1 Introduction
As noted in previous papers [1], the genus Hadrurochactas was created by Pocock [2] for the species Hadrurochactas sclateri. This has Guyana as its type locality. Subsequently, Kraepelin [3,4] placed H. sclateri in synonymy with Chactas schaumii Karsch, which was initially placed in the genus Hadrurochactas and subsequently in the genus Broteochactas. Mello-Leitão [5] followed Kraepelin's decision and cited the species as Broteochactas schaumi. Gonzalez-Sponga [6], however, resurrected the genus Hadrurochactas, a decision that he subsequently maintained [7,8]. Lourenço [9] temporarily accepted Gonzalez-Sponga's opinion, giving as his main reason for doing so the scarcity of material available. Subsequently, this decision was changed and Hadrurochactas considered as a ‘species group’ of Broteochactas [10,11]. In his synthesis of chactid scorpions, Sissom [12] accepted Lourenço's decision, but, in the ‘Catalog of the Scorpions of The World’ [13] he changed his mind and re-established Hadrurochactas as a valid genus. Monod and Lourenço [1] also retained Hadrurochactas as a ‘species group’ of Broteochactas. Since this publication on Hadrurochactas, more precise studies of several scorpion groups have lead different authors independently to consider Hadrurochactas to be a valid genus [14,15]. For this reason, I have followed the example of these authors in the present publication, and accepted the validity of the genus Hadrurochactas.
A new species is described here from ‘Chapada do Araripe’, a ‘Brejo’ located in the State of Pernambuco. The distribution of the genus Hadrurochactas is consequently confirmed as including the ‘Brejo’ formations of northeastern Brazil. The new species is also evidence of a past connection between the Amazonian and Atlantic forests, as already suggested by palaeobotanists [16].
2 The disrupted pattern of distribution of Hadrurochactas species in forest formations of South America
Interesting examples of genera presenting disrupted patterns of distribution can be found among scorpions restricted to savannas or rainforests (e.g. Rhopalurus Thorell, 1876). In the genus Hadrurochactas, close relationships exist between species in the Amazon rainforest and species found in outlying forest islands called ‘Brejos’. These are surrounded by xerophytic formations such as the ‘Caatingas’ of the northeastern Brazil. These ‘Brejos’ hills are covered by forest, because their elevation causes humid air to cool, so that condensation and consequent precipitation take place [17].
The species of Hadrurochactas, like almost all other chactid scorpions, are exclusively restricted to forests. Initially, they were believed to be distributed only in the ‘Guayana lowland floristic province’ as defined by Mori [18] and represented by two species: Hadrurochactas schaumii (Karsch) and Hadrurochactas odoardoi González-Sponga. Subsequently, three new species have been described from elsewhere: Hadrurochactas mapuera (Lourenço) found in the contact zone between Amazon and Guayana, south of the ‘Serra do Tumucumaque’, State of Pará, Brazil (considered to be a sibling species of H. schaumii). Hadrurochactas brejo (Lourenço), the first species to be found outside the previously known range of distribution of the genus (basically the Guayana-Amazon region) was collected in a ‘Brejo’ formation near Maranguape, in the State of Ceará, Brazil. The site is a small hill covered with forest and totally surrounded by xeric ‘Caatingas’. Hadrurochactas polisi (Monod & Lourenço) collected in central Amazon. The description of Hadrurochactas araripe sp. n., again from a ‘Brejo’ formation, confirms the disrupted pattern of distribution presented by the genus. The description of the new species brings further evidence for a possible past connection between the Amazon and the Atlantic forests [19], as already suggested by palaeobotanists. [16].
3 Possible origins and the distribution of the family Chactidae
In view of the evidence of the probable past connection between the Amazonian and the Atlantic forests [16,19], one further question can be addressed: Why has no species of chactid scorpions ever been recorded from the Brazilian Atlantic forest?
