1 Introduction
Stevia (Stevia rebaudiana Bertoni) is a semi bushy perennial herb, which belongs to the family Asteraceae. The plant is also known as “Sweet leaf (in USA)”, “Sweet honey leaf (in Australia)”, “Sweet herb of Paraguay”, estimated to be 300 times sweeter than sucrose [1]. Stevia is native to eastern Paraguay and has been used as a sweetener for many years. Guarani tribes of Paraguay and Brazil used Stevia species primarily S. rebaudiana as a sweetener and source of medicine. Stevia has been introduced as a crop in a number of countries including Japan, China, Korea, Mexico, United States, UK, Indonesia, Canada and all over South America [2,3]. Stevioside, a diterpene glycoside, forms the largest part of sweetener molecules present in the leaves. Other compounds present but in lower concentration are steviolbioside 2, rebaudioside and dulcoside A10 [4]. Stevioside is highly recommended for diabetes, hypoglycemia, high blood pressure and obesity because it remains unmetabolized. In addition, Stevia provides other therapeutic properties such as anti-hyperglycemic, anticancerous [5], antihypersensitive agent [6], contraceptive properties [7] and prevention of dental caries [8].
The very low germination percentage of Stevia seeds [9] restricted its uses for large-scale cultivation. On the other hand, propagation through seeds produces less homogeneous populations, resulting in variations in sweetening levels [10]. Propagation by cutting is also limited because of a lower number of individuals that can be obtained simultaneously from a single plant [11]. Therefore, to overcome the all above-mentioned obstacles, micropropagation becomes a viable alternative for large-scale cultivation of this medicinally important plant.
The focus of this article is to establish an improved micropropagation method of Stevia rebaudiana with an aim to increase biomass and stevioside content through priming by a plant growth retardant, chlorocholine chloride (CCC). Such priming of micropropagated propagules had been recommended to obtain robust and acclimatized plantlets against various environmental stresses [12]. Plant growth retardants play an important role in agriculture and horticulture by reducing unwanted shoot elongation [13]. CCC was reported to increase the number of flowering nodes and time to flower with decreased shoot height [14]. Application of CCC was found to increase the multiple shoot regeneration in Vigna mungo and also showed positive implications on the hardening process [15]. Taxol production in Taxus globosa shoot callus was also elevated by CCC treatment [16]. In Catharanthus roseus, CCC increases biomass and alkaloid production under both in vitro and field grown plants [17]. In view of the all the promising impact of CCC, this is an attempt for the improved micropropagation and stevioside production of Stevia rebaudiana.
2 Materials and methods
2.1 Plant material
Stevia rebaudiana Bertoni seeds were brought from medicinal plant nursery of Government Cinchona Factory located at Mungpoo, Darjeeling, West Bengal.
2.2 Tissue culture
Axenically grown cotyledonary leaves were used as explants in the tissue culture experiment. Seeds were first surface sterilized with a 0.1% (w/v) mercuric chloride solution followed by washing in distilled water several times and then soaked in distilled water for 2–3 h. Soaked seeds were kept in a filter paper inside Petri plates moistened with water for germination. Regenerated cotyledonary leaves (6–7 days old) were then thoroughly washed in sterile distilled water followed by surface sterilization in 0.1% (w/v) mercuric chloride for 2–3 min. After that, leaves were rinsed three to four times in sterile double distilled water then inoculated in culture media. Cultures were kept in culture room illuminated with white fluorescent light (2000 lux) under 16/8 h light/dark and 72% RH condition. Subculturing was routinely done after every 4 to 5 weeks of inoculation.
2.3 Culture medium and hormonal manipulations
MS medium (Murashige and Skoog, 1962) was used as a basal medium for the in vitro culture experiment. The culture medium was provided with 3% (w/v) sucrose and gelled with 0.8–1% (w/v) agar-agar. The pH of the medium was adjusted to 5.7–5.9 by 0.1(N) NaOH. The gelled medium was poured into culture tubes, plugged with non-absorbent cotton and then autoclaved at 121 °C under 15 lb/sq. inch pressure for 15 min. MS medium supplemented with different plant growth promoters like BA and kinetin singly or in combinations were used for multiple shoot induction [18]. For rooting, regenerated microshoots were transferred into the MS medium containing different concentrations of IBA (0.25 mg/l, 0.5 mg/l, 1 mg/l and 3 mg/l) (Figs. 1–4). Moreover, for priming, the regenerated microshoots were transferred into either different concentration of CCC (0.25 mg/l, 0.5 mg/l, 1 mg/l and 3 mg/l) or in CCC + IBA (at same concentrations) supplemented MS medium (Figs. 5–8).

Microshoot transferred in MS medium containing 3 mg/l CCC.

Microshoot transferred in MS medium containing 1 mg/l CCC.

Callusing under microshoot base in MS medium supplemented with 1 mg/l IBA and 3 mg/l CCC.

Subsequent regeneration of microshoots from callus in MS medium supplemented with 1 mg/l IBA and 3 mg/l CCC.

Microshoot regenerated in MS medium supplemented with 1 mg/l IBA + 3 mg/l CCC combination (after 3 weeks of transfer into fresh rooting medium containing 1 mg/l IBA).

