1 Introduction
Members of the class Cephalopoda are characterised by numerous apomorphies (i.e. central nervous system, closed circulatory system, direct development after hatching). Extant cephalopods are classified into two subclasses, Coleoidea and Nautiloidea. Coleoidea include all present forms of squids and cuttlefishes (Decabrachia), and octopuses (Octopoda) (ca 650 species). Their evolution and biodiversity has recently been revised [1].
Nautiloidea, the only extant cephalopods with an external shell, are clearly monophyletic. The number of living nautiloids is small and their present geographic distribution is restricted to the central Indo-Pacific. The specific composition of the genus Nautilus is not yet well established. Some species are known only from the description of drift shells, without observation or description of the animals. Based on shell morphology alone, a total of eleven species and three variants have been named [2]. Saunders (1987) [3] recognised five species (pompilius, scrobiculatus, macromphalus, belauensis, stenomphalus). The type species is N. pompilius (type locality: Ambon), which is also the most widely distributed since it occurs over almost the entire range of distribution of the genus. The other species have more restricted distributions: some occur alone in one region, others coexist with N. pompilius. The number of valid species is still a subject of debate. Partial sequences of 16S rDNA mitochondrial gene (3′ end of the molecule, 449bp; [4]) for some Nautilus species, collected from a variety of geographical localities, have established phylogenetic relationships and revealed that most Nautilus species are grouped into geographical clades. N. scrobiculatus appears as the only clearly distinct species and was placed later in a distinct genus, Allonautilus [5].
Recently, phylogenetic studies based on nuclear and mitochondrial gene sequences have helped clarify cephalopod taxonomy [6,8–10]. 18S rDNA sequences are available in a large range of taxa including cephalopods [9,11]. In order to test the taxonomic status of N. macromphalus and its relationships with other species, the complete 18S rDNA gene of N. macromphalus is described here and its structure compared to that of N. pompilius and N. scrobiculatus from databases.
In parallel with morphological and molecular studies, cytogenetic data are increasingly being used in taxonomic analyses, at all hierarchical levels [12]. The karyotype of N. macromphalus is compared to that of other species [13,14,16,17] and the molecular and karyological data analysed in a taxonomic and phylogenetic context at both intrageneric and suprageneric levels.
2 Materials and methods
Nautilus macromphalus specimens were collected on the outer shelf of New Caledonia (22°33′S; 166°25′E). Small pieces of gill tissue from frozen (−20 °C) specimens were fixed in 70% ethyl alcohol. DNA was extracted as described in Bonnaud et al. [6].
The complete 18S rDNA gene was amplified in polymerase chain reaction using the following primers: ATGCTTGTCTCAAAGATTAAGCC (between region 4 and 5 – Fig. 1) and AATTACCGCGGCTGCTGGCA (helix 20), TACTTGGATAACTGTGGTAA (helix 9) and AAATCGCTCCACCAACTAAGAA (helix 40), GGGCGGACACCGGTAGGATTGAC (helix 37) and AATGATCCTTCCGCAGGTTCAC (helix 50). PCR products were purified and cloned. Several clones of each 18S rDNA portion were sequenced in both strands (EMBL accession number AJ301606). The complete 18S rDNA gene sequence of N. macromphalus (submitted to EMBL under the accession number AJ301606), N. pompilius and N. scrobiculatus (accession numbers AF207641 and AF120504, respectively) were compared taking into account the secondary structure features.

Secondary structure of 18S rDNA molecule of Nautilus, based on secondary structure database [7]. The numbers indicate the loops and helices. The square area (E23-1 to E23-5) indicates large sequences for which it was not possible to establish loops and helices.
For karyotype determination, three mature males were used. One was dissected on the day of capture (A), another was injected with colchicine 0.5% (0.3 ml per 100 g of fresh animal) and kept in aquarium for 24 h prior to dissection (B), the third was brought back alive to Paris and dissected upon arrival (C). Gills, mantle and testis were sampled and directly dilacerated in the hypotonic media (i.e. 0.55% KCl and distilled water) where they were kept for 30 to 70 min prior to fixation with three baths (10, 20, 30 min) of a freshly prepared solution of iced methanol-acetic acid (3:1). The pellet from the last centrifugation was resuspended in 2 ml fixative, then deposited on slides and stained with Giemsa 4%.