It seems evident that there is a gap in this particular forest formation in species of the family Chactidae. In fact, there are very few examples of typically Amazonian scorpions in the Brazilian Atlantic forest [20]. Only groups having a large geographical distribution (such as some basal elements of the family Buthidae), have been recorded from both forest formations. It also appears likely that some widely distributed groups, such as the genera Ananteris Thorell and Tityus C. L. Koch, have a Gondwanian origin. This seems not to be the case with chactids. The proto-elements that are at the base of the extant chactid (sensu strictu), undoubtedly have their origin in Laurasia, as do elements of the family Iuridae [21,22]. The proto-elements of the family Chactidae definitely originated from the North. Subsequently, a remarkable radiation took place in the tropical forests of the South American continent. Some fossil evidence justifies assuming a possible Gondwanian origin for the Chactidae [23]. However, fossil evidence is very often weak and can lead to ‘free interpretations’. These naturally are open to possible misinterpretations. For example, Lourenço [24] assigned Palaeoeuscorpius gallicus from the Lower Cretaceous amber of France, to the Chactoidea. This conclusion was based on a single incomplete pedipalp, which is a rather weak evidence. Neverless, Menon [23], confirmed the previous opinion of Soleglad and Fet [14], according to which, this species is not a member of the Chactoidea. In the same line of thought, the decision to assign Araripescorpius ligabuei Campos from the Lower Cretaceous of Brazil to the family Chactidae, even if based on some trichobothrial evidence, remains a ‘free interpretation’. More than 100 my separate the fossil species from the Lower Cretaceous of Brazil (Santana formation) from the extant species of Chactidae, including Hadrurochactas araripe sp. n. In this same line of thought, it appeared more logical to Carvalho and Lourenço [25] to assign Protoischnurus axelrodorum, also from the Lower Cretaceous of Brazil, to a new family Protoischnuridae, within