Microshoot regenerated in MS medium supplemented with 3 mg/l IBA + 3 mg/l CCC combination (after 3 weeks of transfer into fresh rooting medium containing 1 mg/l IBA).

Difference in length of the microshoots transferred in MS medium supplemented with IBA or regenerated in MS medium containing IBA + CCC combinations.

Initiation of rooting from microshoot base.
2.4 Hardening
Well-rooted plantlets were transplanted into a 100 ml beaker filled with autoclaved soilrite-soil mixture (1:1) and kept in the culture environment till the plantlets resumed growth (Figs. 9–12). After that, the plantlets were transferred to the small pot for full growth followed by final transplantation into the garden. Survival percentage of the plantlets was recorded after 1 month of final transplantation (Figs. 13–15).

Rooting of different microshoots.

Microshoots regenerated in MS medium supplemented with 0.5 mg/l IBA and 3 mg/l CCC transferred to fresh MS medium containing IBA for rooting.

Plantlet transferred to soilrite-soil mixture (1:1) for hardening.

Plantlet resumed growth in hardening mixture.

Plants transferred into the pot showing different growth behavior (plant indicated by arrow is CCC primed).

An established plant in the pot.

Fully established plant in the field (after 4 months of final transplantation).
2.5 Extraction of stevioside
Extraction of stevioside was carried out following the method described in [19] with slight modifications. Briefly, freshly collected Stevia leaves (200 mg) were dried at room temperature (30 °C) and ground into small particle size (1 mm). Such ground leaves were taken into a 10-mL centrifuge tube and extracted three times with water in a boiling water bath set at 60 °C for 1 h. Each extract was cooled to room temperature and centrifuged (for 15 min at 5000 rpm). The supernatants were filtered through Whatman No 1 filter paper and transferred to a 25-mL separating funnel and partitioned against isobutyl alcohol. Then the organic extracts were taken into a pre-weighed beaker and evaporated to dryness under reduced pressure at 50 °C. Evaporated residues (1 mg) were dissolved into a known volume (2 ml) of HPLC grade water and filtered through Millipore microfilter (0.45 μm). The filtrates were then subjected to HPLC analysis.
