1 Introduction
Avian foraging strategies can be influenced by morphology, foraging behaviour, microhabitat selection and resource availability [1]. There is a large body of literature on avian foraging, that is either species specific [2–7] or compares feeding guilds [8–11] living into two different habitats. Foraging specialisation is thought to promote coexistence among closely related and morphologically similar species living in the same habitat [12–14]. Foraging strategies of sympatric species can be partitioned by the identification niche components, such as foraging substrates and techniques used [15–17].
In a seasonally tropical dry forest, potential prey for insectivorous birds can vary in quantity and quality, notably concerning litter arthropods [18,19], and insects associated with new leaves [20]. Arthropod abundance and diversity affect the foraging behaviour of insectivorous bird guilds [21]. Anthropogenic disturbances, such as logging, also influence prey availability for insectivorous birds, including tropical dry forests of western Madagascar [22].
Nine Coua species occur in Madagascar [23]. This endemic genus appears to have no close living relatives [24,25], and include terrestrial as well as arboreal species, occurring in different kinds of forest habitats [26].
I examined the morphology and the foraging ecology of two coua species living in sympatry and studied their foraging behaviour in unlogged and logged dry deciduous forests of western Madagascar. In this paper, I compare: (1) whether the two sympatric species differ in their foraging strategies which can be correlated with morphological differences; and (2) whether these strategies are affected by logging.
2 Materials and methods
2.1 Study site
The study site was in the 10000-ha forestry concession of the Centre de Formation Professionnelle Forestière de Morondava (CFPF), in the Kirindy Forest (44°39′ E, 20°03′ S), 60 km northeast of Morondava in the deciduous forest of west Madagascar (Fig. 1). Rainfall ranges from 300 to 1400 mm per year (80% falling in January–February, the wet season) with an annual average of 800 mm [27,28]. Mean daily maximums are around 36 °C and the minimum around 19 °C, with a daily mean of 22 °C during the dry season and 29 °C during the rainy season [29,30].
Map of Madagascar showing the situation of the Kirindy station where the field study was performed.
The Kirindy River crosses the forest from east to west (Fig. 2). Proximity to water is important in the distribution and structure of the vegetation [31]: vegetation is tallest near the river on humid soils, where trees can reach a height of 25 m [32]. Forest along the river was a gallery forest, while the forest far from the river (>1 km) rarely exceeded 15 m high, with a denser understorey and more deciduous plants [33].
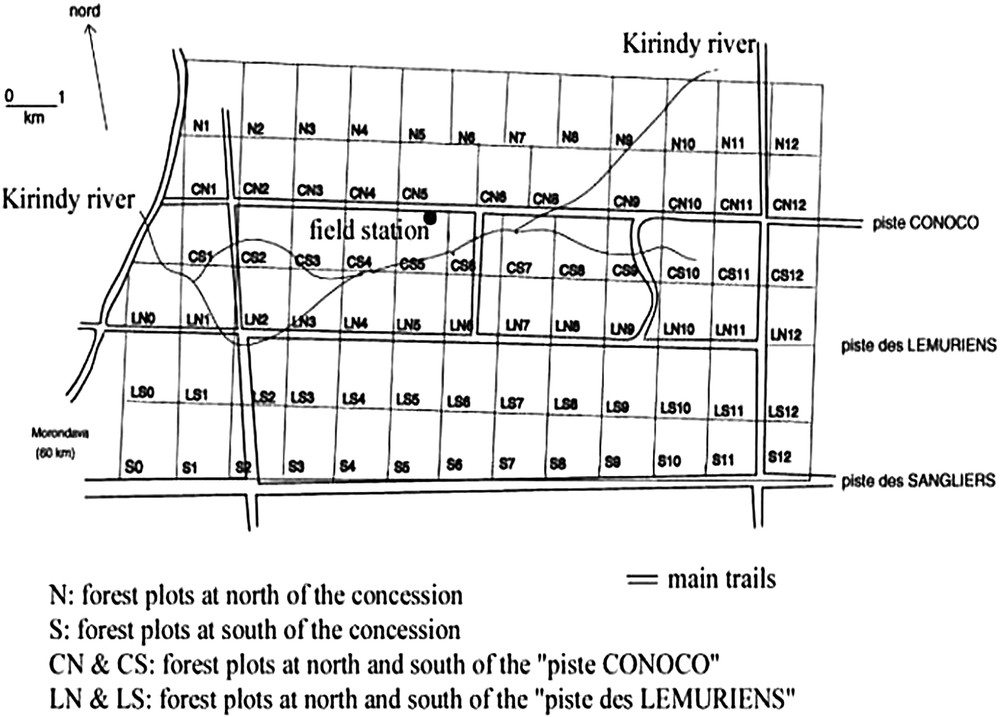
Map of the Kirindy forest (CFPF forest concession). The different forest plots are indicated by letters and figures. The field station was located near the “Piste Conoco”. Field studies were performed in the plots CS6 (logged forest) and CS7 (unlogged forest).