the Scorpionoidea, as a possible proto-element (sedis mutabilis) of both families Scorpionidae and Ischnuridae (now Liochelidae). This position was, however, rejected by Menon [23], on the basis that age does not count: “Mere difference in age is not be a valid criterion on which to create a new family”. Here, we are faced once again, with a conflict between reductionism and divisionism.
A further consideration that should support the case for a non-Gondwanian model for chactids is the remarkable gaps they represent in the faunas of Africa, Australia and especially in Madagascar. Since its rupture from Africa almost 100 my BP, and subsequently from Australia and India [26], Madagascar remained isolated and preserved some of the original lineages that had been present in Gondwana. Only two scorpion lineages, Buthoidea and Scorpionoidea can be observed in Madagascar [27]. These contain typical Gondwanian elements such as the genera Tityobuthus Pocock and Opisthacanthus Peters [26,27]. This suggests that chactoids were never represented in the fauna of Gondwana.
4 Methods
Measurements and illustrations were made using a Wild M5 stereo-microscope with a drawing tube (camera lucida) and an ocular micrometer. Measurements follow those of Stahnke [28] and are given in mm. Trichobothrial notations are those developed by Vachon [29] and the morphological terminology mostly follows that of Hjelle [30].
Taxonomic treatment
Family Chactidae Pocock, 1893
Genus Hadrurochactas Pocock, 1893
Hadrurochactas araripe sp. n. (Figs. 1–4, 6, 8–13)
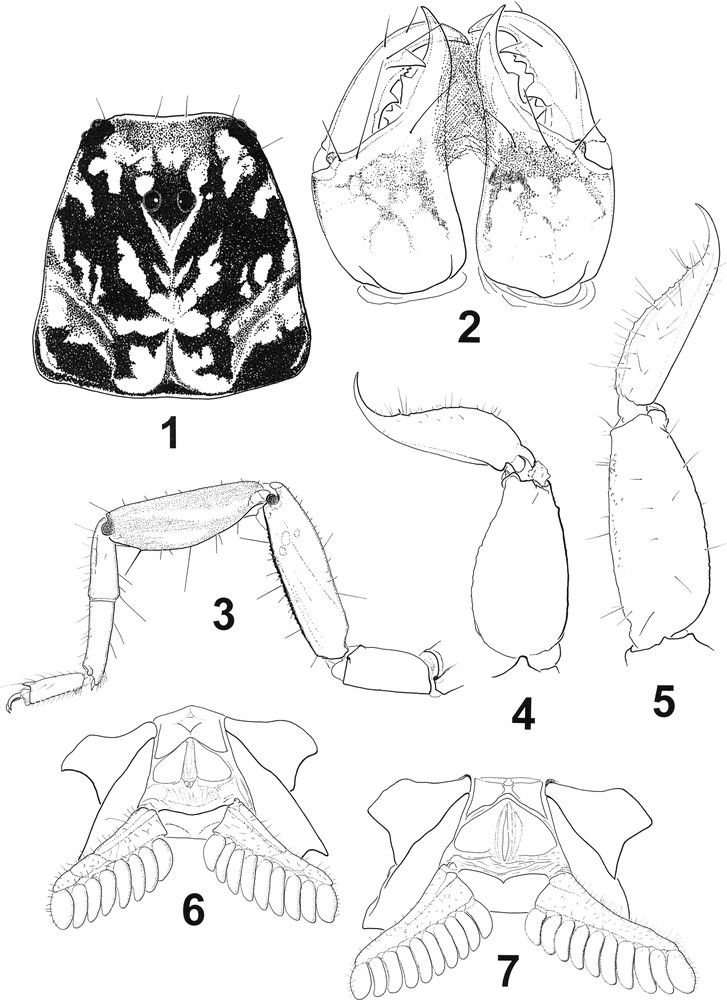
Hadrurochactas araripe sp. n., male holotype. 1. Carapace. 2. Chelicerae. 3. Leg IV. 4-5. Metasomal segment V and telson, lateral aspect. 4. H. araripe sp. n. 5. H. schaumii (male). 6-7. Sternum, genital operculum and pectines. 6. H. araripe sp. n. 7. H. schaumii (male).
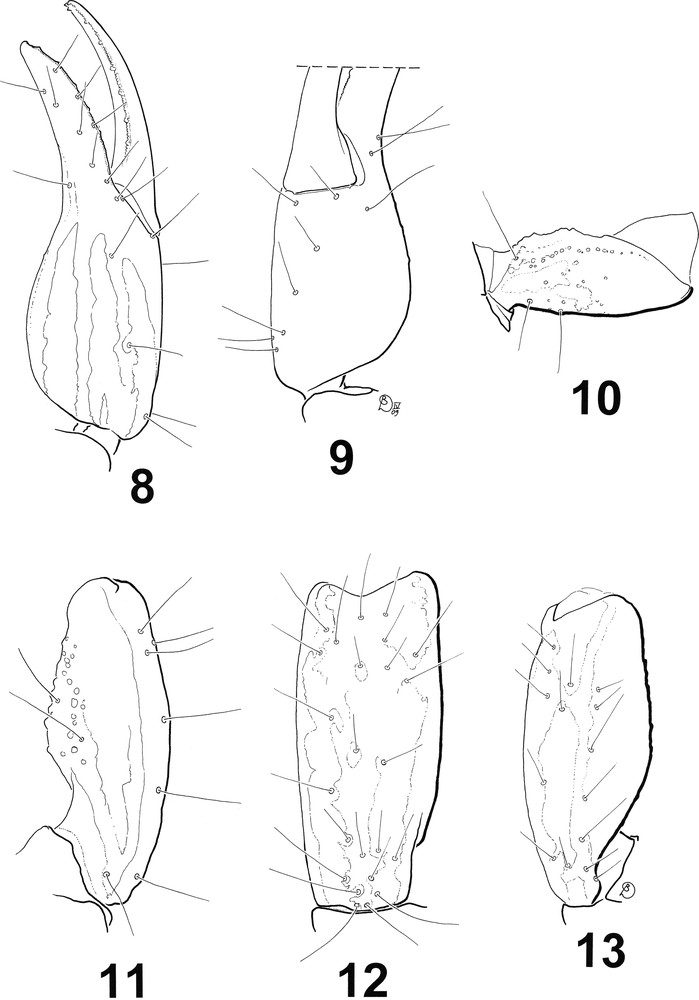
Hadrurochactas araripe sp. n., male holotype. Trichobothrial pattern. 8–9. Chela, dorso-external and ventro-internal aspects. 10. Femur, dorsal aspect. 11–13. Patella, dorsal, external and ventral aspects.