2.6 HPLC conditions
HPLC was performed by using Water's HPLC system (USA) equipped with a 1525 binary HPLC pump, XTerra RP18 column, 4.6 × 250 mm with 5 μm internal diameter, and 2487 duel λ absorbance detector along with the integrated Empower 2 software. The mobile phase in gradient mode started with 84% acetonitrile (A) and 16% water with 0.05% w/v tri-fluroacetic acid (B). The elution changed to 70% A and 30% B within 25 min. The details of gradient elution are given in Table 1.
Gradient elution.
| Time (min) | Elution of A (%) | Elution of B (%) |
| 1–7 | 84 | 16 |
| 7–15 | 75 | 25 |
| 15–20 | 75 | 25 |
| 20–25 | 70 | 30 |
The flow rate was 1 ml/min and the detector was set at 210 nm. The injection volumes were 20 μL throughout the experiment. All the solvents and samples were filtered through 0.45 μm filter (Millipore) before use in HPLC. The identification and quantification of stevioside in plant samples were done by retention time, UV spectra and by comparing the peak area of the sample with that of the standard. Calibration curve for standard stevioside was constructed in the concentration range of 0.025 to 2 mg/ml.
2.7 Statistical analysis
All the experiments were carried out in triplicate. Mean, standard deviations and one-way ANOVA with Duncan's Post Hoc tests were performed to determine the significant differences between the data using SPSS version 17 for Windows. Significant differences were considered at P = 0.05.
3 Chemicals
Acetonitrile (HPLC grade), water (HPLC grade) and tri-fluroacetic acid were purchased from Merck, Germany. Standard stevioside was brought from Sigma-Aldrich (USA). All the other chemicals used were of analytical grade.
4 Results
4.1 Micropropagation
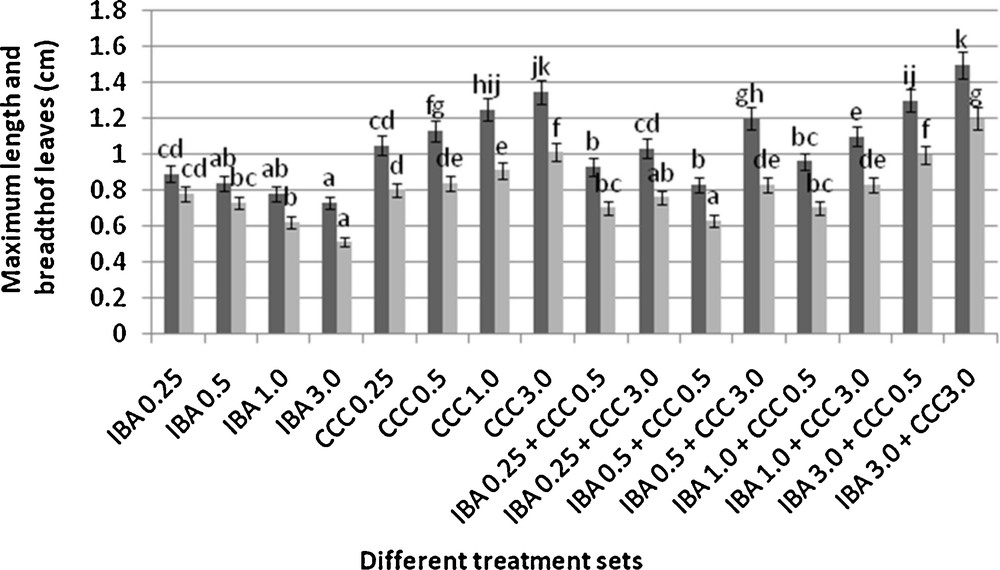
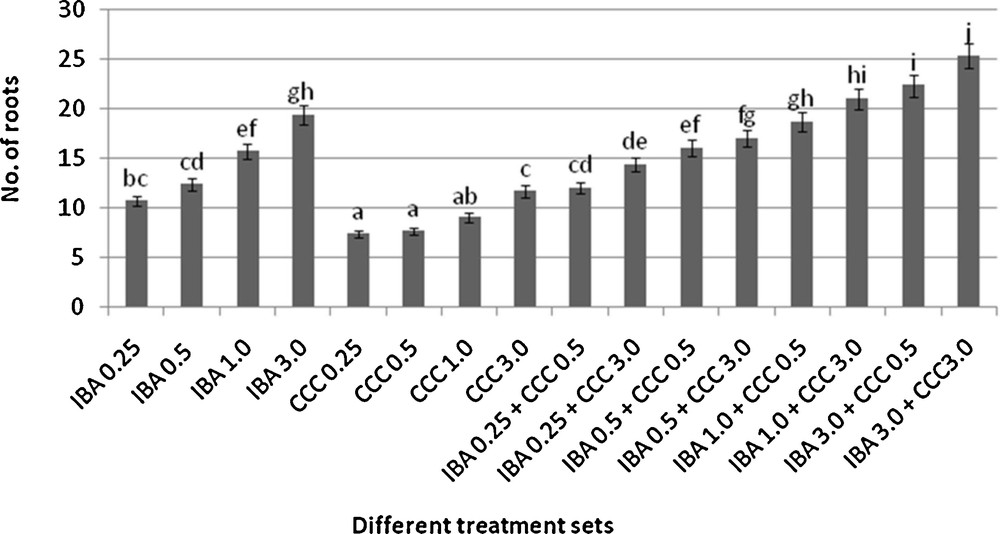
Microshoots regenerated in MS medium supplemented with different combinations of BA and Kinetin [18] were subjected to rooting in MS containing different concentrations of IBA. For in vitro priming, microshoots (2 cm long) were transferred to the MS medium supplemented with various concentrations of CCC singly as well as in combinations with IBA. Comparing the response induced by IBA and CCC separately, it was found that the number of regenerated roots per microshoot was