1 Introduction
Abramowitz et al. [1] were the first to demonstrate the presence of diabetogenic principle in the neurohemal sinus gland in the eyestalks of a crustacean. They found that injection of eyestalk extracts of Uca pugilator induced hyperglycaemia in Callinectes. Since then, several workers observed similar results in different crustaceans [2]. The hormone, which is responsible for hyperglycaemic activity is commonly referred to as the ‘crustacean hyperglycaemic hormone’ (CHH). The chemical nature, mode and site of action of crustacean hyperglycaemic hormone were established [3,4]. The amino acid sequence of hyperglycaemic hormone was first determined from Carcinus maenas [5]. Later, several scientists determined amino acid sequence of CHH in other crustaceans [6–12]. The nucleotide sequences of cloned CHH cDNA have been determined in the lobster Homarus americanus [13,14] and in the prawns Penaeus japonicus [15] and Metapenaeus enois [16,17].
The occurrence of opioid peptides and opioid-like substances in crustaceans has been discovered for the past two decades. The presence of leucine-enkephalin has been first reported [18] in the retinular cells of the spiny lobster Panulirus interruptus and crayfish Procambarus clarkii. Later, methionine-enkephalin-like and leucine-enkephalin-like compounds were reported in the neuro-endocrine cells of eyestalks of the fiddler crab, Uca pugilator [19,20]. Though there are sporadic reports on the identification of opioid peptides in the crustaceans [21], there is little information available on the role of opioid peptides in the regulation of physiology. In Uca pugilator, injection of methionine-enkephalin stimulated the release of red-pigment and black-pigment concentrating hormone [22] and distal retinal pigment dark adapting hormone [23]. Injection of a stable methionine-enkephalin analogue, FK-33824 increased locomotor activity in the land crab, Gecarcinus lateralis [24]. Administration of leucine-enkephalin resulted in hypoglucosemia in Carcinus maenas [25] and hypoglycemia in Procambarus clarkii [26]. Injection of methionine-enkephalin significantly slowed ovarian maturation in Uca pugilator and Procambarus clarkii [27–29]. An antagonistic action of opioid peptides in the regulation of ovarian maturation in the freshwater rice field crab, Oziotelphusa senex senex was also observed [30]. A neurotransmitter role of methionine-enkephalin in regulating haemolymph sugar levels has been demonstrated [31]. It was hypothesized that the methionine-enkephalin induces hyperglycaemia through eyestalks in the estuarine crab, Scylla serrata [32] and in the prawns, Penaeus indicus and Metapenaeus monocerus [33]. Recently, we reported that injection of leucine-enkephalin induces both moulting and vitellogenesis in the freshwater crab Oziotelphusa senex senex [34]. In the present study, we report that the eyestalk is the major source for hyperglycaemic hormone and methionine-enkephalin regulates the carbohydrate metabolism through eyestalk mediation in the freshwater rice field crab, Oziotelphusa senex senex.
2 Materials and methods
Intact crabs were collected from rice fields in and around Tirupati (13°38′N, 79°25′E) and maintained in the laboratory at in large glass aquaria partially filled with tap water. They were acclimatized to laboratory conditions (12:12 light:dark) for at least one week before being used for experimentation. The crabs were fed with sheep meat ad libitum and ambient medium was changed every day. Feeding was stopped one day before the commencement of experiment to avoid changes due to prandial activity. Only intermolt (Stage C4) crabs with a body weight were used. For the present experiments, both intact and eyestalk-ablated crabs were used. Eyestalks were removed by cutting off the stalks at base without prior ligation but with cautery of the wound after operation. No mortalities were observed in the crabs and used for experimentation one day after eyestalk ablation.
Haemolymph was collected from intact and experimental crabs through the arthrodial membrane of the coxa of the third pair of walking leg. The crabs were dissected and tissues like hepatopancreas and muscle were isolated. Tissues were weighed and homogenate was prepared separately to estimate the total carbohydrate (TCHO), glycogen levels and to determine the phosphorylase activity.
