1 Introduction
In severe climatic conditions, plants subjected to stress produce reactive oxygen species (ROS) that lead to cellular damage and are known to be involved in several plant disorders, as well as in senescence processes. ROS can also react with biological molecules, such as DNA, proteins, or lipids, generating mutations and damaging membranes, resulting into cell and tissue injuries [1]. Biotic and abiotic stresses exert a considerable influence on the secondary metabolite pool in plants [2]. This pool varies widely in phenolic composition both qualitatively and quantitatively. Its variations are controlled by genetic factors, plant development, and environmental conditions [3]. Enhanced synthesis of determined secondary metabolites in response to stressful conditions is believed to protect the cellular structures from oxidation [4]. In fact, the adaptation of many plant species to hostile environmental conditions suggests the presence of antioxidative and antimicrobial constituents in their tissues [5,6]. Antioxidants are divided into two main types according to their action. Primary antioxidants can inhibit or delay oxidation by scavenging reactive oxygen species, through reduction. Secondary antioxidants function by binding metal ions, converting hydroperoxides to non-radical species, absorbing UV radiations or deactivating singlet oxygen [7]. Among the various kinds of natural antioxidants, polyphenols have received much attention [8]. Structurally, phenolic compounds comprise an aromatic ring, bearing one or more hydroxyl substituants, and range from simple phenolic molecules to highly polymerized compounds [9]. Phenolic acids, flavonoids, and tannins are regarded as the main dietary phenolic compounds. In addition to this diversity, polyphenols may be associated with various carbohydrates and organic acids [10]. These compounds exhibit a wide range of physiological properties, such as anti-allergic, anti-atherogenic, anti-inflammatory, antimicrobial, antioxidant, anti-thrombotic, cardioprotective, and vasodilatory effects [11].
There is an increasing interest for naturally occurring antioxidants for use in foods or medicinal materials to replace synthetic antioxidants, which are being restricted due to their suspected carcinogenicity [12]. Besides, natural compounds had stronger antioxidant activity than that of synthetic ones [6].
Cynara cardunculus L. (Asteraceae), commonly named ‘cardoon’, is a Mediterranean species that grows naturally in harsh habitat conditions (arid region) with high temperature, elevated salinity and drought in summer [13,14]. This plant is used worldwide and represents a notable ingredient of the Mediterranean diet [15]. In fact, this multipurpose plant is used in several dishes, as soups and/or salads [13]. C. cardunculus flowers are traditionally used for cheese preparation [16], while leaves are particularly known in folklore for their therapeutic potential as diuretic, choleretic, cholagogue, antidiabetic, and antimicrobial agent [15,17]. Yet, data related to the antioxidant and antibacterial activities as well as phenolic composition of this species are scarce [16].
The aim of the present study was to determine total polyphenol, flavonoid, and condensed tannin contents at organ level (leaves, flowers and seeds) and their biological activities in a Tunisian C. cardunculus provenance from El Jem (central arid region of Tunisia). The antioxidant capacity was evaluated by DPPH⋅ radical and O−⋅2 superoxide anion scavenging, and the antibacterial potential by the ability to inhibit the growth of eight human pathogenic bacteria.
2 Materials and methods
2.1 Plant material and preparation for extract
C. cardunculus samples were collected from El Jem (, , 250 km south of Tunis, arid climate). Leaves and flowers were collected in May 2005, while mature seeds were harvested in August 2005. The harvested plants were identified at the Biotechnology Centre of the Technopark of Borj-Cédria, and a voucher specimen [ACC27] was deposited at the Herbarium of the Laboratory of Plant Adaptation to Abiotic Stresses at the Biotechnology Centre.
Leaves and flowers were dried at room temperature for two weeks. Organ extracts were obtained by magnetic stirring for 30 min of 2.5 g of dry organ powder with 25 ml of solvent. Several solvents with different polarities were compared (n-hexane, acetone, methanol, and water). Then, extracts were kept at 4 °C for 24 h, filtered through a Whatman No. 4 filter paper, and evaporated to dryness under vacuum. They were stored at 4 °C until analysis. The yield (%) of evaporated dried extracts was calculated as 100 DWextr/DWsamp, where DWextr is the weight of extract after evaporation of solvent, and DWsamp is the dry weight of organ sample. As pure methanol yielded the highest extracting power (%) as compared to the other solvents (data not shown), only methanolic extracts were used in subsequent analyses.
