Patients with severe heart failure consequent to extensive chronic ischemic lesions of myocardium are at high risk. In many cases, the coronary vascular bed has severe atherosclerotic lesions, total arterial occlusion, or has already suffered surgical interventions, and these patients cannot be submitted to further angioplasty or vascular surgery. Traditional therapy is only palliative, and although considerable improvements have been offered by new pharmacological approaches, the quality of patients' life remains very low, they suffer of effort angina pectoris and heart dysfunction, and frequently need repeated hospitalisations. The heart transplantation, which is often the only therapeutic proposal, is hampered by shortage of donors, high risk, and cost of complex surgical procedures and of post-surgical complications.
Cell therapies have opened new proposals, since in several experimental models improvements of the damaged heart function and of the clinical state have been observed and documented after introduction of different types of cells into the cardiac tissue [1]. Although we are still far from understanding the mechanisms involved in myocardium vascularisation and repair, most experimental studies point towards promising results, and most scientists would agree that there is not any more a question of whether cell therapies should be tried in patients, but rather how they will be tried [2].
From the beginning of this decade onwards, clinical studies were done by several groups. The pioneering study used autologous skeletal muscle myoblasts, surgically introduced into the akinetic scar tissue of patients with severe ventricular dysfunction [3]. This study has shown feasibility of cell therapy, long-term engraftment of implanted myoblasts, and improvement of heart function. The fact that myoblasts differentiated in situ exclusively into skeletal muscle, and that they were not coupled with gap-junctions to adjacent cardiomyocytes has raised concern on efficacy of long-term restoration of heart function, and safety related to complex arrhythmias. These issues are being addressed in ongoing studies.
Alternatively, bone-marrow-derived mononuclear cells have been used in treatment of both acute and chronic ischemic heart disease, introducing cells by transepicardic injection during surgery for revascularization of myocardium, by intracoronary route associated to angioplasty, or by transendocardial injection using appropriate percutaneous delivery systems. Most studies were dedicated to acute infarct of myocardium, reporting a relative improvement of clinical evolution of patients as compared to controls. However, recent extensive grade II–III clinical studies have not always given convincing results [4–6]. This may be related to differences in timing of cell injection, and to the question of retention of the injected cells in the infarcted tissue that needs repair. On the other hand, considerable improvement of the patients' clinical state can be anyhow obtained by rapid and efficient post-infarct intervention using traditional therapies. A large series of double-blind random controlled studies is thus required to reach statistically robust results when introducing a new therapeutic proposal. Many groups, including ours, are working now on standardisation and validation of protocols for cell therapy of acute myocardium infarct, following essentially the guidelines listed by the European Society of Cardiology [7].
In patients with chronic ischemic severe cardiomyopathy, the transepicardial or transendocardial routes of cell administration were preferred, due to difficulties of the vascular access to heavily scarred fibrotic tissue. From the end of 2000 onwards, our group has undertaken a phase I–II clinical trial using transendocardial injection of autologous bone-marrow-derived mononuclear cells (14 patients, 7 controls). After two months, the treated patients had significantly less heart failure and angina, and after four months, they had a sustained and significant improvement of pumping power, exercise capacity, cardiac muscle irrigation, and blood supply to the body [8,9]. The patients continued improving for 12 months, and the overall maintenance of their state has now been confirmed 24 and 48 months after the therapy. Five out of the studied patients had already been listed and waited for cardiac transplantation. After six months, their clinical improvement was such that they were no longer eligible for heart transplantation [10]. Similar results were now reported by other groups that used the intramyocardial cell therapy for ‘no-option’ patients with refractory angina and severe myocardial ischemia [11–15].
Potential mechanisms involved in the cell therapy of severe chronic heart disease are still not understood. In our study, the pattern of the overall improvement of the patient's cardiac functions suggested two possible cues: (a) increased blood perfusion due to angiogenesis, and/or (b) improved heart muscle contractility, consequent to enhanced activity and/or increased number of cardiomyocytes.