2.1 Estimation of haemolymph sugar level
Haemolymph sugar levels were estimated according to the method of Carroll et al. [35] and expressed as mg per 100 ml haemolymph.
2.2 Estimation of tissue carbohydrate content
The tissue total carbohydrate and glycogen levels were estimated [35] in 10% trichloroacetic acid supernatant (5% w/v) and ethanolic precipitate of trichloroacetic acid supernatant respectively.
To 0.5 ml of the centrifuged (4000 rpm for 10 min) clear supernatant, 5.0 ml of anthrone reagent was added and the combination was boiled for 10 min in a water bath. The tubes, with their contents, then were immediately cooled. A standard sample containing a known quantity of molar glucose solution was always run along with the experimental samples. The colour was measured at 620 nm in spectrophotometer (Hitachi model U 2001) against reagent. The level of the content was expressed as mg/g wet weight of fresh tissue.
2.3 Assay of tissue phosphorylase
The activity levels of glycogen phosphorylase were assayed [36] in hepatopancreas and muscle tissues, in the direction of glycogen synthesis, by the determination of the amount of released inorganic phosphate from glucose-1-phosphate.
In brief, 0.4 ml of enzyme was incubated with 2.0 mg glycogen for 20 min at 35 °C, then the reaction was initiated by the addition of 0.2 ml of 0.016 M glucose-1-phosphate (G-1-P) to one tube (phosphorylase ‘a’) and a mixture of 0.2 ml of G-1-P and 0.004 M adenosine-5-monophosphate (phosphorylase ‘ab’) to another tube. The reaction mixture was incubated for 15 min at 37 °C for determining total phosphorylase and for 30 min for active phosphorylase. The reaction was stopped by the addition of 5.0 ml of 5 N sulphuric acid and the released inorganic phosphate was estimated.
2.4 Estimation of protein content
The amount of protein in the enzyme source was estimated [37] using bovine serum albumin as a standard.
2.5 Statistical analysis
The mean, standard deviation (SD) and Student's t-test were made using the SPSS version 10.0 (SPSS Inc., Chicago IC).
3 Results
3.1 Determination and location of hyperglycaemic hormone
Eyestalk ablation resulted in a significant () decrease (−16.11%) in haemolymph sugar level when compared to the controls, whereas injection of eyestalk extract into ablated crabs resulted in a significant increase (24.04%) in haemolymph sugar level (Table 1). From the results, it can be concluded that there is a principle present in the eyestalk, which enhances the haemolymph sugar level.
Effect of eyestalk ablation (1-day ESX) and eyestalk extract (ESE) injection into 1-day ESX crabs (1-day ESX–ESE) on the haemolymph sugar level of Oziotelphusa senex senex
| Intact | 1-day ESX⁎ | 1-day ESX–ESE |
| 70.62±5.09 | 59.24±2.33 | 73.51±3.86 |
| (−16.11) | (24.04) | |
| p<0.001 | p<0.001 |
⁎ For calculation of % change and evaluation of p for 1-day ESX crabs, intact crabs served as controls; for 1-day ESX–ESE crabs, 1-day ESX crabs served as controls.
3.2 Time-dependent hyperglycaemic action of eyestalk extract
Hormones and hormone-like substances are metabolized when secreted internally in the organism or given by the way of injection. Hence it is appropriate to study the effect of hormones at different time intervals after hormone injection. The haemolymph sugar level was measured 1, 2, 4 and 6 h after injecting the eyestalk extract (two eyestalk equivalents) in to intact crabs. A significant () increase in haemolymph sugar level was observed in crabs 1 h post injection. Maximal hyperglycaemic effect was found 2 h after injection of eyestalk extract and haemolymph sugar level reached control level 6 h post injection (Fig. 1). It is evident from the data that the circulating hormone is soon inactivated or large amount of released sugars are taken up by the tissues at a rapid rate. In the subsequent studies, 2-h duration after eyestalk extract injection has been selected as an appropriate time interval.