The study took place in plot CS7 (unlogged forest) and in the contiguous plot CS6, logged in 1980 [34], both in the gallery forest near the river (Fig. 2). The area of each plot was around 100 ha (Fig. 2). Unlogged forest had a closed canopy, with few understorey shrubs. Logged forest had a dense understorey vegetation, and canopy cover was also reduced [35]. Vegetation structure (logged or unlogged forest) will be referred to as habitat. I chose two contiguous plots to decrease the heterogeneity in the vegetation structure characterising this tropical forest at a small scale [36]. The fact that the study was carried out in two plots within close proximity could indicate the vegetation structure was more similar than it would be in different forest plots far from each other. To study the effects of the logging, I studied the vegetation structure by making 7 transects in CS7 and 6 in CS6 [37]. This showed that the unlogged gallery forest had more trees with dbh >20 cm, but fewer trees with dbh between 10 and 20 cm. Logging in the gallery forests induced a decrease of the canopy height and cover, an increase of the understorey vegetation and of the density of lianas (measured as the opposite of visibility). However, after logging, stems did not increase significantly in the understorey level.
I studied the foraging strategies of the two couas species during the wet season which coincided with the couas breeding season and probably with greater prey availability, from 1997 to 1999.
2.2 Study species
Two terrestrial couas were encountered in Kirindy: Coquerel's coua (Coua coquereli) and the Giant Coua (Coua gigas). Both species were encountered in the western forest domain in Madagascar [38], although Giant Coua occur also in the southern scrubland [23]. Chouteau et al. [37] estimated the population sizes of the two species in Kirindy. Coquerel's Coua was more abundant in the logged gallery forest than in the unlogged forest (24.2 versus 13.3 individuals/km2), but Giant Coua was less common in the logged habitat (3.7 versus 5.6 individuals/km2).
I first analysed the morphology of both Coua species to evaluate if there are differences in their morphology and then, if the same morphology could be linked to the use of the same foraging behaviour [14].
To compare the relative proportions of the two species, I analysed 21 specimens of Coquerel's Coua (6 females and 15 males) and 15 specimens of Giant Coua (6 females and 9 males) from the Museum National d'Histoire Naturelle de Paris. I measured three bill variables (length, width and depth), two variables on the legs (tarsus length and medium toe length) and also the total length of the specimens. I standardized each measure by dividing it by the total length of the individual – in order to study only the relative proportion of each variable. This ratio was used in a Mann–Whitney test [39] to compare the relative proportions of each species.
2.3 Data collection
Foraging data was recorded during the rainy season from 1997 and 1999. Observations of foraging events were opportunistic, but I attempted to observe both species at various times of day, although couas were difficult to locate. I obtained several foraging sequences during at least 1 minute, but no more than 5 minutes, with an interval of 30 minutes between two successive foraging sequences on the different sampled individuals. Although some investigators recommend taking only the first foraging event for analysis, I retained all to ensure recording inconspicuous foraging events and to reduce biases towards the most common foraging techniques and substrates used.
