1 Introduction
Thirty years after the discovery of monoclonal antibodies (mAb) by Kohler and Milstein, and a series of pit falls and disillusions, mAb have now reached the therapeutic stage and have become the first market in biotechnology, its major application so far being cancer (Table 1).
Clinical trials based on monoclonal antibody therapy
| Molecular target | Antibody | Applications | Number of trials (% of MAB trials) |
| CD20 | Rituximab | oncology | 71 (24%) |
| Her2/neu | Trastuzumab | oncology | 42 (15%) |
| Pertuzumab | 4 | ||
| VEGFa | Bevacizumab | oncology | 32 (11%) |
| CD52 | Alemtuzumab (CAMPATH-IH) | oncology | 20 (7%) |
| IL2Rα(CD25) | Daclizumab (17.1A) | oncology/psoriasis/ITP | 12 (%) |
a VEGF: Vascular endothelial growth factor.
As reviewed by Teillaud in this issue, technological modifications have been prominent to allow the establishment of mAb as therapeutic tools. For a long time, mAb have been of murine origin, impairing their use in immunocompetent patients due to of the development of human anti-mouse antibodies (HAMA), which, at least, neutralized the activity of the injected mAb and, at most, were responsible for acute inflammatory syndromes upon repeated treatments with murine mAb. It is therefore not surprising that the only real success of murine therapeutic mAb were anti-lymphocytic antibodies that induced strong immunosuppression needed to help controlling the rejection of renal grafts. Thereafter, the development of mouse-human chimeric, then humanized, mAb and the recent appearance of fully human mAb have solved in great part the HAMA problem and opened the way for the use of repeated injections of mAb in a dosing and a schedule allowing us to reach therapeutic efficacy. Finally, increasing knowledge of the mechanisms of action of therapeutic mAb permits to engineer and to target them in the most appropriate way for the disease and the disease modifications that therapists want to achieve. The present review summarizes these approaches for immunotherapy of cancer, particularly of solid tumours, with special emphasis on the role of Fc Receptors.
2 Antigens targeted by therapeutic mAb in human cancer, and mechanisms of their action
Three types of mAb have been developed and are efficiently used in human cancers. A first type is directed against antigens expressed by tumour cells either specifically, such as Her2-neu on breast cancer and other carcinomas, or shared with normal cells, such as differentiation antigens (CD20, CD52, CD19...). The aim of using the type of antibodies is to induce the death of the tumour either by neutralizing the effect of a growth factor or by inducing apoptosis (Fig. 1) or by activating effector mechanisms of the host, such as complement activation (Fig. 2) or killing by macrophages or NK cells through their interaction with cellular receptors for IgG called Fcg receptors (FcgR) (Fig. 3).
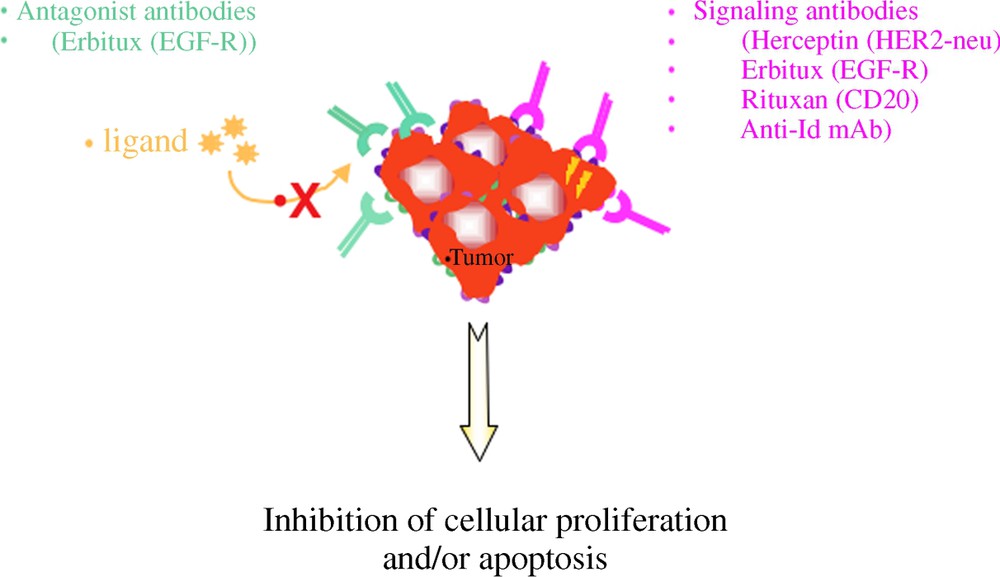
Mechanisms of action of MoAbs in anti-tumour immunotherapy. (1) Direct mechanisms.