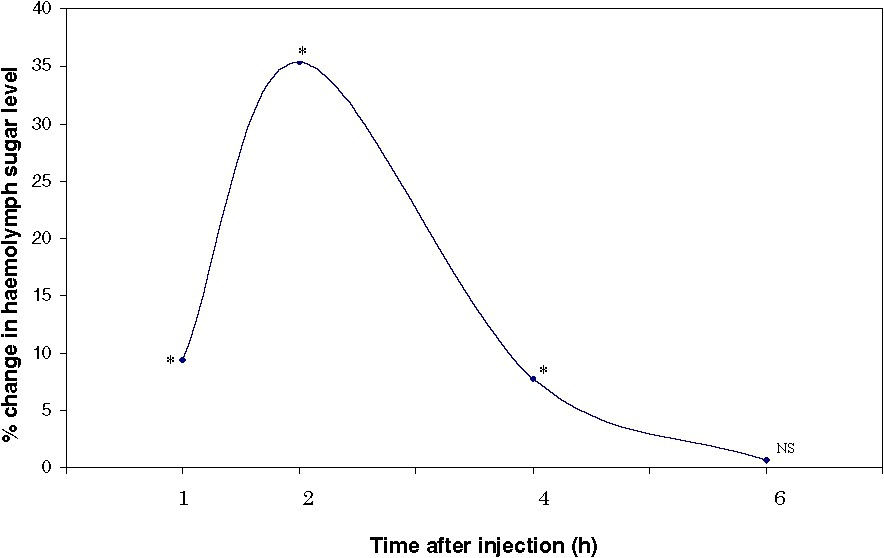
Effect of injection of eyestalk extract on the haemolymph sugar level in Oziotelphusa senex senex at different times post injection (n=12). ∗ Statistically significant at p<0.001. NS Not significant.
3.3 Dose-dependent hyperglycaemic effect
Determination of the dose–response relationship is one of the criteria used for establishing the glandular tissue extract as a hormone. A saturated response commences with a dosage of two eyestalk equivalents of extract in the crabs (Fig. 2). In the subsequent experiments, the concentration of two eyestalk equivalents was selected as the injection dosage.
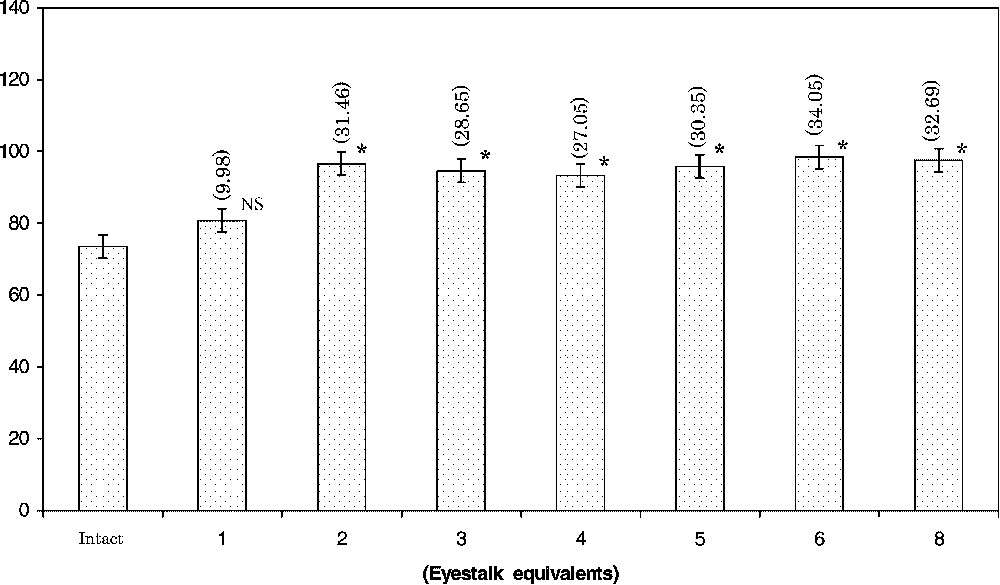
Effect of injection of various concentrations of eyestalk extract (eyestalk equivalents) on the haemolymph sugar level in Oziotelphusa senex senex. Each bar represents mean ± S.D. (n=12). Values in parentheses are percent change from controls. ∗ Statistically significant at p<0.001. NS Not significant.