Due to the difficulty of capturing the birds in order to ring them, only five Coquerel's Coua were ringed in the logged forest and only three in the unlogged forest. All were in a small area (around 10 ha) situated between the two plots, and delimited by some trails (Fig. 2). Four Giant Couas were ringed and identified in the gallery forest – two in each habitat. The proportion of ringed birds compared to the total population in each plot was not known. However, the whole area of each plot (100 ha) was covered in this study in order to obtain data. Measures of foraging variables were performed on all the birds encountered in both plots (or 200 ha). Although it was impossible to evaluate the exact number of individuals recorded and used in the sample, the density of each species, as previously measured [37], indicated that different individuals were potentially used in this study. I obtained several observations on ringed and unringed birds, temporally and spatially separated, so these observations were probably taken from several different individuals and the sample of individuals observed was probably large enough to avoid a possible pseudoreplication in the analysis [37]. I took care to eliminate the individuals known to range over both habitats [40] from the analysis in order to avoid a bias in the measurement and because the delimitation between the disturbed and undisturbed forest was not always clear.
I obtained 66 foraging sequences in the unlogged forest and 54 in the logged forest for Coquerel's coua (respectively 367 and 361 foraging events). For Giant Coua, I obtained 63 sequences in the unlogged forest and 66 in the logged forest (respectively 763 and 551 foraging events).
During these observations I also tried to identify the prey captured by each coua species. However, the proportion of unidentified prey was high, due to the difficulty to identify the smaller captured prey.
2.4 Foraging behaviour
Five foraging variables were recorded: mean foraging height, capture technique, substrates used by the bird, prey size, and rate of capture. Terrestrial couas foraged mainly on or near the ground (in the first 30 cm above the ground). However, some prey was captured higher, by climbing in the understorey vegetation. In order to measure the proportion of prey captured in this way, I measured the height of the places where the birds foraged when they climbed the vegetation to search for food. The proportion of prey captured in the upper levels of vegetation was compared to the total amount of capture. Heights were estimated to the nearest 30 cm, by comparison with the height of the observer.
I recorded the kind of capture techniques the birds employed as follows (modified from [41]): ‘Glean’: prey captured on the substrate without manipulation of the substrate. Gleaned prey were usually spotted nearby (<0.3 m) and the attack did not involve a run or a flight component. ‘Lunge’: prey captured by running on the litter (to catch running prey) or in the air (to catch flying prey). ‘Leap’: prey captured by jumping (without using their wings), from the ground. ‘Sally’: prey captured by a jump, with use of wings. Prey captured by sallying was always higher than prey captured by leaping. ‘Probe’: prey captured after manipulation of the substrate (i.e. by using the bill to move the dead leaves on the litter or to chase the prey into the dead curled leaves). ‘Other’ included techniques not recorded or which did not fit exactly one of the techniques previously described. For analysis purposes, sally and leap were pooled together.
Substrates used were defined as: ‘Ground’ (on and into leaves litter); ‘Leaves’ (green leaves). ‘Trunk’ (defined as the bark of the tree and the stems, excluding leaves). ‘Other’ (including dead trunk and air for flying prey). I assumed that these substrates would harbor different prey types, and some differences in their use could help segregating the foraging strategies used by these two couas.
Prey size was estimated from bill length. Three prey size classes were defined: smaller than 0.5 cm (noted A); 0.5–1.5 cm (B) and longer than 1.5 cm (C). The largest animal prey was easily recognized. Seeds were identified because they were grouped on the ground under a particular tree. Usually, the bird ate them slowly, and I was able to identify the remaining seeds after the bird left the site.
To evaluate the prey availability for the two species, I calculated an index by retaining 20 periods of observation for each species and in each habitat. To ensure independence of data, these periods were selected randomly and were spatially and temporally separated, on different individuals in whole of the study area. These periods were equal at least to 20 minutes in order to reduce the possible bias introduced by inactive behaviour (preening, basking, singing and resting). To assess the index, I used the total number of events of capture observed during 20 minutes, divided by the total duration for each period (i.e. 20 minutes). Comparison was done by using each period as an independent data point. I often could not determine if an attack was successful, so the attack rate refers only to the rate at which prey was attacked, not captured.