lower (11.66 ± 1.52 in 3 mg/l CCC, 19.33 ± 1.5 in 3 mg/l IBA) in MS with only CCC (Table 2), but length of the microshoots (4.7 ± 0.30 cm in 3 mg/l CCC, 9.8 ± 0.43 cm in 3 mg/l IBA) and intermodal distance (0.75 ± 0.05 cm in 3 mg/l CCC, 1.63 ± 0.08 cm in 3 mg/l IBA) was reduced significantly following CCC application (Figs. 1,2,16 and 17). Emergence of typical larger leaves was found (max. length 1.35 ± 0.05 cm and max. breadth 1.01 ± 0.07 cm in 3 mg/l CCC, max. length 0.73 ± 0.02 cm and max. breadth 0.51 ± 0.02 cm in 3 mg/l IBA) to develop with CCC priming (Table 2). Moreover, the fresh weight (198 ± 3 mg in 3 mg/l CCC) of the plantlets (without the weight of the roots) and their chlorophyll retaining capacity (65.33 ± 2.51 days in 3 mg/l CCC) were also enhanced significantly after CCC treatment, as compared with MS with IBA (142 ± 1.73 mg and 25.33 ± 1.53 days, respectively in 3 mg/l IBA) (Table 2).
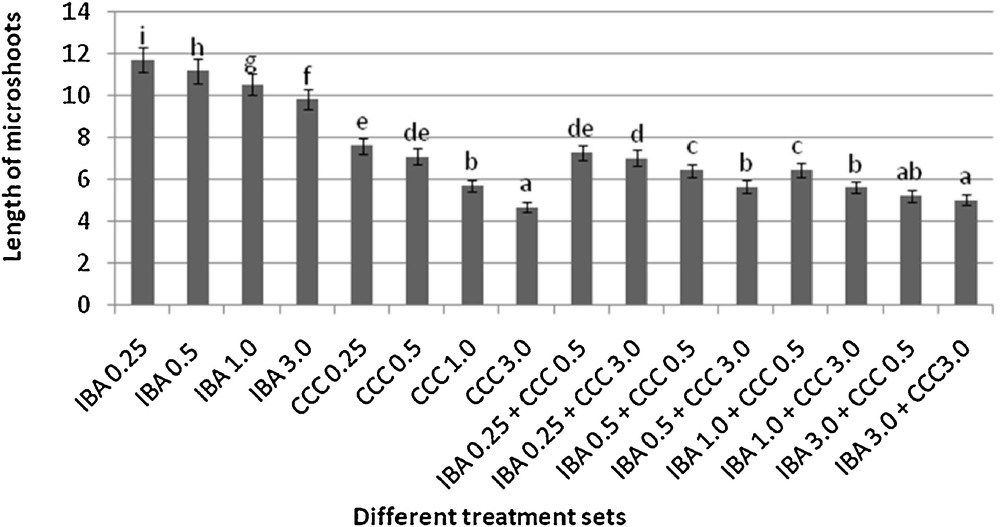
Variation in the length microshoots (mean ± SD). Bars with same alphabet superscripts are not significantly different (P = 0.05).
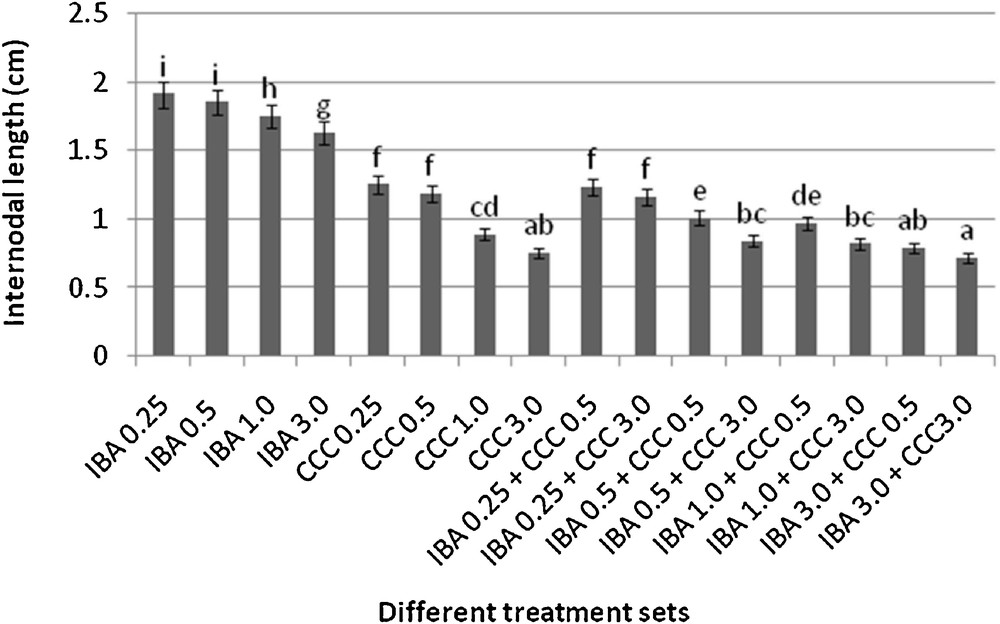
Differences in internodal length of the microshoots (mean ± SD). Bars with same alphabet superscripts are not significantly different (P = 0.05).
Rooting behavior of different microshoots (mean ± SD). Bars with same alphabet superscripts are not significantly different (P = 0.05).
Growth behavior of different microshoots in MS medium supplemented with IBA, CCC or IBA + CCC combinations.
| Treatments (mg/l) | Length of microshoots (cm) | Internodal length (cm) | Maximum length of leaves (cm) | Maximum breadth of leaves (cm) | No. of roots | Fresh weight of the plantlets [mg] | Chlorophyll retaining capacity (in days) |
| IBA 0.25 | 11.7 ± 0.40i | 1.91 ± 0.05i | 0.89 ± 0.02cd | 0.78 ± 0.03cd | 10.66 ± 1.53bc | 16l ± 2cd | 35 ± 2ca |
| IBA 0.5 | 11.16 ± 0.35h | 1.85 ± 0.06i | 0.84 ± 0.01ab | 0.73 ± 0.02bc | 12.33 ± 1.52cd | 154.33 ± 2.51bc | 31 ± 2.02bc |
| IBA 1.0 | 10.53 ± 0.51g | 1.75 ± 0.07h | 0.78 ± 0.03ab | 0.62 ± 0.04b | 15.66 ± 1.54ef | 148.34 ± 2.52ab | 28.33 ± 1.52b |
| IBA 3.0 | 9.8 ± 0.43f | 1.63 ± 0.08g | 0.73 ± 0.02a | 0.51 ± 0.023 | 19.33 ± 1.56gh | 142 ± 1.73a | 25.33 ± 1.53a |