3.4 Source of hyperglycaemia and mode of action of hyperglycaemic hormone
Total carbohydrates and glycogen levels were significantly increased () in hepatopancreas (36.26 and 43.24%, respectively) and muscle (34.58 and 65.15%, respectively) after eyestalk ablation (Table 2), whereas injection of eyestalk extract into ablated crabs significantly decreased total carbohydrates and glycogen levels in hepatopancreas (−24.71 and −26.58%) and in muscle tissue (−26.15 and −34.86%).
Effect of eyestalk ablation (ESX) and injection of eyestalk extract (ESE) into ablated crabs on hepatopancreas and muscle TCHO and glycogen levels of Oziotelphusa senex senex
| Tissue | Intact | 1-day ESX | 1-day ESX–ESE |
| TCHO | |||
| Hepatopancreas | 12.41 ± 0.92 | 16.91±1.43 | 12.73±1.22 |
| (36.26) | (−24.71) | ||
| p<0.001 | p<0.001 | ||
| Muscle | 4.54 ± 0.42 | 6.11±0.52 | 4.51±0.55 |
| (34.58) | (−26.15) | ||
| p<0.001 | p<0.001 | ||
| Glycogen | |||
| Hepatopancreas | 1.48 ± 0.08 | 2.12±0.22 | 1.55±0.14 |
| (43.24) | (−26.88) | ||
| p<0.001 | p<0.001 | ||
| Muscle | 0.66 ± 0.06 | 1.09±0.09 | 0.71±0.08 |
| (65.15) | (−34.86) | ||
| p<0.001 | p<0.001 |
Removal of eyestalks resulted in significant () decrease in phosphorylase activity in hepatopancreas (‘a’ −38.56% and ‘ab’ −11.18%) and in muscle (‘a’ −48.32% and ‘ab’ −39.14%). In contrast, injection of eyestalk extract into ablated crabs resulted in a significant elevation of phosphorylase activity in both hepatopancreas (‘a’ 58.86% and ‘ab’ 9.11%) and muscle (‘a’ 96.94% and ‘ab’ 65.49%) tissues. The ratio of phosphorylase a/ab also increased after eyestalk extract injection into intact crabs when compared to eyestalk-ablated crabs, indicating interconversion from inactive phosphorylase to active phosphorylase (Table 3).
Effect of eyestalk ablation (1-day ESX) and injection of eyestalk extract into ablated crabs (1-day ESX–ESE) on the phosphorylase activity in hepatopancreas and muscle of Oziotelphusa senex senex
| Treatment | Hepatopancreas | Muscle | ||||
| ‘a’ | ‘ab’ | a/ab | ‘a’ | ‘ab’ | a/ab | |
| Intact | 4.59±0.29 | 7.78±0.92 | 0.58 | 2.56±0.31 | 4.19±0.82 | 0.61 |
| 1-day ESX | 2.82±0.31 | 6.91±0.76 | 0.40 | 1.31±0.24 | 2.55±0.31 | 0.51 |
| (−38.56) | (−11.18) | (−48.82) | (−39.14) | |||
| 1-day ESX-ESE | 4.48±0.51 | 7.54±0.86 | 0.59 | 2.58±0.29 | 4.22±0.51 | 0.61 |
| (58.86) | (9.11) | (96.94) | (65.49) | |||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
3.5 Effect of injection of methionine-enkephalin on carbohydrate metabolism in the crab Oziotelphusa senex senex
3.5.1 Effect of methionine-enkephalin on haemolymph sugar level
Injection of methionine-enkephalin into intact crabs resulted in significant hyperglycaemia in a dose-dependent manner when compared to the controls (Fig. 3), whereas injection of physiological saline did not cause any significant effect on haemolymph sugar levels. At doses between 10−10 mol/crab (68.33%) and 10−8 mol/crab (167.85%), the effect of methionine-enkephalin was statistically significant and dose-dependent. For doses lower than 10−10 mol/crab, however, methionine-enkephalin did not elicit any hyperglycaemic response, whereas doses higher than 10−8 mol/crab exhibited a saturated response in inducing hyperglycaemia (Fig. 3). In the subsequent experiments, 10−8 mol/crab was selected as injection dosage.