2.5 Data analysis
For technique and substrate variables expressed as proportions, I used 8 multivariate analyses of variance (MANOVA, [42]), with the different values obtained for techniques or substrates used as dependent variables, to compare the interspecific variations (with the two coua species into each habitat used as independent variables) and to compare the intraspecific variations (with one coua species compared between the two habitats as independent variables). I did not include the ‘others’ categories in the MANOVA to avoid nonindependence of proportions [43]. I used an Arcsin transformation of the square root [] of each proportion p, in order to make the distribution closer to a normal distribution [44]. The number of prey chased by climbing, compared to the total of capture events recorded, the prey size and the nature of prey were analysed by chi-square (procedure FREQ, [39]). The foraging height reached by the birds in the vegetation and the index of capture were calculated by a Mann–Whitney test (procedure NPAR1WAY, [39]).
3 Results
3.1 Morphology and size of the two coua species
No sexual difference was revealed in each species by a preliminary analysis, so I pooled all the individuals to compare the two species. No difference existed in the relative form of the bill and in the relative length of the tarsus for the two species (all , Mann–Whitney test; Table 1). However, the relative length of the medium claw differed between the two species (, ) with a relatively longer claw for Coquerel's Coua.
Morphological measurements of individuals Coua coquereli and Coua gigas.
| Morphological variables | Measurements (cm) | Ratio (Gigas/Coquereli) | Ratio (variable/total length) | Mann–Whitney test | |||
| Coua coquereli () | Coua gigas () | C. coquereli | C. gigas | U | P | ||
| Bill length | 2.55 (±0.11) | 3.60 (±0.17) | 1.41 | 0.067 | 0.066 | 127 | ns |
| Bill depth | 0.95 (±0.07) | 1.44 (±0.08) | 1.51 | 0.025 | 0.026 | 111.5 | ns |
| Bill width | 0.92 (±0.06) | 1.34 (±0.08) | 1.45 | 0.024 | 0.024 | 151.5 | ns |
| Tarsus length | 4.11 (±0.34) | 5.75 (±0.20) | 1.40 | 0.108 | 0.105 | 134 | ns |
| Middle toe length | 2.58 (±0.16) | 3.48 (±0.24) | 1.35 | 0.068 | 0.064 | 98.5 | ns |
| Middle claw length | 1.07 (±0.09) | 1.41 (±0.11) | 1.32 | 0.028 | 0.026 | 78 | * |
| Total length (excluding tail) | 38.20 (±1.91) | 54.75 (±2.83) | 1.43 | – | – |
By its morphology, Giant Coua appeared to be similar by its general form but 45% bigger than Coquerel's Coua (Coquerel's Coua length: mean = 38.20 cm; Giant Coua length: mean = 54.70 cm, Table 1).
All ratios calculated in this study for linear size measurements between the two species were more than 1.3, and range from 1.32 to 1.51 (Table 1).
3.2 Intraspecific differences in foraging behaviour between habitats
No difference was recorded for Coquerel's Coua between unlogged and logged forest in the proportion of prey captured by foraging in the upper level of vegetation (, ; ). However, foraging heights to capture prey in the upper level of vegetation were significantly lower in the logged forest than in the unlogged forest (; ).
No difference was recorded for the size of the prey captured by this species between the unlogged and the logged forest (; , Fig. 3) and for the number of capture events by time unit between the two habitats (, ).