| CCC 0.25 | 7.6 ± 0.45e | 1.25 ± 0.02f | 1.05 ± 0.13cd | 0.80 ± 0.015d | 73 ± 0.5 7a | 165.66 ± 3.05de | 44.66 ± 2.51d |
| CCC 0.5 | 7.1 ± 0.20de | 1.19 ± 0.03f | 1.13 ± 0.15fg | 0.84 ± 0.01de | 7.6 ± 0.573 | 169 ± 2.64e | 52 ± 3e |
| CCC 1.0 | 5.7 ± 0.35b | 0.89 ± 0.07cd | 1.25 ± 0.05hij | 0.91 ± 0.02e | 9 ± lab | 183 ± 2f | 59.66 ± 3.05fg |
| CCC 3.0 | 4.7 ± 0.30a | 0.75 ± 0.05ab | 1.35 ± 0.05jk | 1.01 ± 0.075f | 11.66 ± 1.52c | 198 ± 3g | 65.33 ± 2.51h |
| IBA 0.25 ± CCC 0.5 | 7.26 ± 0.208de | 1.23 ± 0.051 | 0.93 ± 0.152b | 0.7 ± 0.1bc | 12 ± 1cd | 201 ± 4g | 57.33 ± 2.08f |
| IBA 0.25 ± CCC3.0 | 7.03 ± 0.02d | l.l6 ± 0.07f | 1.033 ± 0.115cd | 0.76 ± 0.05ab | 14.33 ± 1.52de | 212 ± 5.09h | 59 ± 2fg |
| IBA 0.5 ± CCC 0.5 | 6.43 ± 0.25c | 1.01 ± 0.72e | 0.833 ± 0.05b | 0.63 ± 0.056a | 16 ± 1ef | 243.66 ± 4.25i | 62.67 ± 1.52gh |
| IBA 0.5 ± CCC3.0 | 5.66 ± 0.25b | 0.84 ± 0.03bc | 1.2 ± 0.1gh | 0.83 ± 0.58de | 17 ± 1.02fg | 265.67 ± 6.8j | 65.62 ± 2.51h |
| IBA 1.0 ± CCC0.5 | 6.46 ± 0.2c | 0.97 ± 0.02de | 0.96 ± 0.208bc | 0.7 ± 0.1bc | 18.66 ± 2.08gh | 279.33 ± 1.52k | 71.33 ± 2.5H |
| IBA 1.0 ± CCC 3.0 | 5.6 ± 0.15b | 0.82 ± 0.02bc | 1.1 ± 0.1e | 0.83 ± 0.05de | 21 ± 2i | 288 ± 21 | 79 ± 2j |
| IBA 3.0 ± CCC 0.5 | 5.2 ± 0.16ab | 0.79 ± 0.015ab | 1.3 ± 0.1ij | 1 ± 0.lf | 22.33 ± 1.15j | 304 ± 4.6m | 82.7 ± 2.08k |
| IBA 3.0 ± CCC3.0 | 5.03 ± 0.17a | 0.72 ± 0.03a | 1.5 ± 0.12k | 1.2 ± 0.1g | 25.33 ± 1.52k | 329 ± 2.01n | 89.34 ± 1.53l |
a Values are mean ± standard deviations of three replicates; means followed by the same letter in ii column are not significantly different (P = 0.05).
Interestingly, friable calli were found to develop from the base of microshoots when transferred to MS medium containing CCC and IBA, (Fig. 3). These friable calli further differentiated into microshoots (Fig. 4). Data regarding the number of regenerated microshoots is not included here as the paper mainly concentrated on growth and viability of the CCC primed microshoots. Such regenerated microshoots possess characteristically reduced intermodal lengths (0.72 ± 0.03 cm in 3 mg/l IBA + 3 mg/l CCC) as compared with microshoots in MS with either IBA (1.63 ± 0.08 cm in 3 mg/l IBA) or CCC (0.75 ± 0.05 cm in 3 mg/l CCC) (Figs. 5, 6 and 7). It was also recorded that the regenerated microshoots in MS medium