3 Results
3.1 Nuclear 18S rDNA gene analysis
The 18S rDNA of Nautilus macromphalus consists of 2246 nucleotides, intermediate between that of N. pompilius (2182 nucleotides) and that of N. scrobiculatus (2485 nucleotides).
The sequences of the three Nautilus species are very similar in the regions identified as constant in mollusc sequences (helix 9, 11 to 23, E23-6 to 27, 31 to 42 – Fig. 1), but they differ both in composition and size in regions known to be the most variable (helix 10, E10-1, regions between helix 23 and E23-6, 43 to 45, 49), leading to differences in loop and hairpin structures.
Hairpin 11, hairpin 17 and helix E10-1 are identical in the three nautilus species, but helix E10-1 is longer in nautiloids (169 nucleotides) than in other molluscs (46 to 69 nucleotides) (Figs. 1 and 2). The 3′ end of the molecule is highly variable (essentially in helices 23 to E23-6 in coleoids). Among cephalopods, only Nautilus has a huge insertion of around 200 nucleotides in this region (almost identical in the three species). From helix 25 to the molecule end, N. pompilius and N. macromphalus sequences are very similar, whereas large insertions are present in N. scrobiculatus, especially in loops 43 and 49 (Fig. 2).
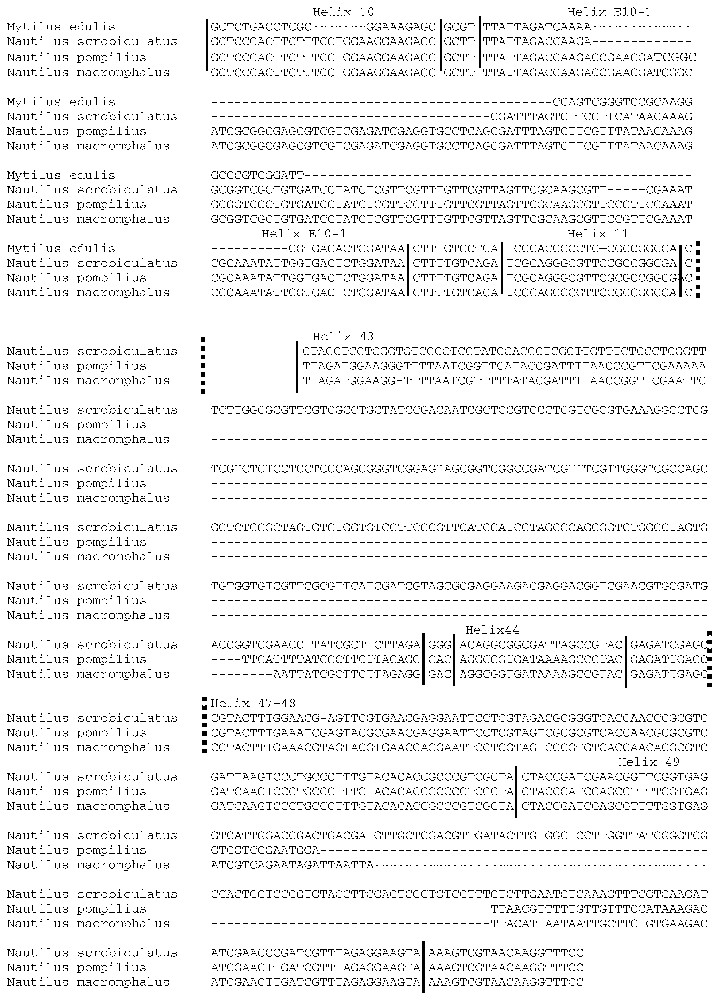
Alignment of the three Nautilus sequences in variable regions: E10-1, 43 and 49.
3.2 Karyotype of Nautilus
The best karyological results are obtained with testis tissue from individuals B and C, after 50- or 60-min hypotonic treatment in distilled water. The chromosomes are condensed into round bodies. They appear rudimentary, including many forms with one arm and only one or two larger bi-armed chromosome pairs.