2.2 Phenolic compounds analysis
2.2.1 Total phenolics content
Total phenolics were assayed using the Folin–Ciocalteu reagent, following Singleton and Rosi's [18] method, based on the reduction of a phosphowolframate–phosphomolybdate complex by phenolics to blue reaction products and slightly modified by Dewanto et al. [19]. An aliquot of diluted sample extract was added to 0.5 ml of distilled water and 0.125 ml of the Folin–Ciocalteu reagent. The mixture was shaken and allowed to stand for 6 min, before addition of 1.25 ml of 7% Na2CO3. The solution was then adjusted with distilled water to a final volume of 3 ml and mixed thoroughly. After incubation in dark, the absorbance at 760 nm was read versus the prepared blank. Total phenolic content of plant parts was expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE g−1 DW) through the calibration curve with gallic acid. All samples were analyzed in three replications.
2.2.2 Total flavonoid content
Total flavonoids were measured using a colorimetric assay developed by Dewanto et al. [19]. An aliquot of diluted sample or standard solution of (+)-catechin was added to 75 μl of NaNO2 solution (7%), and mixed for 6 min, before adding 0.15 ml AlCl3 (10%). After 5 min, 0.5 ml of 1 M NaOH solution was added. The final volume was adjusted to 2.5 ml, thoroughly mixed, and the absorbance of the mixture was determined at 510 nm. Total flavonoids were expressed as mg (+)-catechin equivalent g−1 DW (mg CE g−1 DW), through the calibration curve of (+)-catechin (0–400 μg ml−1 range). All samples were analyzed in three replications.
2.2.3 Total condensed tannins
Procyanidins were measured using the modified vanillin assay described by Sun et al. [20]. Three millilitres of 4% methanol vanillin solution and 1.5 ml of concentrated H2SO4 were added to 50 μl of suitably diluted sample. The mixture was allowed to stand for 15 min, and the absorbance was measured at 500 nm against methanol as a blank. The amount of total condensed tannins was expressed as mg (+)-catechin equivalent g−1 DW. All samples were analyzed in three replications.
2.2.4 Analysis of individual phenolic compounds by analytical RP-HPLC
Dried samples from C. cardunculus leaves were hydrolyzed according to the slightly modified method of Proestos et al. [21]. A dried C. cardunculus sample (0.5 g) was added to 40 ml of a methanolic solution containing BHT (1 mg/ml). Then 10 ml of 6 M HCl were added. The mixture was stirred carefully and then sonicated for 15 min and refluxed in a water bath at 90 °C for 2 h. The obtained mixture was filtered through a 0.45 μm membrane filter and injected to RP-HPLC. Phenolic compounds analysis was carried out using an Agilent Technologies 1100 series liquid chromatograph (RP-HPLC) coupled with an UV-vis multiwavelength detector. The separation was carried out on , 4-μm Hypersil ODS C18 reversed phase column. The mobile phase consisted of acetonitrile (solvent A) and water with 0.2% sulphuric acid (solvent B). The flow rate was kept at 0.5 ml/min. The gradient programme was as follows, 15% A/85% B 0–12 min, 40% A/60% B 12–14 min, 60% A/40% B 14–18 min, 80% A/20% B 18–20 min, 90% A/10% B 20–24 min, 100% A 24–28 min. The injection volume was 20 μl and peaks were monitored at 280 nm. Peaks were identified by congruent retention times compared with standards. Analyses were performed in triplicates.
2.3 Antioxidant activity
2.3.1 DPPH⋅ radical scavenging activity
The diphenylpicrylhydrazyl radical (DPPH⋅) scavenging activity was estimated according to Hanato et al. [22]. The dried plant extract was diluted in pure methanol at different concentrations ranging from 1 to 200 μg ml−1, and then 2 ml were added to 0.5 ml of a 0.2 mmol−1 DPPH⋅ methanolic solution. The mixture was shaken vigorously and left standing at room temperature for 30 min in the dark, and then the absorbance was measured at 517 nm. For each dilution of the extract, the DPPH⋅ scavenging activity was calculated as 100 , where is the absorbance of the control at 30 min, and is the absorbance of the sample at 30 min. The antiradical activity was finally expressed as IC50 (μg ml−1), the extract concentration required to cause a 50% inhibition. A lower IC50 value corresponds to a higher antioxidant activity of the plant extract. All samples were analyzed in three replications.