supplemented with both IBA and CCC produced leaves with maximum leaf surface (max. length 1.5 ± 0.12 cm and max. breadth 1.2 ± 0.1 cm in 3 mg/l IBA and 3 mg/l CCC) (Fig. 19). Rooting efficiency of these regenerated microshoots was also found to be higher as revealed by more roots per microshoots (25.33 ± 1.52 in 3 mg/l IBA and 3 mg/l CCC) in comparison to microshoots transferred to MS medium with either IBA (19.33 ± 1.5 in 3 mg/l IBA) or CCC (11.66 ± 1.52 in 3 mg/l CCC) (Table 2). Both fresh weight and chlorophyll retaining capacity (279.33 ± 1.52 mg and 71.33 ± 2.51 days, respectively in 1 mg/l IBA and 0.5 mg/l CCC) of the microshoots regenerated in MS medium containing both CCC and IBA were significantly higher in comparison to MS with either IBA (148.34 ± 2.52 mg and 28.33 ± 1.52days, respectively in 1 mg/l IBA) or CCC (169 ± 2.64 mg and 52 ± 3 days, respectively in 0.5 mg/l CCC) (Figs. 20 and 21). The highest fresh weight (329 ± 2.01 mg) and chlorophyll retaining capacity (89.34 ± 1.53 days) were noticed in MS medium containing 3 mg/l IBA and 3 mg/l CCC than either MS with 3 mg/l IBA (142 ± 1.73 mg and 25.33 ± 1.53days, respectively) or 3 mg/l CCC (198 ± 3 mg and 65.33 ± 2.51 days, respectively) (Table 2). Lastly, application of CCC was also found to increase the survival percentage (92.3 ± 3.05% in MS with 3 mg/l IBA and 3 mg/l CCC, 83 ± 2% in MS 3 mg/l CCC compared to 50.66 ± 3.51% in MS with 3 mg/l IBA) of plantlets after transplanting to soilrite-soil mixture (1:1) for hardening and subsequently to the pot (Table 3). This is clear from the results that MS in combination with CCC and IBA played a positive role to manifest all the beneficial changes, which definitely have serious commercial prospects.
Changes in the leaf size (mean ± SD). Bars with same alphabet superscripts are not significantly different (P = 0.05).
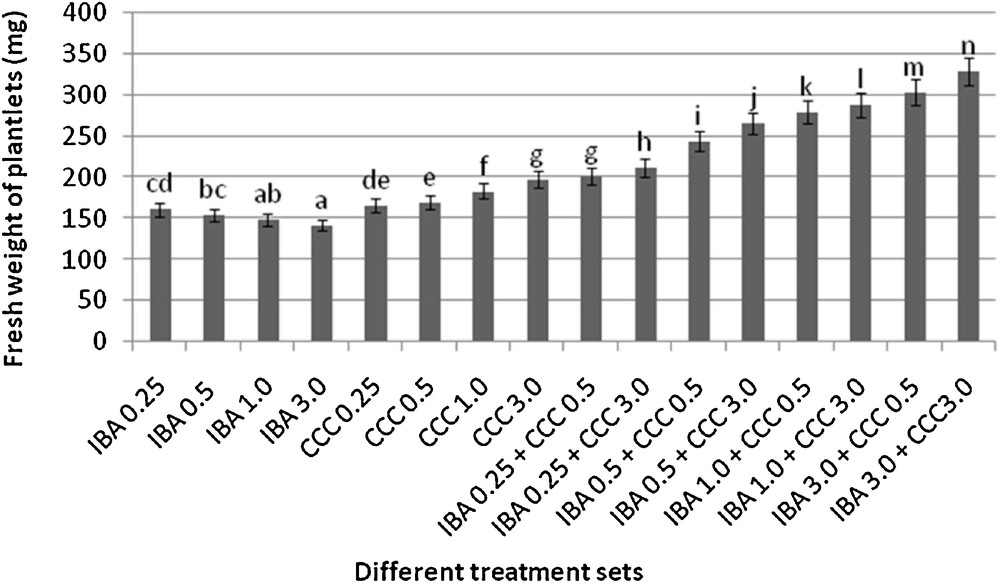
Differences in fresh weight accumulation (after 30 days of respective transfer into the rooting medium, mean ± SD). Bars with same alphabet superscripts are not significantly different (P = 0.05).
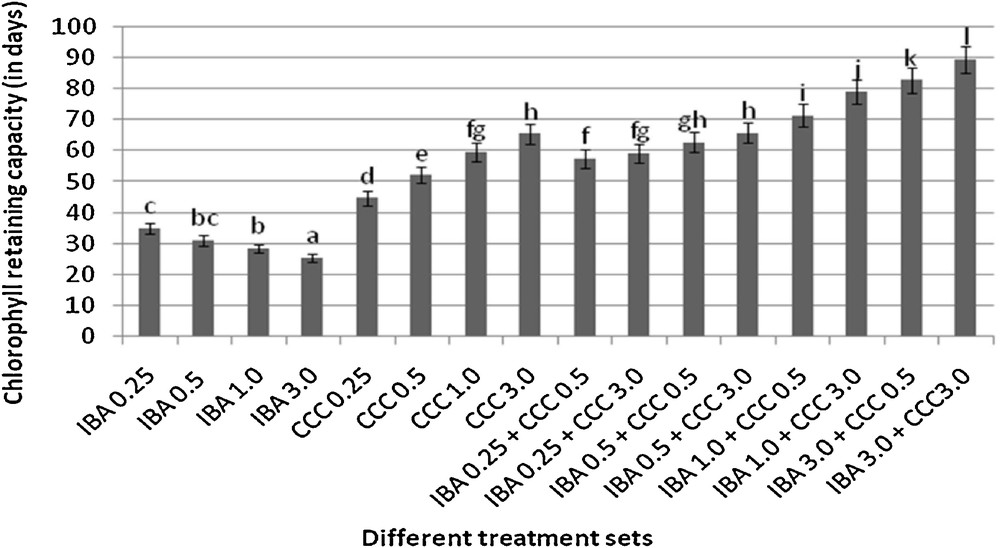
Chlorophyll retaining capacities of different plantlets (mean ± SD). Bars with same alphabet superscripts are not significantly different (P = 0.05).