The transendocardial cell injection was done by NOGA MyoStar Cordis® catheter, and it was preceded by electrical and mechanical mapping of the heart muscle activity, which was repeated four months after the therapy. We could thus locate precisely the improvement of heart muscle electrical and mechanical activities, as well as the position of the cell-injection sites (Fig. 1). All the cell injections were done in viable regions in terms of electrical cell activity, since non-viable sites were considered not to be appropriate to sustain survival and growth of newly introduced cells. We found by NOGA mapping a striking improvement in both electrical activity and contractibility of myocardium of the treated patients, in the regions that received injections as well as in extensive adjacent regions, sometimes quite distant from the injection sites (Fig. 1). We observed an increase in the unipolar voltage that was not statistically significant within the regions of cell injections, but there was a significant increase in voltage in the area surrounding the injection sites. These findings may represent an expansion in myocardial viability, as reflected by an increase in unipolar voltage detected by electromechanical maps, and may have important therapeutic implications (Table 1). Similar result was obtained by segmental echocardiography. In global comparison, the number of segments with abnormal contractility (hypocinetic, acinetic, dyscynetic) was decreased in treated patients as compared to controls. Conversely, in the injected regions, the proportion of segments with normal contractility increased with time after the treatment.
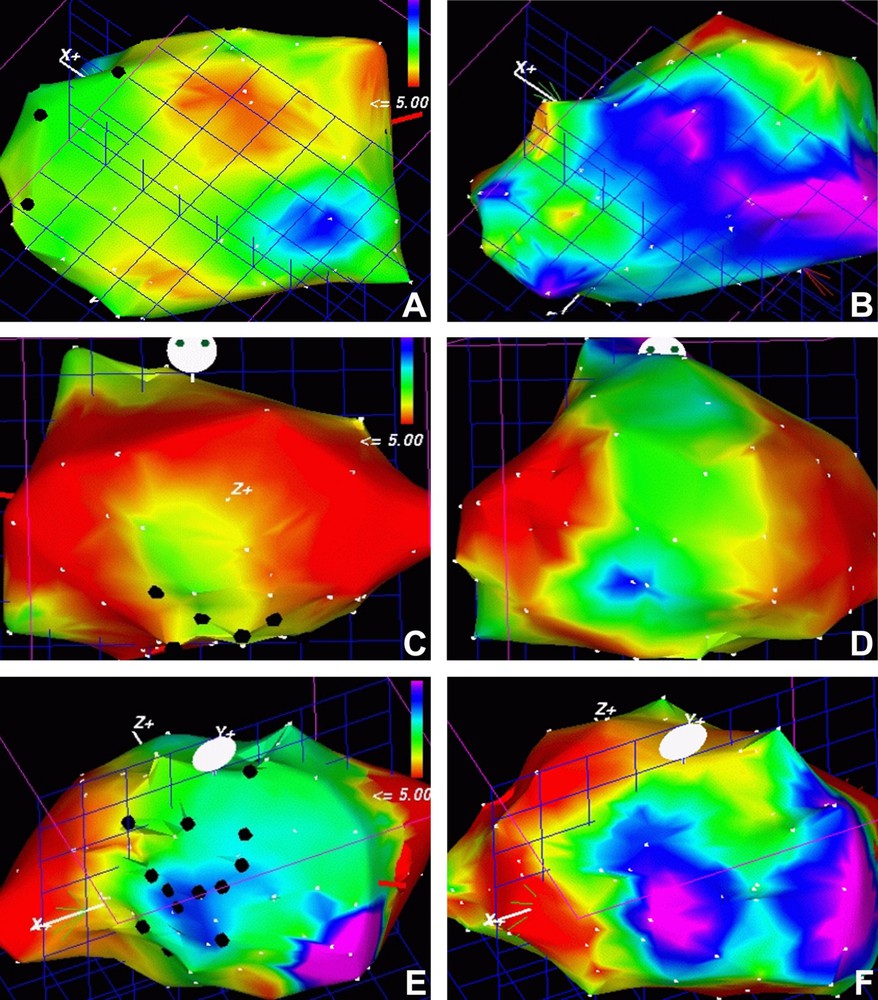
NOGA mapping of the electrical activity of myocardium in three patients before (A, C, E) and four months after the therapy with bone-marrow-derived mononuclear cells (B, D, F), respectively. Black dots represent sites of cell injections. The scale shown in A, C and E represents the electric activity; the red–yellow side of the scale corresponds to areas with null or low activity and the lilac–blue one to those with a high normal activity. Note that the injections have been done in the intermediate activity regions (green), and that the improvement depicted by the colour change in images B, D and F compared to the A, C and F ones involved frequently regions of the myocardium quite distant from the injection site.