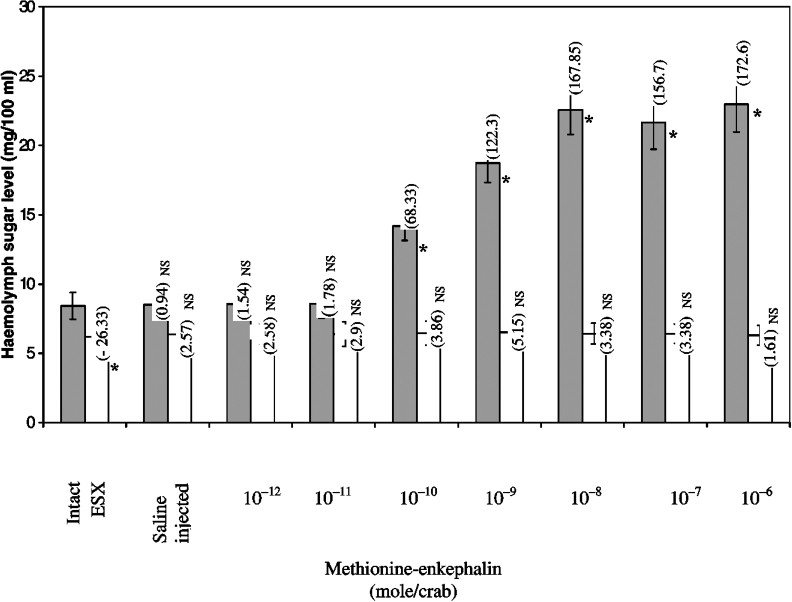
Effect of injection of methionine-enkephalin into intact (closed bars) and ablated (ESX) (open bars) crabs on haemolymph sugar level. Each bar represents mean ± S.D. (n=10). Values in parentheses are percent change. For calculation percent change intact served as control for ESX and intact + met-enk-injected crabs. Whereas ESX crabs served as controls for ESX + met-enk-injected animals. ∗ Statistically significant at p<0.001. NS Not significant.
A time course action of methionine-enkephalin induced hyperglycaemia is presented in Fig. 4. The haemolymph sugar level increased significantly within 1 h after methionine-enkephalin injection and reached a highest peak at 2 h. Haemolymph sugar level declined gradually after 2 h.
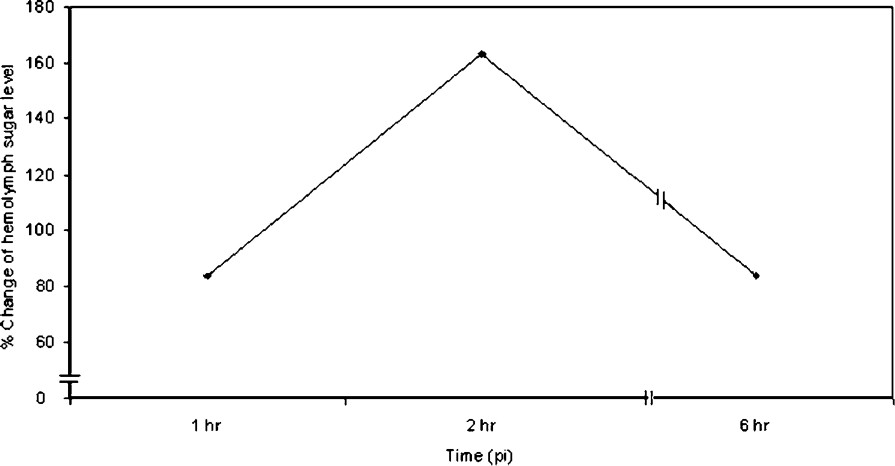
Effect of injection of methionine-enkephalin on the haemolymph sugar level in the crab Oziotelphusa senex senex at different times post injection (n=10).