Survival percentage of different microshoots (mean ± SD).
| Treatment (mg/l) | Survival percentage (%) |
| IBA 0.5 | 49 ± 3.60 |
| IBA 3.0 | 50.66 ± 3.51 |
| CCC 0.5 | 77.33 ± 3.05 |
| CCC 3.0 | 83 ± 2 |
| IBA 3.0 + CCC 0.5 | 88.66 ± 2.08 |
| IBA 3.0 + CCC 3.0 | 92.3 ± 3.05 |
4.2 HPLC analysis
The calibration curve of authentic stevioside in the concentration range of 0.025 to 2 mg/ml was found to be almost linear (r2 = 0.999) (Fig. 22). The identification and quantification of stevioside content in the plant samples were carried out by comparing the retention time (RT) and peak area of sample with that of the standard. The retention times of standard stevioside (0.025 to 2 mg/ml) and S. rebaudiana leaf extracts were found to be similar respectively (Figs. 23 and 24a–24f). Peak area percentages of standard stevioside (0.025 mg/ml, 0.05 mg/ml, 0.2 mg/ml, 0.4 mg/ml and 0.6 mg/ml) were 44.68%, 56.3%, 74.02%, 86.7%, and 95.1%, respectively almost similar to 45.5%, 55.2%, 74.8%, 87.3% and 94.88% of plant extracts (Tables 4 and 5). HPLC data of standard stevioside and plant extracts proved that CCC priming had promoted the stevioside content as compared with only IBA containing sets (Fig. 25). A significantly higher (2.1 mg/g fresh weight) stevioside content was found in microshoots transferred to MS with only CCC in comparison to MS with only IBA (0.2 mg/g fresh weight) (Table 5). Interestingly, the highest stevioside accumulation (2.9 mg/g fresh weight) was recorded in microshoots grown in MS medium with IBA and CCC combinations compared to MS with either CCC or IBA (Table 5). It was revealed that stevioside production increased almost about 15 times in leaves grown in MS medium with 3 mg/l IBA and 3 mg/l CCC (2.9 mg/g fresh weight) in comparison to MS with only 3 mg/l IBA (0.2 mg/g fresh weight). However, an about 1.4 fold increased stevioside content was noticed in MS medium with 3 mg/l IBA and 3 mg/l CCC (2.9 mg/g fresh weight) as against MS with only 3 mg/l CCC (2.1 mg/g fresh weight) (Fig. 25).
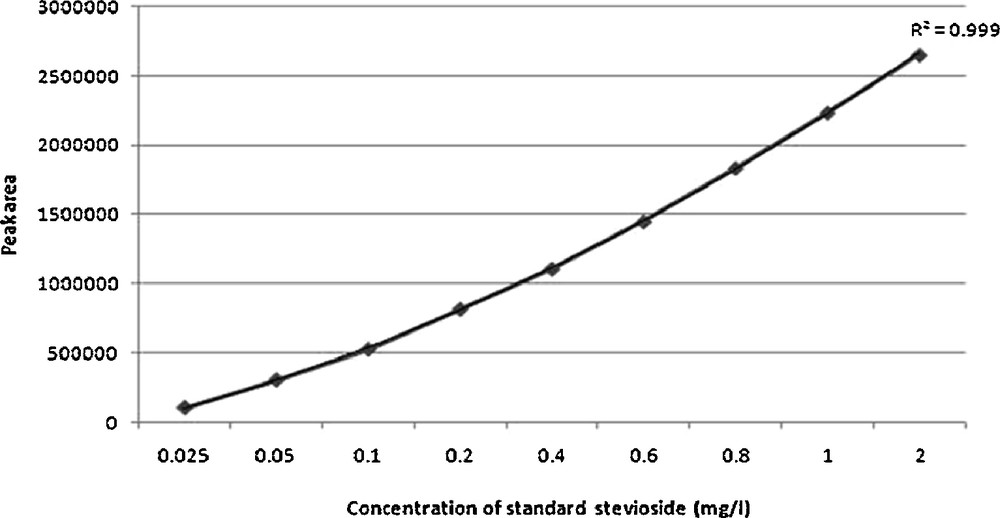
Calibration curve of standard stevioside (0.025 mg/l–2 mg/l).
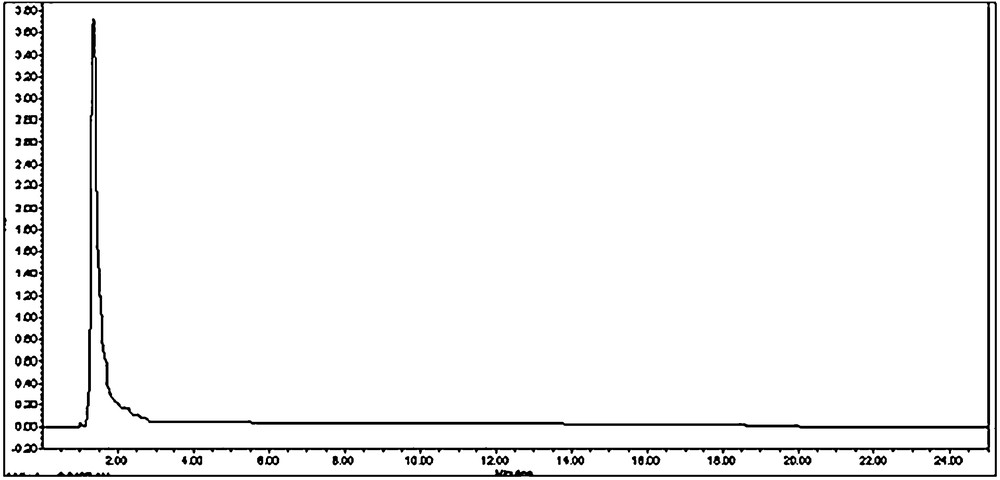
HPLC chromatogram of standard stevioside (2 mg/ml).
a–f: HPLC chromatogram of plant extracts.
