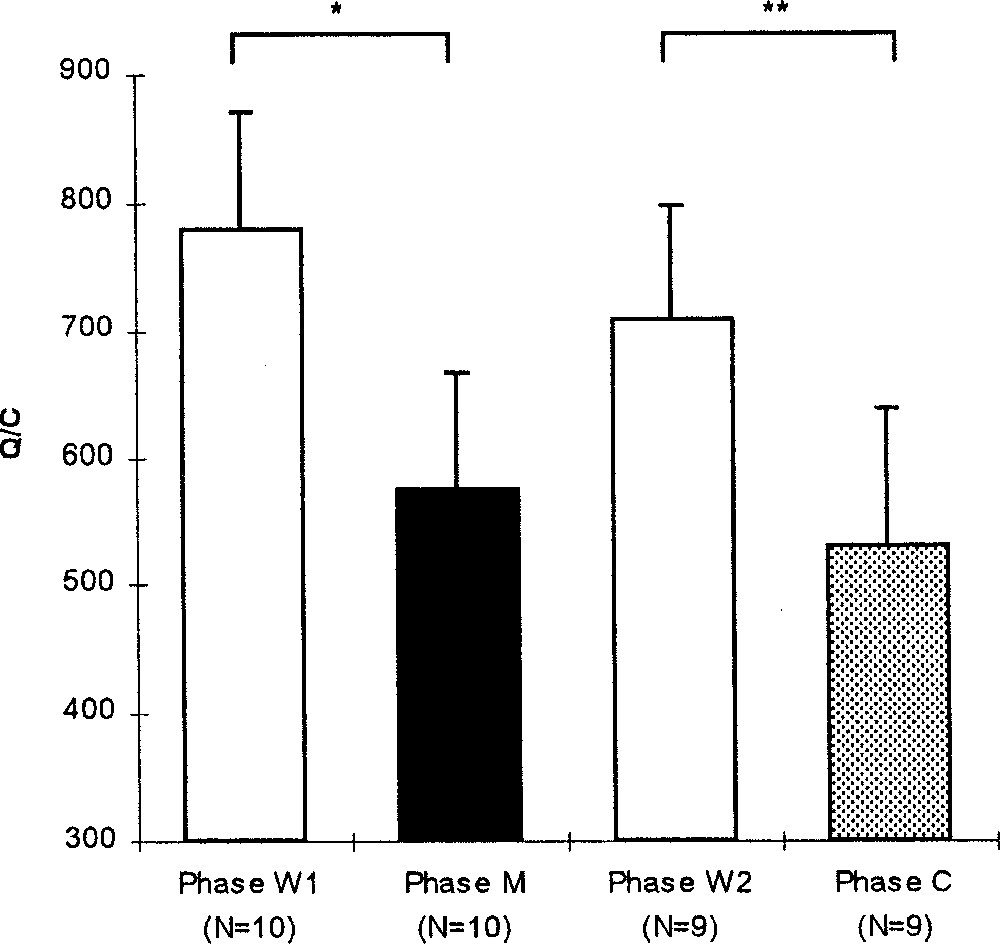
Mean (+SE) level of activity per cycle during the experimental phases: before (W1), during (M) and after (W2) the melatonin treatment and during the control phase (C). Wilcoxon: *, p < 0.05; **: p < 0.01.
4 Discussion
Our results show that the cyclic administration of exogenous melatonin synchronised the circadian rhythm of feeding activity in the rhythmic free running quail, leading to a circadian period of about 24 h. Moreover, when we stopped the melatonin treatment as well as during the control phase, the circadian period went back to the initial value. As these experiments were carried out on castrated male birds expressing a very short circadian period in dim light, and seeing that the triggering was rapid and effective in spite of a difference of 1 h between the endogenous period and that of the cyclic melatonin administration, this demonstrates the important function of this hormone on feeding rhythm. There was also a phase controlled by cyclic melatonin feeding beginning when the hormonal signal decreased. The activity offset was less regular: feeding can stop, whereas exogenous melatonin had not yet increased. This could perhaps depend on the individual duration of active phase or this could also be linked to the circadian melatonin endogenous signal 〚17〛.