The number of chromosomes in N. macromphalus is 52, the same as in N. pompilius [15], i.e. the lowest among cephalopods. Within coleoids, the number of chromosomes is distinctly lower in Octopoda than in Decabrachia. Mapped on a consensus tree (Fig. 2) built from morphological and molecular phylogenies [6,18,19], chromosome number appears significantly correlated with the lineage history.
4 Discussion
Coupled with gene-sequence analyses, insertions and/or the composition of hairpins and loops are beginning to be considered of interest in phylogenetic reconstruction, systematic grouping and for explanation of molecular mechanisms in an evolutionary perspective [11,20,21]. Partial sequences of mitochondrial and nuclear genes for Nautilus species are now available [4,22]; however, total sequences are rare.
The present results show that 18S rDNA is longer in Nautilus than in any known mollusc, including other cephalopods, where the size of 18S rDNA is around 1850 nucleotides [9].
Being a highly conserved molecule, it was expected that 18S rDNA sequences would be very similar amongst Nautilus species. This is the case for Nautilus macromphalus and N. pompilius, but the N. scrobiculatus 18S rDNA sequence shows significant insertions mainly in the last region of the molecule (Fig. 2). This region could be an interesting tool to estimate genetic variation between Nautilus species, like helix 17 within Rhabditidae (Nematoda) [20] or helix 23 within Rotifera-Acanthocephala [11].
Although five to six morphotypes are generally recognised based on shell morphology (size, colour pattern, general shape and form of the umbilical region), only three species are universally accepted: (1) Nautilus pompilius Linnaeus, 1758, the type species of the genus, distributed over almost the entire range of the genus and possessing a closed umbilical region; (2) N. macromphalus Sowerby, 1849, endemic to New Caledonia, with an intermediate umbilicus opening; (3) N. scrobiculatus [Lighfoot 1786], endemic to Papua-New Guinea, sympatric with N. pompilius, with a large and open umbilical area.
In nautiloids, phenotypic variations do however not necessarily relate to genetic variability. N. pompilius and N. macromphalus are two forms easily recognised by their shell characteristics but neither mitochondrial genes [4] nor 18S (present study) nucleotide differences correspond to the morphological species variation, suggesting a close phylogenetic affinity between these morphologically distinct species. Moreover, the chromosome number is the same, indicating that there has not been a drastic transformation of the genome. Therefore, N. macromphalus is probably a geographic variant of N. pompilius, a hypothesis supported by 16S mitochondrial gene analysis [4].
The N. scrobiculatus 18S rDNA sequence shows specific large insertions in some loops leading to a unique form among nautiloids. Moreover, there is intraspecific variation in helix E10-1 (unlike N. pompilius, data not shown): the database complete sequence is identical to that of the other Nautiloids (169 nt long), whereas the partial 18S rDNA sequence previously obtained from another specimen [22] is only 116 nucleotides long (gaps of 48 and 5 nucleotides – Fig. 2). An intraspecific variation was also observed between 16S rDNA sequences obtained from different specimens of N. scrobiculatus [4]. Polymorphism could be at the origin of speciation events. Ward and Saunders [5] proposed to elevate N. scrobiculatus to a generic rank, coining the genus Allonautilus. Although this raised some controversy [23], the present differences in 18S rDNA sequences observed between N. scrobiculatus and the other representatives of the genus, its apparent intraspecific variability, its morphological characters and the phylogenetic analysis of the different species based on 16S [4], all warrant that N. scrobiculatus is to be placed in the distinct genus, Allonautilus. The karyotype of N. scrobiculatus is still to be established.
A. scrobiculatus is often considered as representing the oldest lineage in Nautilus evolution. Therefore, its larger 18S rDNA gene with many insertions could be the ancestral form of 18S, suggesting that the tendency in nautiloid evolution might be a reduction of the molecule size. Similarly, the ancestral form is the large rDNA gene of nautiloids (compared to other cephalopods), with many insertions. The loss of partial sequences in loops during evolution has lead to smaller rDNA molecules in the coleoid lineage.