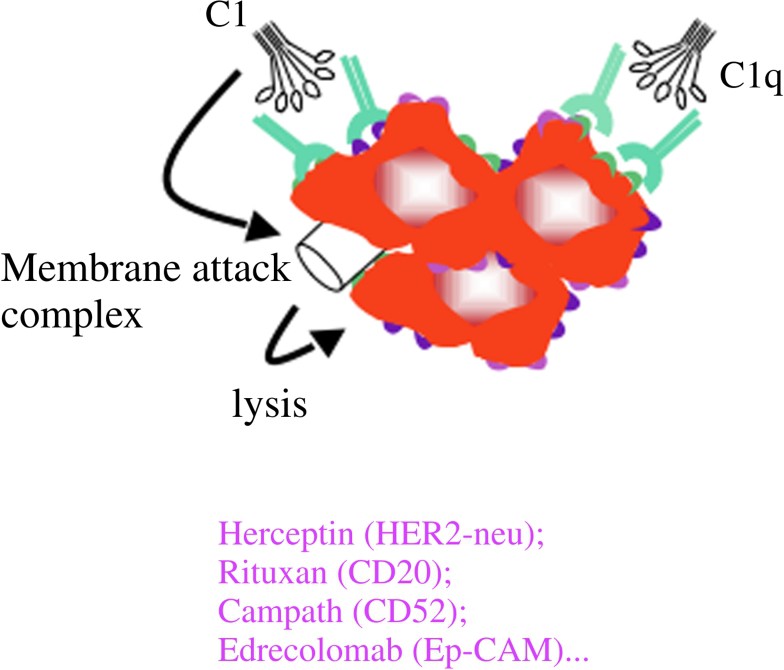
Mechanisms of action of MoAbs in anti-tumour immunotherapy. (2) Indirect mechanisms: CDC; complement-mediated lysis.
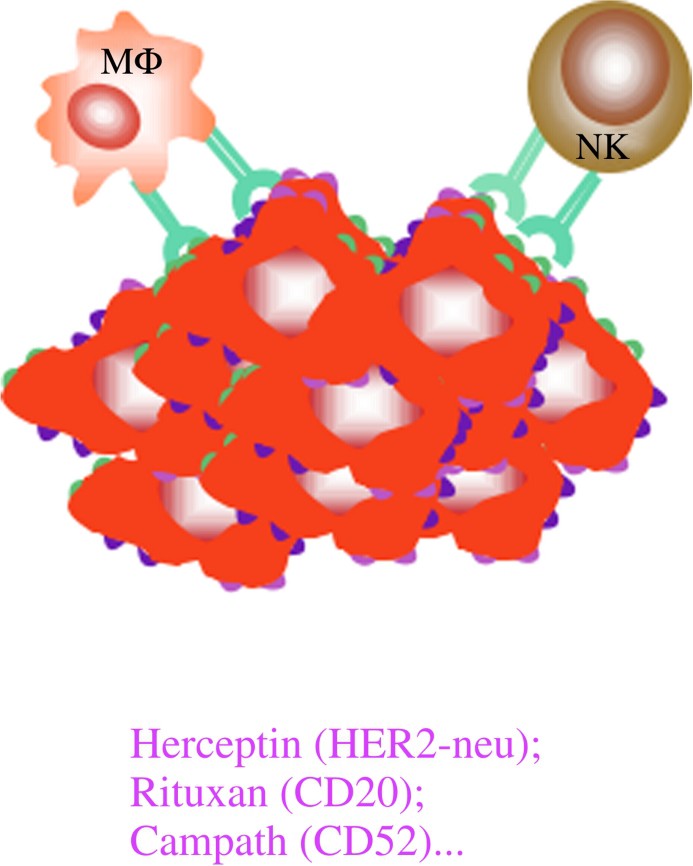
Mechanisms of action of MoAbs in anti-tumour immunotherapy. (3) Indirect mechanisms: ADCC.
The second type of therapeutic mAb targets the stroma reaction around the tumour. The leader of this type is a mAb that neutralizes V-EGF, a tumour-derived factor that increases neo-angiogenesis that the tumour needs for its high requirements in oxygen and instruments. Neutralization of V-EGF blocks angiogenesis and results of hypoxic of the tumour (Fig. 4). Other mAb are being tested, such as those targeting the receptors for V-EGF or neutralizing adhesion molecules or proteases used by the tumour cells to invade its environment.
