1 Introduction
Cold-hardiness is the ability of organisms to survive short or prolonged exposures to low temperatures, with or without freezing 〚1–8〛. Some species maintain their body fluids supercooled above the temperature of crystallisation (Tc), or supercooling point, the temperature at which spontaneous freezing occurs. Others freeze and survive ice formation in the body fluids below their Tc. The mode of freezing tolerance is much less widespread than freezing susceptibility, and generally associated with a rather high Tc. Freezing susceptibility is probably a primitive property of species native to warm areas for supercooling is an innate capacity, dependent especially on the molecular concentration in the body fluids 〚9〛. Freezing tolerance is an outstanding and probably derived feature; this is the most successful mode of frost resistance in regions where insects are exposed to very low temperatures for long periods, as in arctic regions 〚2, 6, 10〛.
2 The evolution of frost resistance
There are three basic reasons to consider that freezing tolerance arose after freezing susceptibility in arthropods during evolution.
- • (i) Phylogenetically. Among the earliest fossil hexapods, which date from Devonian, all the Collembola studied so far are freezing-susceptible 〚11〛. Primitive arthropods like mites, spiders, millipedes are also freezing-susceptible. A small size also enhances supercooling ability for physical reasons 〚12〛. In insects, freezing tolerance appears in a few species of large Exopterygota, e.g. the New Zealand alpine weta Hemideina maori (Orthoptera: Stenopelmatidae) 〚13〛, but is more common in higher winged insect orders (Endopterygota) such as Coleoptera, Hymenoptera, Lepidoptera, and Diptera.
- • (ii) Ontogenetically. All arthropod eggs are reportedly acknowledged freezing-susceptible 〚7〛, as also are newly hatched larvae; freezing tolerance, if need be, starts in overwintering larvae 〚14〛. The theory that the ontogeny of an individual recapitulates the phylogeny of its group cannot be expressed as a general principle, but this Haeckel’s biogenetic law of recapitulation 〚15〛 can be invoked as a convergent argument, suggesting that the young developmental stages currently reproduce the frost resistance mode of their early ancestors. Identifying the real nature of the repetition of past phylogenetic stages in ontogenetic stages of descendants unquestionably deserves a much greater attention in ecophysiology.
- • (iii) Ecologically. In temperate and cold regions, as the seasons progress, some species switch from freezing susceptibility in summer to freezing tolerance in winter as in the case of the wood mould long-lived Osmoderma eremita (Coleoptera: Cetoniidae) larvae in a temperate deciduous forest near by Paris 〚16〛. The reverse is not true, i.e. freezing-susceptible species in winter are never freezing-tolerant in summer. In fact, the freezing-tolerant species do not survive freezing all year long in the field. They generally alternate potential freezing susceptibility in summer and freezing tolerance in winter. Some species, as the overwintering larvae of Cucujus clavipes (Coleoptera: Cucujidae) and Dendroides canadensis (Coleoptera: Pyrochroidae), are even freezing-tolerant in harsh winters, but freezing-susceptible in most years 〚6〛. Only very few insect species are known to be capable of a year-round freezing tolerance: the adults of the New Zealand alpine weta 〚13〛, the larvae and adults of the New Zealand alpine cockroach Celatoblatta quinquemaculata 〚17〛, the adults of Phyllodecta laticollis in Norway (Coleoptera: Chrysomelidae) 〚18〛 and the woolly-bear larvae of Gynaephora groenlandica (Lepidoptera: Lymantriidae) 〚19〛. In this last case, the caterpillars, living in the Canadian High Arctic Archipelago and Greenland, survive freezing throughout the year, but are more freezing-tolerant in winter than in summer.
Avoiding freezing by supercooling or tolerating ice formation in the body tissues and fluids is not a guarantee of winter survival, if we consider the cumulative effect of cold. In a rather recent review 〚20〛, it was suggested that there are five distinct situations representing decreasing levels of cold-hardiness, where death may occur: (i) at some temperature below the Tc, (ii) when the organism freezes, (iii) after prolonged chilling at moderate to low sub-zero temperatures above the Tc, (iv) after brief chilling at moderate to high sub-zero temperature, (v) when temperatures are too low to maintain normal metabolism and the species is unable to enter dormancy. This classification is based on ecologically relevant criteria and emphasises that an evolutionary approach based strictly on the two modes of frost resistance, i.e. freezing susceptibility vs freezing tolerance, is oversimplified 〚9〛.