2.3.2 Superoxide anion radical-scavenging activity
The superoxide quenching activity was assessed using the method described by Duh and Yen [23]. The reaction mixture contained 0.2 ml of extract diluted in pure methanol at different concentrations (1–200 μg ml−1), and 0.2 ml of 60 μM PMS solution, 0.2 ml of 677 μM NADH, and 0.2 ml of 144 μM NBT, all in phosphate buffer (0.1 M, pH 7.4). After incubation at ambient temperature for 5 min, the absorbance was read at 560 nm against a blank. The inhibition percentage of superoxide anion generation was calculated as for DPPH⋅ scavenging, and the antioxidant activity in each organ extract was expressed as IC50. All samples were analyzed in three replications.
2.4 Antibacterial activity tests
The antibacterial activity of leaf extracts was screened by the agar disk diffusion assay [24] against eight human pathogenic bacteria, including Gram-positive cocci Staphylococcus epidermidis Institute Pasteur Collection 106510, Staphylococcus aureus ATCC 25923, Micrococcus luteus NCIMB 8166, Enterococcus feacalis ATCC 29212, Listeria monocytogenes ATCC 19115, and Gram-negative bacteria Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC27853, Salmonella thyphymurium LT2.
The bacterial strains were first grown on Muller Hinton medium (MHI) at 37 °C for 24 h prior to seeding onto the nutrient agar. One or several colonies of the indicator bacteria were transferred into an API suspension medium and adjusted to the 0.5 McFarland turbidity standards with a Densimat (BioMérieux). A sterile 6-mm-diameter filter disk (Whatman paper #3) was placed on the infusion agar seeded with bacteria, and 10 μl of each extract suspended in methanol was dropped onto each paper disk (500 μg per disk). The treated Petri dishes were kept at 4 °C for 1 h, and incubated at 37 °C for 24 h. The antibacterial activity was assessed by measuring the zone of growth inhibition surrounding the disks. Standard disks of gentamycin (10 UI) served as positive antibiotic controls according to CASFM 2005 guidelines. Disks with 10 μl of pure methanol were used as negative controls. Each experiment was carried out in triplicate.
2.5 Analysis
A one-way analysis of variance (ANOVA) using the STATI-CF statistical program, with the organ as factor, was achieved for the following parameters: polyphenol, flavonoid, and tannin contents, as well as IC50 on superoxide and DPPH scavenging activities. Means of these parameters were compared using the Newman–Keuls test at the level, when significant differences were found. Concerning antibacterial activity, data are reported as the mean ± SD of three measurements.
3 Results
3.1 Analysis of phenolic compounds
3.1.1 Methanolic extract yield and total phenolic content in the studied organs
The yield of the methanolic extract in the studied organs depended on their type (Table 1). The highest yield was registered in leaves (ca. 35%), while that of flowers and seeds was 3.5 to 4.6 times lower. All organs exhibited high polyphenol content, comprised between 7.5 and 15 mg GAE g−1 DW (Table 1). Leaf and seed methanolic extracts displayed similar polyphenol content (ca. 14–15 mg GAE g−1 DW), while that of the flower extract only approached 10 mg GAE g−1 DW.
Phenolic compounds from cardoon aerial organs. Yield of methanolic extracts, total polyphenol, flavonoid and condensed tannin contents in leaves, flowers and seeds of Cynara cardunculus
| Organs | Yield (%) | Polyphenol content | Flavonoid content | Tannin content |
| (mg GAE g−1 DW) | (mg CE g−1 DW) | |||
| Leaves | 34.72 | |||
| Flowers | 7.56 | |||
| Seeds | 10.08 |
3.1.2 Total flavonoid content
Flavonoid content in the studied organs ranged from 6 to 10 mg CE g−1 DW (Table 1). It was lower in flowers than in leaves and seeds. Yet, flavonoids represented a larger part of total polyphenol content in flowers (80%) than in the latter organs (61% and 68%, respectively).
3.1.3 Condensed tannins in methanolic extract
Condensed tannins were present in all studied plant organs, although in lower abundance than flavonoids, particularly in flowers (Table 1). Seeds and leaves showed the highest tannin content (ca. 2 mg CE g−1 DW).