Unipolar voltage as shown by electromechanical mapping (NOGA), before and four months after the mononuclear bone-marrow cell injections
| Unipolar voltage | P | ||
| Total area | 10.5 ± 3.5 mV | 10.3 ± 2.7 mV | 0.65 |
| Injection area | 10.2 ± 3.4 mV | 9.9 ± 3 mV | 0.32 |
| Adjacent area | 8.12 ± 2 mV | 9.8 ± 3 mV | 0.04 |
On the other hand, one of the patients deceased of a stroke 11 months after the treatment, and anatomopathological study of his heart could be done [16]. We could thus compare the ventricular anterolateral wall structure, where the cells were injected, with the posterior wall that had also suffered previous infarcts and was also involved by fibrotic cicatrisation, but did not receive any treatment. The comparative analysis suggested both neoangiogenesis and neocardiomyogenesis. While the posterior wall fibrotic tissue had only rare thin-walled blood vessels, as expected for chronic cicatricial tissue, the anterolateral wall had an arborescent branching vascular system running parallel to the cardiomyocytes (Fig. 2A and B). The overall density of capillaries and of lager vessels with smooth muscle α-actin-positive mural cells was significantly higher in the regions that had received cell injection as compared to those that had not () [16]. A striking characteristic of these vessels was hyperplasia of the mural cell layer, intensely labelled for smooth muscle α-actin (Fig. 2C). These morphological data strongly suggested formation of new blood vessels, their maturation, and an enhanced proliferation of pericytes, mural and adventitial cells. Moreover, these abluminal vascular cells had two distinct characteristics. In the regions where cardiomyocytes were present, they appeared to detach from the blood vessel wall and disseminate among the cardiac muscle bundles (Fig. 2D). Simultaneously, while still within the blood vessel wall, they expressed the cytoskeletal components typical of cardiomyocytes, such as desmin, troponin, and sarcomeric α-actinin (Fig. 2E–G). While cells were still small and fibroblastoid, the distribution of these cytoskeletal elements was diffuse, but in vicinity of cardiomyocytes, these cells apparently grew and organised the skeleton similar to cardiac cells (Fig. 2F, H). Similar to all the post-hoc anatomopathological studies, we can only hypothesize that these data suggest that mural and adventitial cells, which are known to have a broad capacity of differentiation [17], may generate new cardiomyocytes when stimulated by the adjacent regenerating cardiac tissue. Within the limits of studies on humans, we understand that taken together these data suggest a long-term sequential regeneration of the cardiac vascular tree and muscle.