The circadian activity of the two arrhythmic birds did not significantly change with the melatonin treatment, although we confirmed the administration of melatonin by liquid intake. Arrythmicity in quail reared in dim light could be different to arrhythmicity observed in constant darkness and that due to enucleation 〚10, 14〛. In constant dim light, the rhythmic process, which is not working, cannot be synchronised.
The clarity of the rhythm did not vary throughout the experiment, except during the control phase, where it decreased. This could be due to the disruptions of the approximately 23-h period rhythm by the 24-h period indoors in the chambers twice a day in order to change the drink. This was not improved by melatonin administration, perhaps for the same reason or because the end of the active phase can proceed, increasing melatonin.
Finally, the level of activity decreased with melatonin administration, melatonin showing an inhibitory effect on feeding activity, as already demonstrated in former experiments 〚9〛.
So, these results support the hypothesis that melatonin, as hormonal output of the oscillatory system in quail, controls the temporal organisation of some overt rhythms, such as that of the feeding activity.
Our results are similar to those of Underwood and Edmonds 〚10〛, who worked on body temperature and locomotor activity in sexually developed females. The synchronisation of feeding activity via melatonin secretion in immature females must be checked before extending this result as a specific regulation process for feeding activity. Nevertheless, our results are contrary to the recent ones of Murakami’s team 〚19〛, who found no triggering of the locomotor rhythm after periodic melatonin administration in free running quails. However, they administrated the hormone by daily subcutaneous injection. This method may have worked in mammals, like rats for instance 〚20〛, but would not work in Japanese quail. Moreover, the time of injection in the endogenous circadian organisation can be important.
If daily melatonin rhythm can synchronise the feeding cycle, what could be the role of this hormone when the rhythm is free running? We found a very slight difference of plasma melatonin during the inactive (37.5 pg ml–1) and the active (16.1 pg ml–1) phases of rhythmic birds maintained under constant dim light. These levels are much lower than the concentrations found previously by Meyer and Millam 〚18〛 in birds maintained in the same conditions or in constant darkness (approximately 100 pg ml–1) but are close to the concentrations measured by Kumar and Follett 〚17〛 after four cycles in birds maintained in constant darkness (15–50 pg ml–1). Moreover, it appeared that the amplitude of plasma melatonin under light-dark conditions depended on the duration of the photophase. It seems that under constant dim green light, a circadian rhythm of plasma melatonin can persist with very small variations, which could be the results of some melatonin production by the retina and the pineal gland. In our experiment, we found a link between the ratio of the plasma melatonin levels during the inactive and active phases and the clarity of the endogenous rhythm. These slight variations could be necessary and sufficient to induce circadian rhythm in these conditions. These results were supported by the fact that we found constant levels in plasma melatonin in the arrhythmic birds. However, this has to be checked, seeing that in our experimental conditions only two birds were arrhythmic.
The duration of the active phase is also an important criteria for circadian activity, but is difficult to estimate for the less rhythmic birds. We noticed on the actograms of the more rhythmic quails that there seemed to be the same values of duration in free running conditions and after synchronisation by melatonin. Thus, it seemed to be controlled by another factor than melatonin. Our previous studies of selection supported the idea that this feature of ‘duration of the active phase’ could be also genetically controlled 〚15〛. If the duration of the active phase seems to be an individual characteristic, we can also observe that during the M phase, if the active phase began when water replaced the melatonin solution, it can also stop even if melatonin was not yet available. The triggering of the beginning of the active phase by the melatonin fall seems more efficient. Nevertheless, other experiments are needed to confirm such an hypothesis.