3.1.4 Phenolic compounds identification in C. cardunculus leaves
According to the retention time of calibration standards (Table 2), leaf extracts presented a chemical profile composed of fifteen identified phenolic compounds, including gallic, sinapic, chlorogenic, syringic, vanillic, rosmarinic, trans-cinnamic, ferulic and p-coumaric acids as well as epicatechin, quercetrin, quercetin, apigenin, amentoflavone and flavones. The chromatogram also shows some other peaks apart from the 17 standards studied (Fig. 1); work is in progress to identify them. The analysis of the typical HPLC chromatogram depicted that syringic and trans-cinnamic acid are the major leaf phenolic acids and epicatechin as well as quercetrin are the major flavonoids.
Retention time of seventeen standards phenolic acids and flavonoids
| Standards | R.T. (min) | |
| 1 | Gallic acid | 5.26 |
| 2 | Sinapic acid | 6.63 |
| 3 | Chlorogenic acid | 8.2 |
| 4 | Catechin | 8.41 |
| 5 | Epicatechin | 9.31 |
| 6 | Syringic acid | 9.67 |
| 7 | Vanillic acid | 10.89 |
| 8 | Rosmarinic acid | 11.80 |
| 9 | Isoquercitin | 12.06 |
| 10 | p-Coumaric acid | 12.60 |
| 11 | Ferulic acid | 13.12 |
| 12 | Quercetrin | 13.30 |
| 13 | Quercetin | 17.67 |
| 14 | trans-Cinnamic acid | 18.84 |
| 15 | Apigenin | 19.18 |
| 16 | Amentoflavone | 19.46 |
| 17 | Flavone | 23.07 |
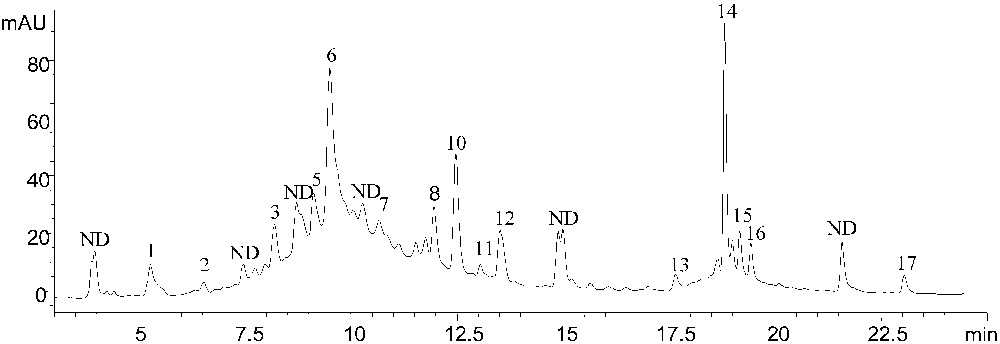
RP-HPLC chromatograms for Cynara cardunculus leaf methanolic extract. Signal was collected at 280 nm. Peaks numbers corresponding to: 1, gallic acid, 2, sinapic acid, 3, chlorogenic acid, 5, epicatechin, 6, syringic acid, 7, vanillic acid, 8, rosmarinic acid, 10, p-coumaric acid, 11, ferulic acid, 12, quercetrin, 13, quercetin, 14, trans-cinnamic acid, 15, apigenin, 16, amentoflavone, 17, flavones. ND, not determined.
3.2 Antioxidant activity
3.2.1 Free radical-scavenging activity of extracts using DPPH⋅
The magnitude of DPPH⋅ radical's quenching activity depended on the plant organs (Table 3), with IC50 values extending over a 5-fold range. Seed extracts displayed the highest DPPH⋅ quenching activity as compared to leaves and flowers, which showed, respectively, 2.3 and 5-times higher IC50 values.
Antioxidative activity of two methanolic extracts concentrations in cardoon aerial organs (1 and 200 μg ml−1). Antioxidative activity expressed as IC50 values for DPPH⋅ radicals and superoxide anion (O−⋅2) scavenging activity
| Organ | 1 μg ml−1 | 200 1 μg ml−1 | IC50 | |||
| DPPH⋅ | O⋅−2 | DPPH⋅ | O⋅−2 | DPPH⋅ | O⋅−2 | |
| Leaves | 6.51 | 34.6 | 96.7 | 85.8 | 53b | |
| Flowers | 7.44 | 21.4 | 64.4 | 83.8 | 118a | |
| Seeds | 12.0 | 27.1 | 99.5 | 97.8 | 23c |
3.2.2 Superoxide anion-scavenging activity
High O−⋅2 quenching activity was found for the different plant organs (Table 3). The IC50 value varied significantly between organs, leaf extracts displayed the smaller IC50 value (1.5 μg ml−1), being four and six times more efficient in scavenging activity than the extract from seeds and flowers, respectively.