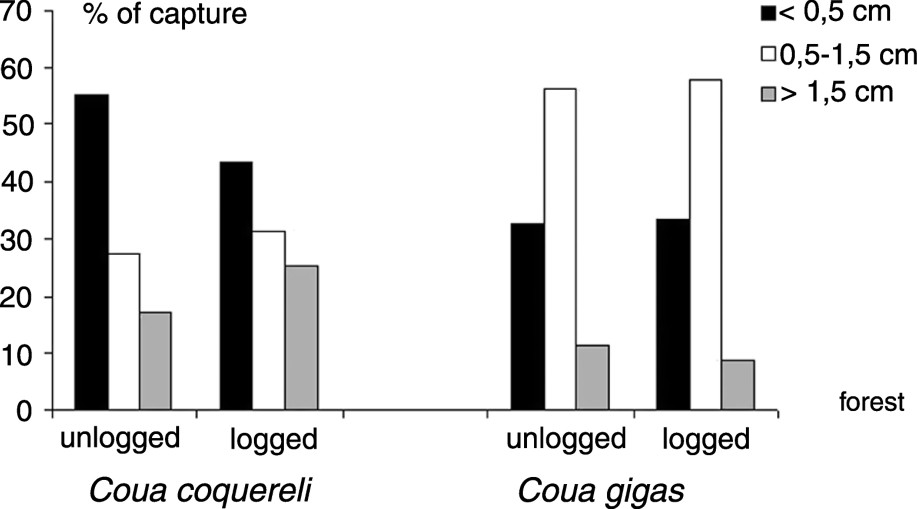
Size of the prey captured by each coua species, in logged and unlogged gallery forest in Kirindy during the rainy season.
Techniques used by Coquerel's Coua differed significantly between the two habitats (, ). Coquerel's Coua used glean and probe more often in the unlogged habitat (, ), but leap and sally were more often used in the logged habitat (, ). For this species, there was no difference for the substrates used between the two habitats (, ).
Diet differed between the two habitats (Fig. 4). Coquerel's coua fed more often on caterpillars in the unlogged forest (, ); They fed on seeds and homopterans in the logged forest by foraging often on the sweet secretion produced by a cicada Phromnia rosea, a common Flatidae found often in the disturbed habitats (Hladik [36]).

Nature of prey captured by each coua species in the unlogged and the logged forest in Kirindy during the rainy season.
Giant Coua did not forage in the upper levels of vegetation in the logged forest (Table 2): this species captured always the prey on or near the ground and did not climb to search prey into the understorey vegetation.
Analysis of variation in proportions of foraging variables used by the two coua species between the two habitats (with number of foraging events recorded between brackets) and interspecific comparison in each habitat.
| Variables | Coua coquereli | Coua gigas | Interspecific comparison | |||||
| Unlogged forest (367) | Logged forest (361) | Result | Unlogged forest (763) | Logged forest (551) | Result | Into unlogged forest | Into logged forest | |
| % of prey captured by foraging in the upper levels of vegetation | 8.6 | 8.6 | 3.5 | 0 | – | ** | – | |
| Mean height of capture when climbing (m) | 3.30 ± 2.40 | 1.60 ± 1.40 | U = 119* | 3.30 ± 3.20 | 0 | – | U = 396 ns | – |
| Prey size | See Fig. 3 | See Fig. 3 | See Fig. 3 | See Fig. 3 | *** | *** | ||
| Index of capture (attack/min) | 0.31 ± 0.15 | 0.23 ± 0.08 | U = 141.5 ns | 0.41 ± 0.17 | 0.42 ± 0.19 | U = 194.5 ns | U = 496.5* | U = 71*** |
| Techniques used | ||||||||
| Glean | 0.79 ± 0.12 | 0.70 ± 0.20 | F = 11.47*** | 0.81 ± 0.12 | 0.84 ± 0.07 | F = 2.21 ns | F = 0.63 ns | F = 29.5*** |
| Leap + Sally | 0.05 ± 0.05 | 0.09 ± 0.06 | F = 15.10*** | 0.09 ± 0.10 | 0.09 ± 0.09 | F = 0.14 ns | F = 10.09** | F = 0.71 ns |
| Lunge | 0.03 ± 0.05 | 0.09 ± 0.21 | F = 1.62 ns | 0.03 ± 0.03 | 0 | F = 84.80*** | F = 0.46 ns | F = 20.64*** |
| Probe | 0.08 ± 0.12 | 0.03 ± 0.06 | F = 7.51** | 0.01 ± 0.03 | 0.015 ± 0.03 | F = 0.36 ns | F = 15.92*** | F = 1.98 ns |
| Other | 0.05 ± 0.06 | 0.09 ± 0.07 | – | 0.06 ± 0.05 | 0.045 ± 0.05 | – | – | – |
| *** | *** | *** | *** | |||||
| Substrates used | ||||||||
| Ground | 0.71 ± 0.21 | 0.63 ± 0.17 | F = 3.27 ns | 0.77 ± 0.16 | 0.82 ± 0.07 | F = 4.22* | F = 3.63 ns | F = 65.67*** |
| Leaf | 0.22 ± 0.21 | 0.22 ± 0.11 | F = 0.015 ns | 0.14 ± 0.09 | 0.11 ± 0.05 | F = 9.08** | F = 6.38* | F = 26.34*** |
| Trunk | 0.06 ± 0.05 | 0.12 ± 0.10 | F = 2.95 ns | 0.08 ± 0.04 | 0.06 ± 0.03 | F = 0.68 ns | F = 7.42** | F = 0.09 ns |
| Other | 0.005 ± 0.02 | 0.03 ± 0.05 | – | 0.01 ± 0.03 | 0.01 ± 0.02 | – | – | – |
| ** | *** | *** |