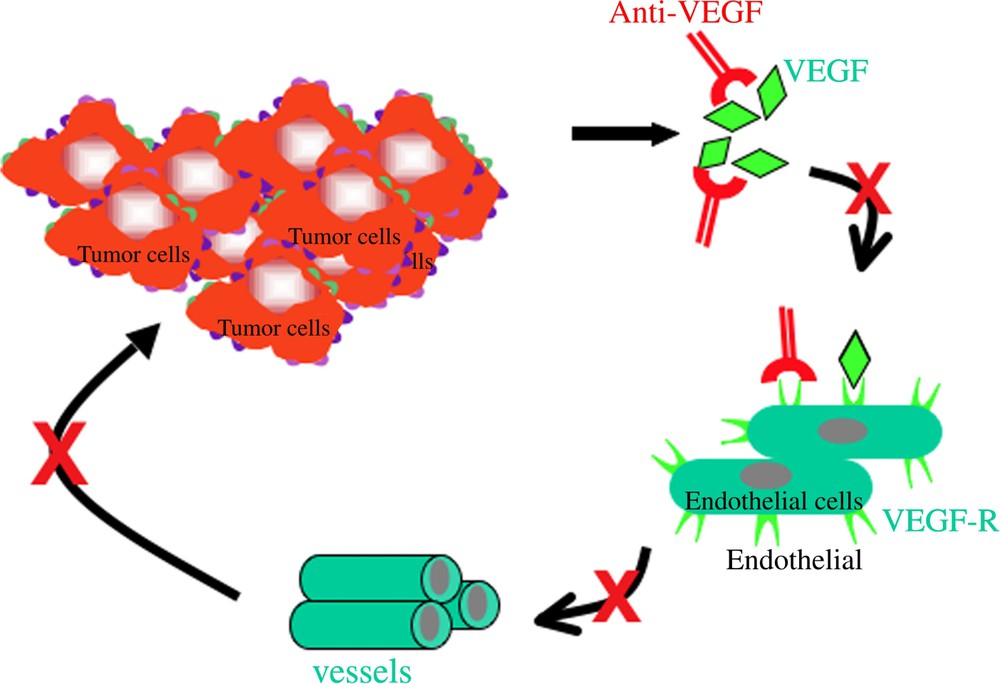
Mechanisms of action of MoAbs in anti-tumour immunotherapy. (4) Indirect mechanisms.
The third type of therapeutic mAb addresses the potentiation of the immune reactions against tumours. It is now established that there are immune reactions in patients against their non-tumours. It has been strongly suggested 35 years ago in acute leukaemia [1], demonstrated in viral-associated tumours, such as Burkitt lymphoma, cervical carcinoma or hepatocarcinoma, and a recent study of almost 1000 patients have established the case in colorectal cancer [2].
However, in growing cancers, the immune reaction is inefficient and therapeutic vaccines have still low efficacy. Several mechanisms are used by the tumour to escape the immune system. A first way is to induce lymphocytes that will inhibit the immune reactions. These suppressor, also called regulatory, lymphocytes express specific markers (CD25, CTLA-4, PD1) that can be used as targets of mAb that will inhibit or destroy these cells allowing a better immune response to the tumour (Fig. 5). As expected, however, the first therapeutic trials, especially with a human mAb directed against CTLA-4, have shown that therapeutic efficacy in cancer is accompanied by auto-immunity due to the fact that regulatory cells also control anti-self reactivity.
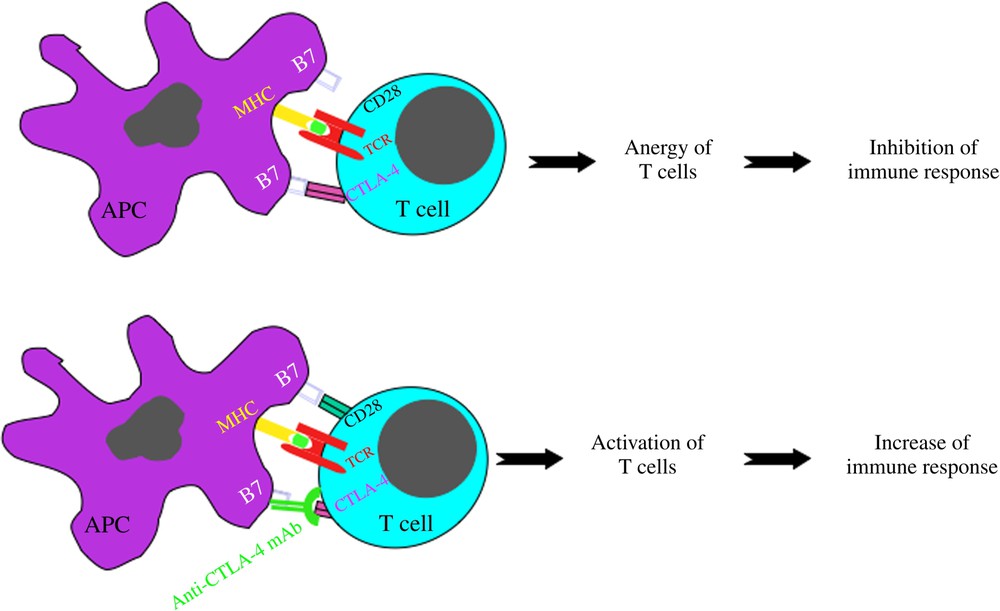
Mechanisms of action of MoAbs in anti-tumour immunotherapy. (5) Indirect mechanisms: immunostimulatory mAb.