3.3 Relationship between content in total phenolic compounds and antioxidant activities
The relationships between polyphenol, flavonoid and tannin contents and DPPH⋅ or superoxide scavenging activity (using IC50 values) were studied using linear regression analysis (Table 4). Significant negative correlation appeared between IC50 for DPPH⋅ scavenging and total phenolic (), flavonoid (), and tannin () contents. The O−⋅2 quenching activity was less tightly correlated with the concentration of the three types of compounds ( for total phenolics; for flavonoids; for tannins).
Relationship between phenolic compound concentration in Cynara cardunculus extracts and their antioxidant activity. Correlation coefficients (r values) of total polyphenol, flavonoid and condensed tannin concentration, with DPPH⋅ or O−⋅2 quenching activity expressed as IC50 values
| Polyphenols | Flavonoids | Condensed tannins | |||
| DPPH⋅ | O−⋅2 | DPPH⋅ | O−⋅2 | DPPH⋅ | O−⋅2 |
| −0.93 | −0.81 | −0.98 | −0.68 | −0.96 | −0.75 |
3.4 Antibacterial activity against human pathogenic bacteria
The leaf extract was active against Cocci Gram+ bacteria, with major activity against S. aureus. It was ineffective against Salmonella thyphymurium LT2. Low activities were observed against Gram bacteria (Table 5).
Inhibitory effect of leaf extracts on eight human pathogen bacteria, compared to that of positive standard (gentamycin)
| Bacterial strains | Diameter of growth inhibition (mm ± SD)a | |
| Gentamycin | Leaf extract | |
| S. aureus ATCC25923 | 18.3±0,6 | 25.7±0.6 |
| S. epidermidis CIP106510 | 11.7±0.6 | 20. 3 |
| Micrococcus luteus NCIMB 8166 | 27.7±1.5 | 21.7±0.6 |
| E. coli ATCC 35218 | 23 | 22.3 |
| E. feacalis ATCC29212 | not determined | 16.3±0.6 |
| L. monocytogenes ATCC19115 | 0 | 9.3±0.6 |
| P. aeruginosa ATCC 27853 | 18 | 13.7±0.6 |
| Salmonella thyphymurium LT2 | 24.7±0.6 | 0 |
a Data are reported as means ± SD of three measurements.
4 Discussion
In the present study, we determined the phenolic composition of customarily consumed organs of a cardoon (C. cardunculus) from an arid region in Tunisia, and we measured the actual antioxidant and antibacterial activities present in these organs.
Differences in polarity (and thus different extractability) of the antioxidative components may explain why extraction yield and antioxidant activity of the extracts differ. The solubility of phenolic compounds is actually governed by the type of solvent used, the degree of polymerization of phenolics, as well as by the interaction of phenolics with other food constituents and formation of insoluble complex [25]. For that purpose, methanol was recommended and frequently used for the extraction of phenolics [3,25].
As previously reported [15], the phenolic content was strongly dependent on cardoon organs. Leaves and seeds showed the same phenolic contents, twice those of flowers. In the same way, Maisuthisakul et al. [6] found the highest contents in total phenolic compounds and flavonoids in seed extract (about three times those in flowers). According to Bano et al. [26], the distribution of secondary metabolites may change during plant development.
Irrespective of the organs, and despite being variable (7 to 15 mg GAE g−1 DW), the polyphenol content in cardoon was higher than in several species cited in the literature. Djeridane et al. [27] found that this abundance is characteristic of the Asteraceae family. This may be related to the hard climate conditions of Asteraceae usual habitat (hot temperature, high solar exposure, drought, salinity), which stimulate the biosynthesis of secondary metabolites such as polyphenols. Indeed, the phenolic contents of a plant depend on a number of intrinsic (genetic) and extrinsic (environmental, handling and storage) factors [15,28].