3 A practical typology
From an evolutionary viewpoint, we suggest that there are not two, but three basic modes of frost resistance (Figs. 1–3):
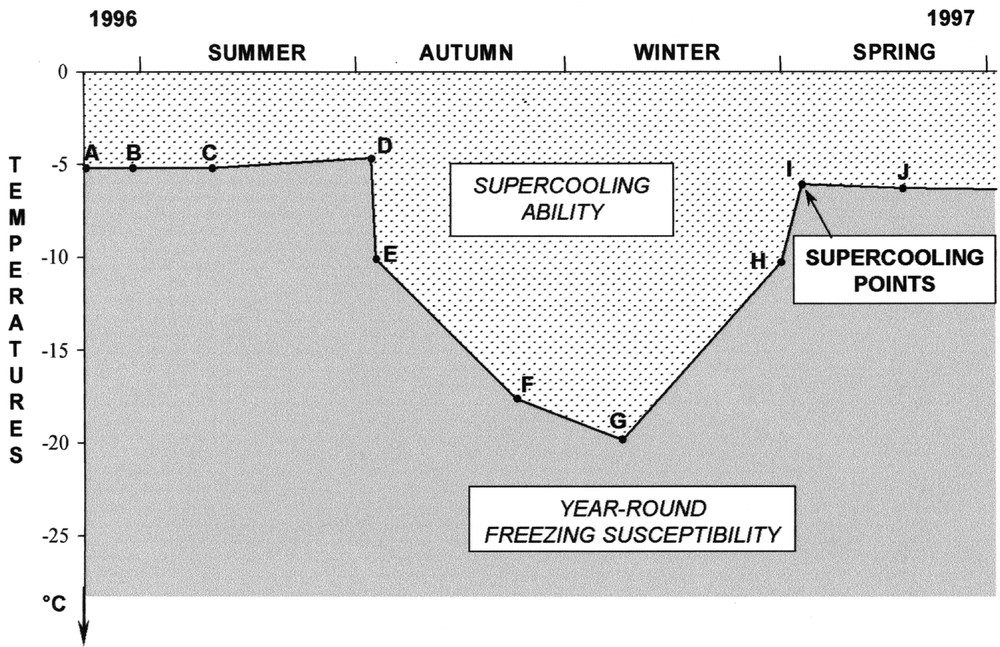
Year-round freezing-susceptible species. Example: the beetle Gnorimus nobilis L. (Coleoptera: Cetoniidae). A, B: First instar larvae with food-filled gut; C: second instar larvae with food-filled gut; D: third instar larvae with food-filled gut; E, F, G: third instar larvae with empty gut; H: third instar larvae with empty gut, inside a mould cell; I: nymphae with empty gut, inside a mould cell; J: newly-emerged adults. Supercooling point is the temperature at which ice crystallisation begins. Data from 〚16〛.
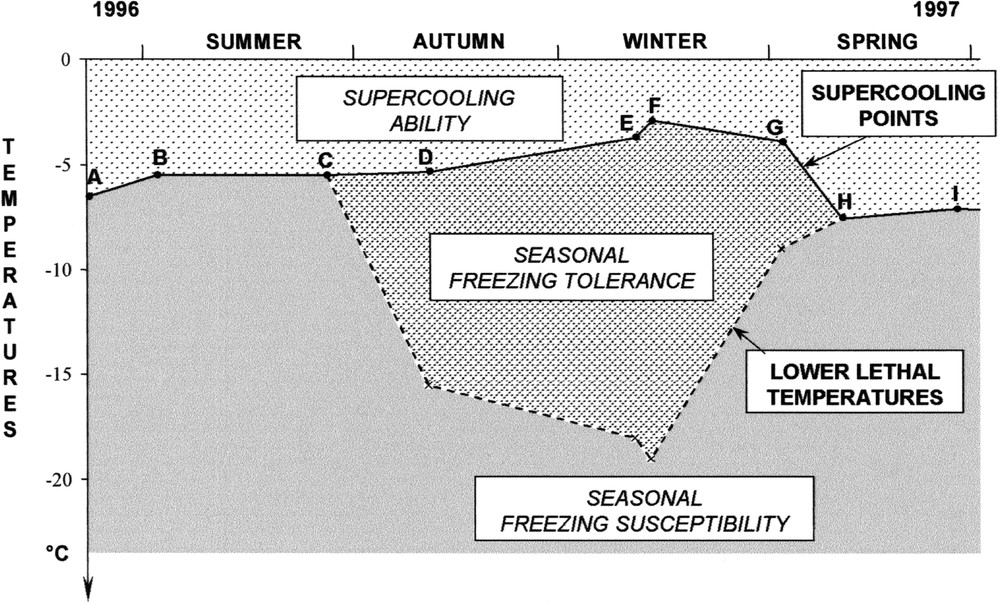
Seasonal freezing-susceptible and freezing-tolerant species. Example: the beetle Osmoderma eremita S. (Coleoptera: Cetoniidae). A: Second instar larvae with food-filled gut; B, C, D, E: third instar larvae with food-filled gut; F, G: third instar larvae with food-filled gut, inside a mould cell; H: nymphae with empty gut, inside a mould cell; I: newly emerged adults. Lower lethal temperature is the freezing temperature at which no survivor is observed. Data from 〚16〛.

Year-round freezing-tolerant species. Example: the cockroach Celatoblatta quinquemaculata J. (Dictyoptera: Blattidae): no specific instar was targeted, and age and size of cockroaches were not recorded. In this study, lower lethal temperature is the freezing temperature at which an insect has a 50% probability of survival. Redrawn from 〚17〛.