3.5.2 Effect of injection of methionine-enkephalin on levels of tissue carbohydrate and phosphorylase activity
Total carbohydrates and glycogen levels in the hepatopancreas and muscle tissues of crabs that received methionine-enkephalin were significantly () decreased when compared to the controls (Table 4), with an increase in the tissue phosphorylase activity (Table 5).
Effect of eyestalk ablation (1-day ESX) and injection of methionine-enkephalin into intact and ablated crabs on hepatopancreas and muscle total carbohydrate (TCHO) and glycogen levels of Oziotelphusa senex senex
| Group | Total carbohydrate (TCHO) | Glycogen | ||
| Hepatopancreas | Muscle | Hepatopancreas | Muscle | |
| Intact | 13.66±1.54 | 4.39±0.53 | 1.22±0.10 | 0.66±0.06 |
| 1-day ESX | 17.89a±1.94 | 6.26a±0.71 | 2.04a±0.29 | 1.01a±0.09 |
| (30.96) | (42.59) | (67.21) | (53.03) | |
| Intact + Met-enk | 9.47a±1.49 | 3.12a±0.92 | 0.64a±0.21 | 0.41a±0.08 |
| (−30.67) | (−28.92) | (−47.54) | (−37.87) | |
| 1-day ESX + Met-enk | 17.44b±1.43 | 6.29b±0.56 | 2.11b±0.18 | 1.13b±0.10 |
| (−2.51) | (0.47) | (3.43) | (11.88) |
a Significant () compared with normal.
b Not significant compared with ESX crabs.
Effect of eyestalk ablation (1-day ESX) and injection of methionine-enkephalin into intact and 1-day ESX crabs on hepatopancreas and muscle phosphorylase activity levels of Oziotelphusa senex senex
| Treatment | Hepatopancreas | Muscle | ||||
| ‘a’ | ‘ab’ | a/ab | ‘a’ | ‘ab’ | a/ab | |
| Intact | 2.62±0.29 | 4.52±0.41 | 0.57 | 1.92±0.09 | 2.49±0.45 | 0.77 |
| 1-day ESX | 1.72a±0.34 | 4.06a±0.44 | 0.42 | 0.99a±0.08 | 2.20a±0.32 | 0.45 |
| (−34.35) | (−10.17) | (−48.43) | (−11.64) | |||
| Intact + Met-enk | 3.63a±0.34 | 5.69a±0.52 | 0.63 | 3.01a±0.12 | 3.66a±0.42 | 0.82 |
| (38.54) | (25.85) | (56.77) | (46.95) | |||
| 1-day ESX + Met-enk | 1.69b±0.11 | 4.10b±0.09 | 0.41 | 1.01b±0.09 | 2.22b±0.34 | 0.45 |
| (−1.74) | (0.98) | (2.02) | (0.9) |
a Significant at when compared with normal.
b Not significant compared with 1-day ESX crabs.
3.5.3 Effect of injection of methionine-enkephalin into ablated crabs on carbohydrate metabolism
Injection of methionine-enkephalin into eyestalk-ablated crabs resulted in insignificant change in the levels of haemolymph sugar and tissue TCHO and glycogen and activity levels of phosphorylase when compared with eyestalk-ablated crabs (Tables 4 and 5).
4 Discussion
Bilateral eyestalk ablation resulted in significant hypoglycemia in the crab, O. senex senex. Injection of eyestalk extract resulted in increase in the haemolymph sugar level in eyestalk-ablated crabs indicating the presence of hyperglycaemic hormone in the eyestalks of crabs. Similar results were reported in crabs [30,31,38–42] prawns [33,43] crayfishes [25,44,45] and in shrimps [46].