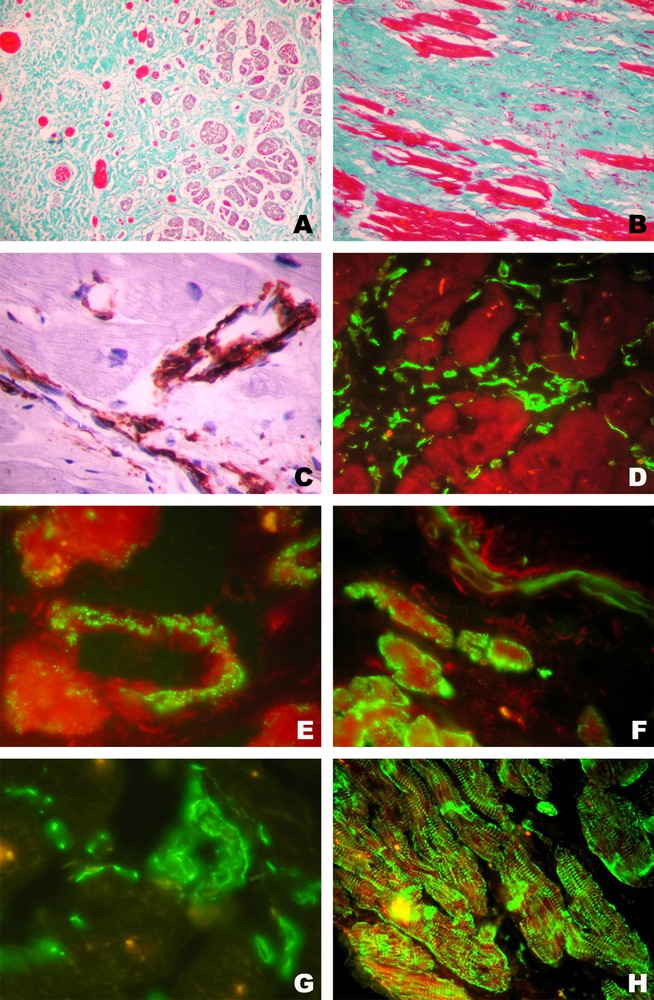
Anatomopathological analysis of the heart tissue of a patient that had deceased 11 months after the therapy. All the images represent areas adjacent to the sites of cell injections. A. Gomori-stained sections perpendicular to cardiomyocytes. Numerous blood vessels in the fibrotic cicatricial area are parallel to heart-cell bundles. Note numerous small cardiomyocytes in the region adjacent to the vascular tree (Original magnification 100×). B. Gomori-stained section of the region at the interface between cardiomyocytes and a fibrotic scar, parallel to the cardiomyocyte bundles. Numerous capillaries and dense cell infiltrate of fibroblastoid cells are seen (original magnification: 400×). C. Immunoenzymatic reaction for smooth muscle α-actin in small blood vessels of the myocardium. Note hyperplasia of pericytes and mural cells (original magnification: 1000×). D. Immunofluorescent reaction for vimentin depicting perivascular cells and numerous fibroblastoid cells migrating among cardiomyocytes (original magnification: 400×). E. Immunofluorescent reaction for troponin depicting positive reaction in mural cells of a blood vessel adjacent to cardiomyocytes (original magnification: 1000×). F. Immunofluorescent reaction for troponin depicting a faint positive reaction in cells of a small blood vessel (upper right) and intense reaction in small cardiomyocyte-like cells (lower left) (original magnification: 400×). G. Immunofluorescent reaction for sarcomeric α-actinin in the wall of a small blood vessel and isolated migrating fibroblastoid cells (original magnification: 1000×). H. Immunofluorescent reaction for desmin depicting small intensely staining cells among cardiomyocytes (original magnification: 400×).
We have further addressed the question of the quantitative and qualitative aspects of the injected cells in relation to the clinical evolution of patients. In a further series of five patients with severe chronic heart disease submitted to cell therapy, we found that the total number of cells correlated with the overall improvement of heart function. However, while increasing the number of injected cells, when reaching a total of 100 million cells, we noted a small and transient presence of circulating markers of inflammatory reaction to stress. Although no adverse effects were observed and normal marker-levels were reached rapidly and spontaneously, we found that this was the upper limit of the total number of injected cells under our protocol. Therefore, we examined the possibility to proceed to positive and/or negative selection of cells that could benefit the outcome of the therapy.