- • (i) permanent or year-round freezing-susceptible species, which occur from the equator to higher latitudes and altitudes, and includes three of the five above cold-hardiness classes (i.e. freezing avoidance, chill tolerance and chill susceptibility) (Fig. 1);
- • (ii) alternative or seasonal freezing-susceptible (in summer) and freezing-tolerant (in winter) species, which are less common and live mostly in temperate, alpine and circumpolar regions (Fig. 2). Seasonal freezing tolerance also arises in terrestrially hibernating vertebrates, like amphibians and reptiles 〚21〛. Among the few freezing-tolerant amphibians, the wood frog, Rana sylvatica, can survive the freezing of 65–70% of its body water at temperatures as low as –3 to –6 °C and can tolerate freezing exposures lasting more than four weeks 〚22〛. R.E. Lee (personal communication) recalls that most amphibians freeze inoculatively through contact with external ice at the melting point of their body fluids;
- • (iii) permanent or year-round freezing-tolerant species, of which only four cases in insects have been described so far (see above) (Fig. 3).
4 To freeze or not to freeze?
Following this Shakespearian interrogation 〚4, 5, 23, 24〛, a complementary issue (how many kinds of frozen?) has recently been clarified 〚24, 25〛. The next intriguing question might be now: what are the selective advantages, if any, to be a freezing-susceptible or a freezing-tolerant species? We have to keep in mind that freezing tolerance may have evolved many times in taxonomic isolates. For instance, freezing tolerance was found in all arctic larvae of Chironomidae (Diptera); in temperate areas, freezing-tolerant larvae were nevertheless freezing-susceptible in summer 〚26〛. If we take into account the cumulative cold effect, freezing tolerance is more beneficial than freezing susceptibility: in the freezing-tolerant species Osmoderma eremita (Coleoptera: Cetoniidae), third-instar larvae with a mean Tc of –5 °C, placed at –10 °C for 12 days were all alive 〚16〛, when in many so-called freezing-susceptible species, extensive prefreeze mortality is a common pattern 〚27〛. We have also to emphasise that freezing susceptibility and freezing tolerance are not fundamentally conflicting modalities as overwintering survival closely depends on the regulation of ice nucleation – either by avoidance or acceptance – in the body extra-cellular fluids of ectothermic organisms 〚28–35〛.
5 Conclusion
Cold hardiness studies in terrestrial arthropods have resulted in a tremendous increasing literature. Our objective was not to confuse an already complicated situation as regards subtle freezing resistance strategies, but on the contrary to suggest the use of a simple consensual typology. When Salt 〚1〛, more than forty years ago, while recognising the importance of chilling and cold acclimation, simply suggested two principles of freezing resistance, i.e. freezing tolerance and avoidance of freezing by supercooling, Bale 〚20, 36〛 characterises five classes of insect cold hardiness, Sinclair 〚25〛 considers that freezing tolerance may be divisible by itself into four groups, and Nedved 〚37〛 describes eight classes of cold tolerance, named after Snow White and the Seven Dwarfs. It is now necessary to assess the heuristic value of these different classifications, including the position we defend here, and to go deeper into the physiological knowledge of prolonged exposures to cold 〚32, 38〛.
About the occurrence of possible phylogenetic constraints in cold hardiness strategies, Block 〚28, 39〛 had suggested in the eighties that freezing tolerance might be restricted to holometabolous insects. Recent works 〚25, 40〛 do not support this idea, but also emphasise the general weakness of phylogenetic signals. A better comprehension of the physiological processes which may act as a link between arthropods phylogeny and cold hardiness is hence highly desirable, even if the task is difficult: as convincingly emphasised by P. Dejours, the nature of the milieu entails special designs and functions. “Many physiological traits, although supported by quite different biochemical and morphological structures, are convergent and related to the environment characteristics” 〚41〛. Moreover, broad scale investigations of physiological tolerances (e.g. cold hardiness differences between northern and southern hemispheres) are currently the subject of convincing studies 〚40, 42, 43〛.
A last point must be mentioned: cold hardiness abilities and diapause syndrome are frequently associated, mainly because they both participate to insects winter survival in temperate areas. However, the relationship between cold hardiness and diapause is complex: the fact that many insects display a great cold hardiness during diapause does not mean that cold hardiness is always a component of the diapause syndrome 〚44, 45〛. The position defended in this paper, i.e. the likely derived condition of freezing tolerance, may cast new light on this relationship. We suggest that, even if some insect diapausing species remain freezing-susceptible during winter, freezing tolerance only occurs in diapausing ones. We currently examine, from a physiological point of view, the validity of this assumption and its possible ecological consequences.
Acknowledgements
We thank H.V. Danks, R.E. Lee, Jr., J. Roy and B.J. Sinclair for helpful critical commentary on previous versions of the manuscript. An anonymous reviewer provided valuable comments.