No difference was recorded for the size of the prey captured by this species between the unlogged and the logged forest (, , Fig. 3), and for the number of capture events by time unit between the two habitats (, ).
Techniques used by Giant Coua differed significantly between the two habitats (, ). This difference was due to the use of lunge, which was used in the unlogged forest (see Table 2) but not recorded in the logged one (, ). The substrates used by this species differed significantly between the two habitats (, ) with ground significantly much more often used in the unlogged habitat but with the leaves more often used in the logged habitat.
Diet did not differ significantly between the two habitats (Fig. 4) although it seems that Giant Coua captured more seeds and more Orthoptera in the logged habitat.
3.3 Comparative foraging behaviour
In both habitats, Coquerel's Coua climbed more often than Giant Coua to search for prey ( in the unlogged forest; ; ; not calculated in the logged forest, due to the fact I recorded no climbing for Giant Coua in this habitat). There was no difference in the mean height reached by climbing between the two species in the unlogged habitat (, ), but a significant difference between the two species in the logged forest, with Coquerel's Coua climbing in the understorey vegetation whereas Giant coua was always foraging at the ground level in this habitat (Table 2).
Differences were significant between the two species for the prey size captured ( in the unlogged forest; in the logged forest; ; ): Giant Coua always captured more medium-size prey than Coquerel's Coua, but large prey (>1.5 cm) were more often captured by Coquerel's Coua (Fig. 3). These large prey were mainly caterpilars and orthopteras. However, the seeds eaten by Giant Coua were mainly around 1 cm, and contributed to an important proportion of the medium-size prey category captured by Giant Coua. I was able to estimate sizes for 82% of prey taken by Coquerel's Coua and 89% for the Giant Coua.
Regarding the index of capture, Giant Coua was always more efficient than Coquerel's Coua in both habitats ( in the unlogged forest, ; in the logged forest, ); Giant Coua captured more prey more frequently than Coquerel's Coua (Table 2).
The foraging techniques of the two species differed between habitats ( in the unlogged forest; in the logged forest; ). In the unlogged forest, Coquerel's Coua used probe (, ) more often than Giant Coua (Table 2), but Giant Coua used leap and/or sally more often (, ). In the logged forest, Giant Coua gleaned (, ) more often than Coquerel's Coua. However, Coquerel's Coua used lunge (, ) more often in the logged habitat.
I recorded a significant difference in the substrates used by the two species in the unlogged habitat (, ): Coquerel's Coua took prey from leaves more often than Giant Coua (, ) but Giant Coua more often used the trunk (, , Table 2). I also recorded a significant difference in the substrates used by the two species in the logged forest (; ). Coquerel's Coua took prey from leaves more often than Giant Coua (, , Table 2) and Giant Coua used ground more often than Coquerel's Coua (, , Table 2).