The effect of eyestalk hormone(s) on tissue carbohydrate levels and phosphorylase activity has been studied in Cambarus affinis [47] and the glycogen phosphorylase system in Callinectes danae [48]. It was reported earlier that eyestalk removal inactivates the phosphorylase system and activates the glycogen synthase in crabs [47,49]. Whereas injection of eyestalk extracts into eyestalk-ablated animals activate phosphorylase and inactivate glycogen synthase. Stimulation of uptake and incorporation of glucose 14C into glycogen fraction of muscle tissue with a decrease in haemolymph sugar levels were also observed after eyestalk ablation [49]. Whereas injection of eyestalk extract reversed these changes. It was also observed that the hyperglycaemic hormone of eyestalks of the crustaceans enhances the activity of the phosphorylase system in insect tissues [50,51].
In the present study, bilateral eyestalk ablation resulted in elevated levels of TCHO and glycogen levels, with a decrease in the activity of phosphorylase levels in the tissues of crab, Oziotelphusa senex senex, whereas the injection of eyestalk extract into eyestalk-ablated crabs reversed these changes. The elevated levels of tissue phosphorylase after eyestalk extract injecting results in stimulation of glycogenolysis. This leads to liberation of glucose and these molecules leaks into haemolymph and causes hyperglycaemia. Hohnke and Scheer [52] suggested that the primary function of the crustacean hyperglycaemic hormone is not to elevate haemolymph sugar level, but to elevate intra-cellular glucose through the degradation glycogen by activating the phosphorylase system. The resultant glucose molecules leak into the haemolymph, causing hyperglycaemia. This view also supported by other workers [53,54].
Injection of methionine-enkephalin elicited hyperglycaemic response in Oziotelphusa senex senex in a dose-dependent manner. Methionine-enkephalin induced hyperglycaemia has also been demonstrated in the estuarine crab, Scylla serrata [32], in prawns, Penaeus indicus and Metapenaeus monocerus [33], and in fiddler crab Uca lactea annulipes [42]. It was hypothesized that the hyperglycaemic effect of methionine-enkephalin is due to release of hyperglycaemic hormone from eyestalks [32,33]. Methionine-enkephalin analogue FK-33824 also significantly increased the haemolymph sugar level in the fiddler crab, Uca lactea annulipes [42], whereas methionine-enkephalin antagonist naloxone blocked the release of hyperglycaemic hormone from the eyestalk [42,55].
An increase in phosphorylase activity and decrease in glycogen and TCHO levels in hepatopancreas and muscle of Oziotelphusa senex senex were observed after the injection of methionine-enkephalin is in agreement with earlier findings in the mud crab Scylla serrata [32] and in prawns Penaeus indicus and Metapenaeus monocerus [33]. Since methionine-enkephalin exerts its effect only in intact crabs but not in eyestalk-ablated crabs it can be hypothesized that methionine-enkphalin acts by triggering the release of hyperglycaemic hormone from the sinus gland of the eyestalks.
In summary, the results clearly demonstrate that eyestalks are the source for hyperglycaemic hormone and methionine-enkephalin induces hyperglycaemia in intact crabs, but not in eyestalk less crabs. This indicates that methionine-enkphalin regulates the release of hyperglycaemic hormone from the X-organ sinus gland complex of the eyestalk there by inducing hyperglycaemia.
Acknowledgements
We thank Prof. K.V.S. Sarma, Department of Statistics, Sri Venkateswara University, Tirupati, India, for statistical analysis of data. This work was generously supported by a Department of Science and Technology grant (SP/SO/CO4/96) sanctioned to P.S.R. and represents part of a dissertation by B. Kishori in fulfillment of the requirements for the Degree of Doctor of Philosophy at Department of Biotechnology, Sri Venkateswara University. Mr S. Umasankar provided valuable technical assistance.