A preliminary backcross study was done in order to establish the correlation among the relative content of different cell phenotypes in the injected bone-marrow-derived cell populations with different parameters of the patients' cardiac improvements. The studied parameters and the bone-marrow cell characteristics are listed in Table 2. The number of positive or negative correlations with the outcome of the studied parameters is listed in Table 3. The most striking result is the fact that the relative number of injected cells that have a potential cytotoxic activity, namely CD8+ lymphocytes and the CD56+ natural killer cells, were clearly correlated with the less efficient therapy, and their negative selection seems to be advantageous. Although CD4+ T-lymphocytes and CD19+ B-cells correlated less clearly with the poor evolution of the therapy, they represent a major fraction of mononuclear cells, and their negative selection may permit introduction of a larger quantity of cells that are more prone to stimulate heart tissue regeneration. It is quite striking that the cell phenotypes, whose presence is clearly correlated with the improved therapy, belong to a minor cell fraction containing typical bone marrow progenitor cells.
Characterisation of injected cells and patients' evolution
| Bone-marrow mononuclear cell phenotype markers monitored by flow cytometry | Cardiac function markers monitored 2, 4, 6, and 12 monthsafter the therapy |
| CD34 | Ventricular extra-systoles |
| HLA DR | Unipolar voltage |
| c-kit | Ischemia |
| CD45 | Fibrosis |
| CD14 | Brain natriuretic peptide |
| CD3 | QRT |
| CD4 | Final systolic volume |
| CD8 | Final diastolic volume |
| CD19 | Scores: |
| CD56 | NYHA |
| Functional assays: | CCSAS |
| CFU-GM | Life Quality Quests: |
| CFU-F | SF36 |
| Minnesota |
Statistically significant (Spearman's ρ) correlation among the characteristics of the injected bone-marrow cells and the above-the-average improvement of the studied parameters of cardiac function or clinical evolution of the patients
| Positive | Negative | |
| Total cell number & cell density | 9 | |
| CD34+ | 6 | |
| CD34- CD45- ckit+ | 8 | |
| CFU-F | 5 | |
| CD4 | 2 | 4 |
| CD8 | 17 | |
| CD56 | 12 | |
| CD19 | 4 |
It should be noted that the myocardium functional regeneration was found to be a long-lasting phenomenon; the above-described improvements extended up to 12 months, and the benefits of the therapy were maintained for up to four years. The anatomopathological analysis that clearly indicated an ongoing process of angiogenesis and regeneration of myocardium was done 11 months after the therapy; it thus represents a late evolution of the tissues in cell injected and in adjacent areas. The bone marrow progenitors may directly participate in the progressive regeneration processes, and/or they may trigger the mobilisation and activation of the resident cell progenitors, whose presence in human and murine cardiac tissue has been demonstrated [18,19]. These issues are object of ongoing studies.
What is the future of the bone-marrow cell therapies in ‘no-option’ patients with severe ischemic heart failure? In this group of patients, the studies reported by now deal with a relatively small number of cases, but the results are consistent and supported by robust statistical data. In our study, ANOVA analysis of the global scores of treated and control patients at 2, 4, 6 and 12 months after treatment, following the standards of the New York Heart Association (NYHA) and of the Canadian Cardiovascular Society Angina Score (CCSAS), reached P values , respectively. This is not surprising, since the control group of patients submitted to standard medical treatment have no chance of improvement, and any positive evolution in the treated group is relevant. As already stated by the Nagoya group for the same profile of patients [15], we “cannot have a placebo group for ethical issues.” New studies should thus follow the recommendation of the European Society of Cardiology, falling into the category of small studies to address specific hypotheses that would have arisen from basic science experiments [7]. Meanwhile, we have no options to offer to such patients, and the reported clinical studies have clearly shown the absence of adverse effects and significant benefits of the therapy. In the case of ‘no-option’ severe cardiac patients, it might be now reasonable to discuss the possibility of application of the ‘principle of compassion’, and search to reach a consensus on the profile of patients to which this principle could be applied and to whom this therapeutic approach could be offered under rigorous controls.