There was also a difference between the two couas in the kind of prey eaten (Fig. 4). Giant Coua ate seeds, and the biggest eaten seeds (between 0.5 and 1.5 cm), such as Capurodendron madagascariensis and Buxus madagascariensis, were found in its diet but, not in that of Coquerel's Coua. Other prey captured by Giant Coua, but not by Coquerel's Coua, included snails and some small vertebrates such as frog and chameleons (Furcifer sp.). Coquerel's Coua fed mainly on arthropods (caterpillars, orthopterans), but ate some unidentified black seeds (<0.5 cm). The fact that some seeds were incorporated in the diet of both species indicated they were not strictly insectivorous birds. Although I regularly saw the birds foraging along anthills, but ants were not consumed by either species. I also observed no aggressive interaction between the two couas species when they encountered each other.
4 Discussion
In this study, the two coua species differed by the foraging strategy used in the unlogged and the logged forest. The proportions of techniques and substrates used, the ability to climb in the upper vegetation layers and the diet differed between the species. However, the small proportion of prey captured in the upper level of vegetation by both species (<10% of all the capture) indicated the two species were mainly ground foragers.
4.1 Morphology and foraging strategies
Coquerel's Coua and Giant Coua encountered in Kirindy were similar in morphology, but differed in size, with Giant Coua being bigger than Coquerel's Coua. Some differences in morphology among sympatric and congeneric birds (i.e. beak size and structure) are often interpreted as a mechanism to maintain species coexistence through adaptation to different foraging behaviours and distinct feeding niche differentiation [14]. Body size is also an important factor to structure ecological communities and to promote coexistence among them [45]. Some previous works have showed that interaction between sympatric birds similar in morphology, physiology and behaviour but with similar body size (i.e. a low body mass difference) will be high and coexist less frequently in local communities. In contrast, species with different body masses have fewer interactions between them, and have different energetic requirements and different capacities in terms of foraging [46]. Although there is not direct proof that the coexistence of species in communities is structured by differences in body mass, evidence of size-related resource division supports the conclusion that different size classes in the animal community may promote coexistence [47].
My results are in connection with the controversial discussion of “Hutchinson's rule”. Hutchinson [48] showed in a study on size ratios of sympatric and congeneric species that they often can be described by a factor of approximately 1.3. He concluded that size ratios may “tentatively be used as an indicator of the kind of difference necessary to permit two species to co-occur in different niches but at the same level of the food web”. The ratios obtained here perfectly support Hutchinson's postulate that species exploiting a similar resource should differ by a certain minimum difference in size, if size describes an ecological relevant feature.
In such a case of interactions between two sympatric species, the larger species has an advantage because of its increased feeding ability, and eat more prey or capture bigger prey, a situation known as asymmetric competition [46]. In addition, energy-consuming techniques are most frequently used by species of lower body mass, presumably because of their energetic constraints [14]. In this study, I recorded that Giant Coua, the largest species, tended also to eat different and smaller prey than Coquerel's Coua. However, this species tended also to eat food more often that Coquerel's Coua. Giant Coua has to capture prey more often than Coquerel's Coua. No aggressive interaction was recorded when individuals of both species encountered. This study suggested that the difference of body size between the two couas, allowed them to coexist in the same habitat. Foraging sites are probably also more important to consider when explaining the coexistence between the two couas. They tended to use the same main substrates, but I found they used different microhabitats to forage [35], and captured probably different prey.
Morphology could be also regarded as having adapted to enable more efficient exploitation of certain microhabitats [14]. If the techniques used were linked to morphology, as suggested by Martin and Karr [49], the two species in Kirindy would have to use the same pattern of techniques with similar proportions for each one. The results obtained for these two species do not support this hypothesis, because Coquerel's Coua and Giant Coua tended to use the same techniques but in different proportions: although gleaning was used as main technique by both species, the proportion of other techniques differed. Energetically expensive techniques, such as probing, sallying and leaping, differed between the two species. Coquerel's Coua, the smaller species, tended to be a more active forager than Giant Coua, which used more probing in the unlogged forest than Giant Coua.