Version abrégée
L’implication de la mélatonine dans le contrôle de la rythmicité circadienne a été démontrée chez de nombreux oiseaux, mais les résultats peuvent être très variables selon les espèces. Chez la caille japonaise, observée en conditions photopériodiques, les niveaux de mélatonine plasmatique sont élevés au cours de la nuit, alors que l’oiseau est inactif au moins pour son comportement alimentaire, et faibles le jour. Chez cette espèce, deux sources principales de sécrétion de mélatonine existent : la glande pinéale et la rétine, ce qui rend difficile l’annulation expérimentale de tout signal endogène. En effet, l’ablation de la glande pinéale seule ne provoque pas de changement du rythme d’activité locomotrice en libre-cours en obscurité continue. En revanche, une énucléation bilatérale provoque de grandes variations, allant jusqu’à la disparition de ce rythme et de celui de la température corporelle. Par ailleurs, des expériences d’administration de mélatonine exogène selon un rythme de 24 h ont montré la possibilité de synchroniser le rythme circadien de température corporelle chez des femelles sexuellement développées. Cependant, l’influence des stéroïdes sur l’organisation temporelle circadienne est maintenant bien établie. Ainsi, nous nous proposons de rechercher le rôle de la mélatonine chez la caille japonaise en observant l’effet d’une administration cyclique de cette hormone dans l’eau de boisson. L’expérience sera menée sur des individus issus de parents ayant montré une rythmicité nette en libre cours, ce qui favorise l’obtention de descendants aux mêmes caractéristiques. Des mâles castrés seront testés afin d’annuler tout effet des stéroïdes sexuels. De plus des oiseaux castrés en libre cours expriment des périodes du comportement alimentaire relativement courtes, ce qui permet de bien visualiser une synchronisation effective.
Pour cela, 10 mâles castrés ont été maintenus en lumière tamisée constante. L’expérimentation est alors divisée en cinq phases successives, durant chacune 10 j. Les oiseaux ont reçu comme boisson une solution de mélatonine concentrée à 15 μg ml–1 (phase M) ou une solution contrôle (phase C) durant 9 h sur un cycle de 24 h, avec de l’eau à disposition durant les 13 h restantes. Avant et après chacune de ces deux phases, les oiseaux disposaient d’eau en continu (respectivement phases W1, W2 et W3). Pour vérifier l’ingestion de mélatonine, nous avons mesuré la consommation de boisson à chaque changement de boisson. De plus, pour doser la concentration de mélatonine plasmatique, des prélèvements sanguins ont été réalisés durant la phase W1 pendant les phases active et inactive, ainsi que durant la phase M avant et après la mise à disposition de la solution de mélatonine. L’activité alimentaire individuelle est enregistrée automatiquement à l’aide de détecteurs à infrarouge au-dessus de la mangeoire et connectés à un ordinateur, qui comptabilise toutes les 12 min le nombre de coupures de faisceaux. L’activité est ensuite analysée par analyse de spectre.
Au début de l’expérience, huit cailles ont présenté une activité alimentaire rythmée, avec une période circadienne de 22,9 ± 0,2 h, les deux autres oiseaux étant arythmiques au niveau circadien. Au cours de la phase M, la concentration de mélatonine plasmatique dosée durant la phase de traitement hormonal confirme l’ingestion de l’hormone. Durant cette phase, pour les oiseaux rythmés, la période circadienne s’est allongée jusqu’à 24 h (23,9 ± 0,2 h), la phase inactive des oiseaux correspondant à la période de disponibilité en mélatonine. Pendant les phases W2, C et W3, la période circadienne n’était pas différente de celle exprimée pendant la phase W1. La clarté du rythme circadien n’a pas changé, sauf pendant la phase C au cours de laquelle elle a diminué de manière significative, ce qui peut être expliqué par le dérangement des oiseaux lors du changement de boisson. De plus, pendant la phase W1, nous avons trouvé une relation positive significative entre la clarté du rythme circadien et le rapport entre les niveaux plasmatiques de mélatonine pendant la phase inactive et la phase active. Enfin, la quantité d’activité a diminué significativement pendant les phases M et C.
Nos résultats montrent une synchronisation du rythme circadien d’activité alimentaire par l’administration cyclique de mélatonine exogène chez des cailles castrées en libre cours. Ils supportent l’hypothèse du rôle de cette hormone dans la régulation du système circadien.