Another way by which tumours escape the immune attack is by either modulating their surface antigens, or by inhibiting complement cytotoxicity or by ectopically expressing Fcg receptors. The latter major mechanism is discussed thereafter.
3 Roles of Fcγ receptors in antibody-based therapies
There has been a renewed interest since few years in the use of mAbs in the diagnostic and treatment of various tumours. The most impressive clinical results have been obtained with Rituximab, a chimeric anti-CD20 mAb that recognizes the human oncoprotein HER-2/neu over-expressed in some breast cancers and other tumours, induces clinical responses. Other mAbs such as Campath-1H or 17-1A produced encouraging results in the treatment of chronic lymphocytic leukaemia or colorectal carcinoma, respectively. There is increasing evidence that the Fc portion of the anti-tumour mAbs is a major component of their therapeutic activity, through binding to FcgRs expressed by effector cells present in the tumour microenvironment. The polymorphisms of FγRIIIb (Val/Phe158) and FcγRIIa (His/Arg131) that affect binding of IgG-immune complexes predict the response to Rituximab in patients with follicular lymphoma, supporting the hypothesis that ADCC by NK cells and macrophages plays an important role in the clinical effect. As demonstrated in Fcg receptor-deficient mice, the anti-tumour effects of Rituximax and Herceptin require the presence of the signal transducing g chain to activate FcγRI and FcγRIII expressed on monocytes/macrophages and NK cells and are down regulated by inhibitory FcγRIIb at the monocyte/macrophage level. The improved efficacy in tumour eradication of bispecific molecules that have one arm specific for tumour cells and the other specific for FcγRs on immune effector cells, further illustrates the major role of FcγRs in immunotherapy.
In view of their pivotal role in the activation and in the regulation of IgG-dependent effector responses, FcγR provide new tools, not only to predict response to antibody-based therapies, but also to manipulate patient's response to treatment [3].
4 Ectopic expression of FcγR on non-haematopoietic tumour cells
The first studies indicating that non-haematopoietic tumours may express FcRs that were performed on a variety of experimental tumours and of human cancers. However, the ectopic expression of FcγRs by non-haematopoietic tumour cells was a controversy because of the presence of FcγR positive inflammatory cells at the tumour site and because FcγR expression was lost during short-term culture of tumour cells in vitro. We reinvestigated the expression of FcγRs on human tumour cells of non-haematopoietic origin. We found that tumour cells from about 40% of human metastatic melanoma tested express inhibitory FcgRIIb1 in vivo and ex vivo [4].
In earlier studies using Polyoma virus-induced mouse tumours expressing FcγRIIb1, it was shown that this receptor confers an in vivo growth advantage to tumour cells and increases their malignancy. It was hypothesized that increased tumorigenicity mediated by FcγRIIb1 could involve immunological mechanisms. For example, FcγRIIb1 expressed by tumour cells could block complement-dependent lysis of tumour cells or could protect tumour cells from ADCC by binding the Fc portion of Abs covering tumour cells. In nude mice, we have shown that FcγRIIb1 expression by human metastatic melanomas has a profound down regulatory impact on tumour growth and uptake in nude mice. This effect is under the control of T-independent IgG anti-tumour antibodies. Conversely, in immunocompetent mice, expression of FcγR on murine melanoma cells inhibits ADCC and favours tumour growth in the presence of anti-melanoma antibodies [5].
5 Conclusions
The use of therapeutic mAb in oncology is an exploding field, since in its infancy, which will grow not only by the identification of novel targets on tumour cells, their stroma and the immune host response, but also by engineering the mAb to increase their binding to FcγR and producing mAb to inhibit the molecules involved in the tumour resistance to the immune attack, such as complement inhibition of FcR. After a quarter of century of scepticism and doubts, the therapeutic mAb have entered for a long time in our modern therapeutic arsenal in all fields of human medicine.