In the case where birds are very different in size, the phenomenon that small species tend to forage higher in the vegetation than their larger relative is confirmed by other studies [14]. Birds of a large size will have some difficulties to move about in vegetation, and will only forage above ground where there is an abundance of easily obtained prey. It might also be caused by an association of small body size with thin branches. However, in this study, Giant Coua was the larger bird, but could forage higher than Coquerel's Coua in the unlogged forest, but this species lost this ability in the logged forest, suggesting it was less efficient to see and climb in the dense, disturbed understorey vegetation.
4.2 Influence of logging on foraging strategies
Selective logging had an influence on foraging behaviour of both couas species. For Coquerel's Coua, logging modified its propensity to climb high in the understorey vegetation. Coquerel's Coua also changed the proportion of techniques used, with a greater use of energetically costly capture techniques, such leap and sally in the logged forest. However, the number of capture events per time unit were equal in the two habitats, suggesting that logging did not modify prey availability for this species. Logged forest could be apparently favourable for this species, because I measured a greater density for this species in this habitat [37].
Giant Coua also modified its foraging strategy, with fewer opportunities to climb in the understorey vegetation, and by modifying the proportion of the foraging techniques and the substrates used. Restriction in the techniques used concerned only ‘lunge’, an energy-expensive technique [41] used to capture big prey able to escape quickly, such as myriapods. This technique was not used by this species in the logged forest, because dense vegetation in this habitat (due to the consequent modification of the vegetation structure) and the big size of this bird prevented it from running and therefore using this efficient technique. The proportions of the other techniques did not change between the two habitats. The fact that the number of capture events did not change between the two habitats indicated this species was able to maintain apparently an efficient foraging by capturing alternative prey as caterpillars and orthoptera. However, the density of the Giant Coua is lower in the logged forest [37] suggesting this habitat was probably not suitable for this species.
The present study demonstrated that density and structure of vegetation play an important part in determining which foraging techniques are used. Coquerel's Coua avoided foraging in some large areas of unlogged gallery forest where Giant Coua was encountered and where this species could easily use techniques such as lunge to capture prey. These areas were characterized by their lack of dense shrub layers and where Giant Coua foraged in open microhabitats, with no dense understorey vegetation, whereas Coquerel's Coua preferred to forage in dense understorey vegetation, where this species can capture caterpillars and other prey, especially by climbing [35]. Logging increases dense shrubs layers in the logged parcels of the forest [37] favouring Coquerel's Coua by increasing the number of favorable microhabitats where this species forages, even if prey seem more difficult to capture in the logged forest and need energetically costly capture techniques. In the logged forest, Giant Coua had less available microhabitats to exploit successfully and had to cover a greater home-range to find the ideal microhabitats to forage efficiently.
Their behavioural flexibility and diverse foraging repertoires allow couas to exploit successfully the disturbed habitat. However, the results obtained here suggest these species are potentially vulnerable to some habitat management changes made in their habitat, such as logging or burning, which greatly modify the habitat structure as well as reducing the forest area. If logging was practised in the Malagasy dry forest at a higher rate, these couas could be susceptible to become endangered.
Acknowledgements
I thank the “Commission Tripartite” of the Malagasy Government, particularly the late Madame Fleurette Andriatsilavo for permission to work in Madagascar. The staff of WWF and Steven M. Goodman provided logistic support and very pleasant hospitality in Antananarivo. Olivier Langrand initiated the study and advised on the methods. Jean-Marc Thiollay made some comments on the first version of the manuscript. I thank the Deutsches PrimatenZentrum of Gottingen, especially Jörg Ganzhorn and Peter Kappeler, for the permission to use their station in the Kirindy forest and to take advantage of their logistics; the Centre de Formation Professionnelle en Foresterie in Morondava for facilitating access to the Kirindy concession. I would also like to thank all the people from the Kirindy forest and the German students and the Malagasy people for facilitating my accommodation during my stay in the forest. Emilien Marc provided some data during my absence. Chris Birkinshaw (Missouri Botanical Garden) helped with seed identifications. Benjamin Bravery (ISZS) and Dorothy Hunt helped to improve the English.