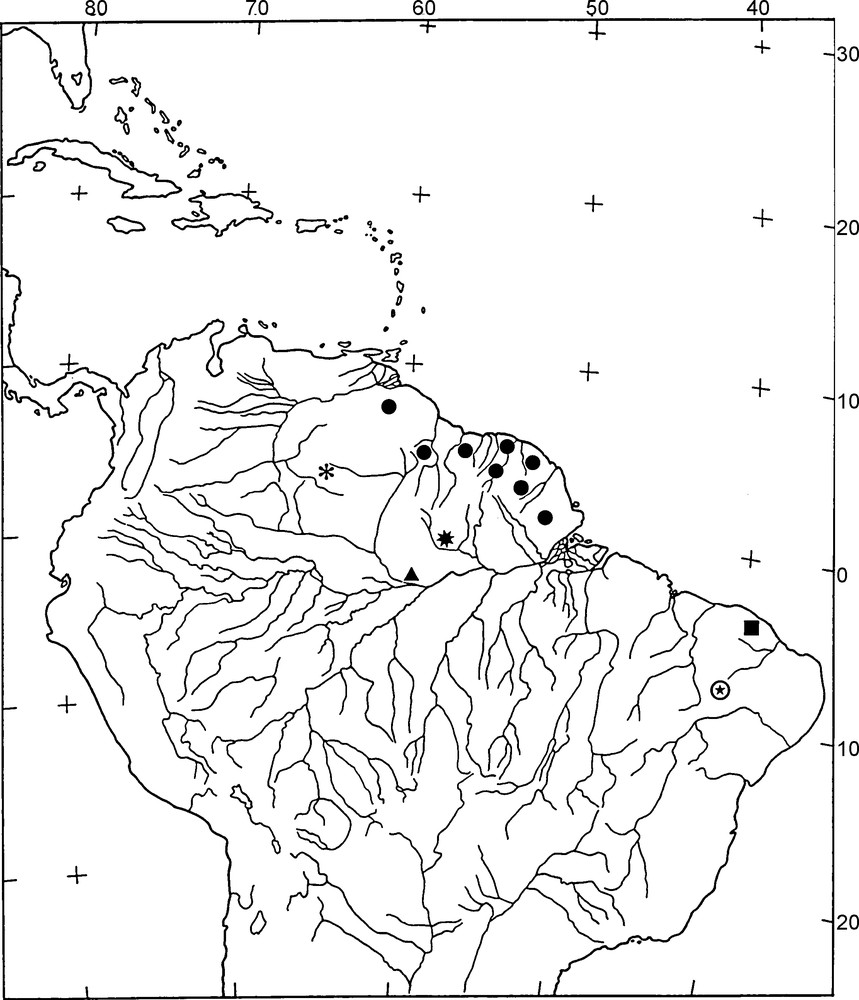
Map of Northern South America showing the distribution of Hadrurochactas species. H. schaumii (black circle). H. odoardoi (black flower). H. mapuera (black star). H. brejo (black square). H. polisi (black triangle). H. araripe sp. n. (circle with black star). For a precise delimitation of the morphoclimatic regions of tropical South America refer to Lourenço [32].
Male holotype: Brazil, State of Pernanbuco, ‘Chapada (serra) do Araripe’, IX/1963 (collected by local people).
The type should be deposited in the collections of the Muséum national d’histoire naturelle, Paris.
Etymology: The specific name is placed in apposition to the generic name and refers to Araripe, the location in which the new species was collected.
Comparative material: H. brejo: female holotype; Brazil, Ceará, Maranguape Mts. (W. M. Mann, Stanford Expedition). H. schaumii: 2 males, 2 females; French Guyana, St Eugène research station, on the stream named Courcibo (J.-C. de Massary leg).
Diagnosis: Small scorpions, 19.3 mm in total length, including telson. Coloration reddish-yellow to reddish-brown, densely spotted on pedipalps and chelicerae. Body and appendages weakly granulated. Telson weakly granulated with small-sized spine-like granules and one larger spinoid granule on the ventral side, under the aculeus. Pectines with 9-8 teeth on male. Fixed and movable fingers of pedipalps with 5-6 rows of linear granules; three accessory granules on the extremity of fingers. Trichobothrial pattern of type C, neobothriotaxic (majorante) ‘major neobothriotaxy’. Legs with long thin setae.