Gene sequences, chromosome number and morphology can all be used as phylogenetic indicators [23], conservatism of chromosome number being reported for many mollusc groups. The chromosome numbers of cephalopods (52–112) are the highest among Mollusca (28–32) [16] and their progression appears non-random, following the successive appearance of the lineages (Fig. 3). As Nautilus displays the lowest number of chromosomes among cephalopods, it can be speculated that a small number of chromosomes, shared by Nautilus and octopods, is the ancestral state for cephalopods. However, within octopods, when the chromosome number is almost the same, the forms are clearly different. Eledone cirrhosa and Octopus vulgaris have a higher proportion of ‘small’ chromosomes than O. ocellatus. The number of chromosomes and the proportion of ‘large’ chromosomes are higher in Decabrachia than in Octopoda. Thus, both number and morphology appear to change radically during evolution. If Nautilus chromosome characteristics, i.e. small number and small size, are considered to be close to the ancestral state, then the decabrachian karyotype probably results from chromosome duplication events having potentially occurred at the base of sepiids and teuthoids.
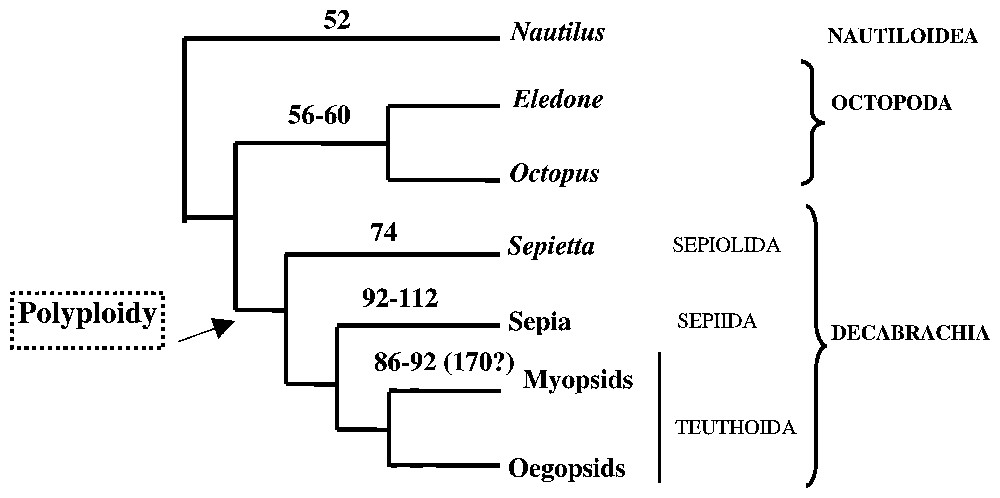
Relationships between chromosome number and phylogenetic analyses, based on a consensus tree built from molecular (16S rDNA [18]) and morphological [19] phylogenies. Chromosome numbers are figured on the corresponding branches. This tree is rooted on Nautilus as ancestral karyotype.
Polyploidy leads to gene duplications. In many ‘recent’ cephalopods (mainly Decabrachia), the existence of different forms of 18S rDNA genes, identified as ‘pseudogenes’ has been demonstrated [9]. Preliminary in situ hybridisation results indicate that Nautilus is apparently the only cephalopod with only one form of 18S rRNA (Bonnaud, unpublished data). Accordingly, ‘pseudogenes’ of 18S rDNA in Decabrachia might be a consequence of a genomic duplication, their chromosome number being higher than in Nautilus.
The presence of insertions in 18S rDNA and the small number of chromosomes could be an ancestral condition in Nautilus and suggest that the derived state is an increased chromosome number and a shorter 18S rDNA gene. Parallel to additional molecular studies, karyological data on a wider range of cephalopod species could be of considerable help to further understand the relationships of mollusc groups in an evolutionary perspective.
Acknowledgements
We are grateful to Pascale Joannot for providing Nautilus specimens and all the facilities at the Aquarium of Nouméa. We thank Lauriana Levy for helping acquiring chromosome images of Nautilus, Cendrine Hudelot and Madeleine Martin for technical support, Mark Norman for critical reading of the manuscript.