Several methods have been developed to evaluate the antioxidant activity, using the quenching of synthetic or generated radicals in polar organic solvents such as methanol [29]. In this study, the DPPH⋅ and regenerated O−⋅2 superoxide methods were used to assess the antiradical activities of plant extracts. These activities were organ-dependent, seeds and leaves showing higher antioxidant activities than flowers. DPPH⋅ assays revealed that a considerable antiradical activity was extracted from the different plant parts. Seeds, and to a lower extent, leaves, have the highest antiradical activity. Seeds are known to display higher antiradical ability than the other organs of herbs and vegetables [6,29]. The O−⋅2 quenching capacity of methanolic extracts was also important in all organs and higher than DPPH⋅ scavenging activity. Leaves showed the lowest IC50 value, followed by seeds. These findings may be due to the higher content in phenolic compounds of leaves and seeds as compared to flowers. However, using the same superoxide inhibition test, Valentão et al. [16] found a much lower activity (IC50 100 times higher) in leaves of a Portuguese cardoon ecotype. This difference may be related to the extract solvent polarity (methanol versus water) and/or to the more severe climatic conditions prevailing at El Jem (Tunisia) than at Aroucas (Portugal).
The correlation level between the phenolic content and antioxidant activity between the plant organs is an interesting aspect, which supports the hypothesis that the former compounds contribute directly to antioxidant activity [23]. In our study, the correlation coefficient between phenolics (total polyphenol, flavonoids, and condensed tannins) and IC50 values of the DPPH⋅ quenching activity was highly significant (−0.93, −0.98, and −0.96, respectively), indicating that polyphenolics may play an important role in free radical scavenging [30]. The same type of linear correlation between antioxidant activities and phenolic contents has been found in whole-plant extracts, fruits, vegetables, and beverages [31]. Bahorun [32] pointed out the relationship between antioxidant activities of extracts from vegetative and reproductive organs of Crataegus monogyna and total phenolic, proanthocyanidin, catechin, flavonoid and phenolic acid contents. In our study, the tighter correlations were found with total phenols, while activities in leaves were influenced by flavonoids and those in reproductive organs were influenced by condensed tannins and catechins. However, O⋅−2 scavenging capacity and phenolic compounds were moderately correlated, as the correlation coefficient ranged from −0.68 to −0.81, suggesting that other compounds participate in the antioxidative activity. Mau et al. [33] found in methanolic extracts from Ling Chih and Baby Ling Chih a naturally antioxidant mixture including ascorbic acid, tocopherols, and total phenols, the latter fraction containing the major antioxidant components. In addition, Djeridane et al. [27] explained the unclear relationship between the antioxidant activity and total phenolics in several ways: the total phenolic fraction does not incorporate all the antioxidants, and synergistic interactions between the antioxidants in the mixture make the antioxidant activity not only dependent on the concentration, but also on the structure and the nature of the antioxidants.
The antibacterial activities of leaf extract were tested against human pathogenic bacteria. This extract was effective against Gram (+) and Gram (−) bacteria, with a major activity against S. aureus, S. epidermidis, Micrococcus luteus, and E. coli, but no activity against Salmonella thyphymurium LT2. The antibacterial activity of leaves may be related to the action of antibiotic compounds or to the presence of metabolic toxins. It may be related to the high level of phenolic components in leaves. Cowan [34] showed that several classes of polyphenol such as phenolic acids, flavonoids and tannins serve as plant defence mechanism against pathogenic microorganisms, insects, and herbivores. In fact, the site and the number of hydroxyl groups on the phenol components increased the toxicity against the microorganisms.
As leaves are the most comestible cardoon organ showing a good antioxidant activity in this study, a HPLC analysis of phenolics in this organ was carried out. This analysis pointed out the major phenolic compounds, which are principally syringic and trans-cinnamic acids (major phenolic acids) in addition to epicatechin and quercetrin as major flavonoids. Regarding to this results, C. cardunculus leaves antioxidant and antibacterial activities may be attributed to its major phenolic compounds. Actually, syringic acid gave strong positive correlation with some antioxidant tests, including DPPH−⋅ and ABTS+⋅ scavenging activities and reducing power [35,36]. Moreover, Oszmianski et al. [37] defined epicatechin as a powerful radical-scavenging compound, which is also able to increase the total antioxidant capacity and act like a powerful ferric-reducing agent [38].
5 Conclusion
Various plant organs (leaves, seeds and flowers) of C. cardunculus showed different levels of total polyphenol, total flavonoid and condensed tannin contents and antioxidant activity. There was a strong linear relationship between total phenolics and antiradical capacity. High phenolic content is thus an important factor in determining the antioxidant activity of this plant. Moreover, C. cardunculus leaves exhibited an interesting power against several human pathogenic bacteria, possibly due to their specific phenolic composition. As a dietary species, C. cardunculus appears not only interesting with respect to its antibacterial activity, but also as a good source of health-promoting polyphenols.