Relationships: Hadrurochactas araripe sp. n. can be distinguished from the other species of the genus, and in particular from H. brejo and H. schaumii by the following combination of characters: (1) globally smaller size; the new species is one of the two smallest species in the genus (see measurements after description); (2) body, pedipalps and chelicerae densely spotted; (3) metasomal segment V and telson weakly granulated to smooth; telson short and bulky; (4) ventral surface of metasomal segment V sparsely granular; (5) ventral side of telson with small-sized spine-like granules and one larger spinoid granule under the aculeus; (6) pectines with only 8-9 teeth.
Description based on male holotype
Coloration. General coloration reddish-yellow to reddish-brown, densely spotted on pedipalps and chelicerae. Prosoma reddish-brown with several dark spots. Mesosoma: Tergites reddish-brown with confluent dark spots; sternites III to V yellowish, sternites VI and VII reddish-yellow to dark reddish; coxapophysis and sternum reddish-yellow; genital operculum and pectines pale yellow. Metasoma: all segments reddish-brown; carinae dark, almost blackish; telson reddish to reddish-brown; aculeus dark, almost blackish. Chelicerae yellowish with variegated brownish spots; fingers yellowish with reddish teeth. Pedipalps reddish-brown with dark longitudinal stripes on femur, patella and chela. Legs yellowish with very diffused pale brown spots; basitarsus and tarsus pale yellow.
Morphology. Carapace weakly granular to smooth; anterior margin very weakly emarginated; carinae absent; all furrows weakly pronounced; postero-median furrow finely granular; median ocular tubercle distinctly anterior to the centre of the carapace; two pairs of small lateral eyes. Mesosoma. All tergites smooth with a few indistinct granules on the posterior margin; tergite VII with four vestigial carinae which carry granules on the posterior half; posterior half of tergite VII finely granular, with some larger granules interspersed. Venter. Genital operculum longitudinally divided, each half with a sub-triangular shape; pectines with 9-8 teeth, fulcra absent; all sternites smooth with rounded spiracles, carinae absent. Metasoma. Dorsal carinae granular on segments I-IV, absent on segment V; dorsolateral carinae granular on all segments; ventrolateral carinae weakly pronounced or absent on all segments; ventrolateral and ventral carinae absent on all segments; dorsal surface smooth on all segments; lateral surfaces weakly granular to smooth on segments I to IV; very slightly granular on segment V; ventral surface smooth on segments I to IV; with some thin granules on V. Telson short and bulky, with small-sized spine-like granules and one larger spinoid granule under the aculeus; dorsal side smooth, aculeus relatively short. Cheliceral dentition characteristic of the family Chactidae [31]. Pedipalp femur pentacarinate, moderately granular; patella and chela with weakly marked to unconspicuous carinae; fixed and movable fingers with 5-6 rows of linear granules; three accessory granules on the extremity of fingers. Trichobothrial pattern of type C, neobothriotaxic (majorante) ‘major neobothriotaxy’ [29]. Legs with long thin setae.
Morphometric values (in mm) of the male holotype. Total length, 19.3 (including telson). Carapace: length, 2.7; anterior width, 1.6; posterior width, 2.7. Mesosoma length, 5.0. Metasomal segments. I: length, 1.0; width, 2.1; II: length, 1.2; width, 2.1; III: length, 1.3; width, 2.1; IV: length, 1.9; width, 2.1; V: length, 3.0; width, 2.0; depth, 1.7. Vesicle: length, 3.2; width, 1.5; depth, 0.9. Pedipalp: femur length, 1.9, width, 0.8; patella length, 2.3, width, 0.9; chela length, 3.6, width, 1.2, depth, 1.2; movable finger length, 2.0.
Acknowledgements
I am most grateful to Prof. John L. Cloudsley-Thompson, London, for useful comments to the manuscript, to Bernard Duhem, Muséum, who prepared the remarkable illustrations and to Elise-Anne Leguin, Muséum, for the help with the preparation of the plates.