HPLC characteristics of standard stevioside.
| Standard stevioside (mg/ml) | Peak area | Peak area % | Retention time (min) |
| 0.025 | 107,067 | 44.68 | 1.32 |
| 0.05 | 307,067 | 56.3 | 1.32 |
| 0.1 | 527,067 | 66.3 | 1.36 |
| 0.2 | 817,067 | 74.02 | 1.34 |
| 0.4 | 1,107,067 | 86.7 | 1.32 |
| 0.6 | 1,447,353 | 95.1 | 1.32 |
| 0.8 | 1,833,386 | 98.3 | 1.32 |
| 1 | 2,233,732 | 100 | 1.36 |
| 2 | 2,650,811 | 100 | 1.34 |
HPLC characteristics of plant extracts and determination of stevioside content.
| Treatment (mg/l) | Peak area % | Peak area | Retention time (min) | Stevioside content (mg/g fresh weight) |
| IBA 0.5 | 45.51 | 117,187 | 1.32 | 0.15 |
| IBA 3.0 | 55.2 | 296,851 | 1.32 | 0.2 |
| CCC 0.5 | 74.8 | 857,183 | 1.36 | 1.05 |
| CCC 3.0 | 87.33 | 1,329,017 | 1.32 | 2.1 |
| IBA 1.0 + CCC 3.0 | 93 | 1,248,313 | 1.4 | 2.5 |
| IBA 3.0 + CCC 3.0 | 94.88 | 1,438,264 | 1.34 | 2.9 |
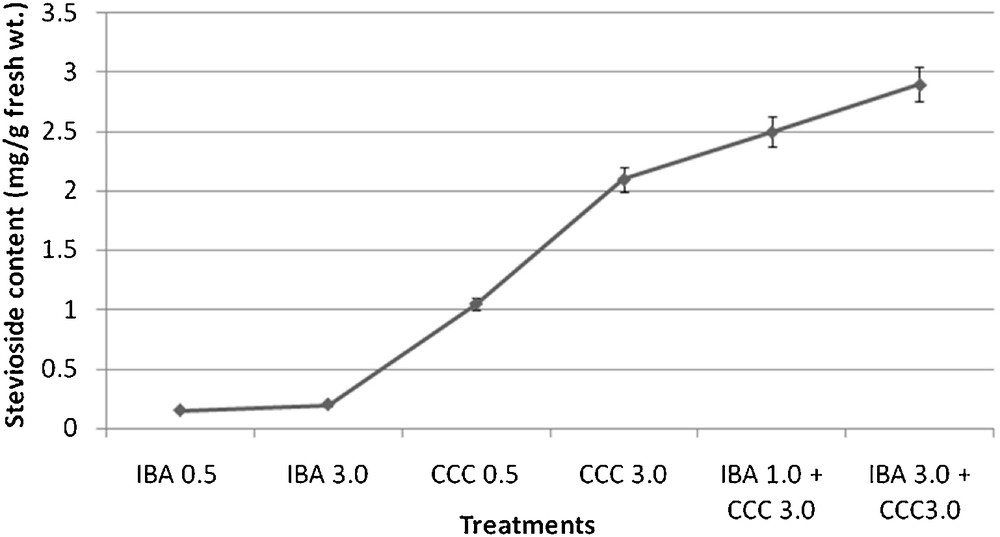
Stevioside accumulation in plant extracts (mean ± SD).
5 Discussion
Present investigation discusses the use and effect of a popular plant growth retardant CCC, on in vitro regenerated microshoots of Stevia rebaudiana. CCC in combinations with IBA found to be most effective for inducing certain beneficial changes like desirable reduction in stem elongation, profuse rooting, bigger leaf size, increased fresh weight of the plantlets, longer chlorophyll retaining capacity and higher stevioside production in comparison to MS with only CCC or IBA (Tables 2 and 5). CCC treatment significantly reduces the internodal length of the microshoots (0.72 ± 0.03 cm in 3 mg/l IBA + 3 mg/l CCC, 0.75 ± 0.05 cm in 3 mg/l CCC) in comparison to MS medium with 3 mg/l IBA (1.63 ± 0.08 cm) due to its anti-gibberellin activity [20]. Remarkable increase in fresh weight of the microshoots (around 2.3 fold) was recorded in MS medium with 3 mg/l IBA and 3 mg/l CCC combination (329 ± 2.01 mg) and 1.4 fold in MS with 3 mg/l CCC (198 ± 3 mg) over the combination of MS with 3 mg/l IBA (142 ± 1.73 mg). Such increment of the fresh weight was due to formation of larger leaves after CCC application (Table 2). Moreover, CCC treatment produced visibly stouter S. rebaudiana plantlets (Fig. 5). These dwarf and robust plantlets are more desirable than slender, delicate plantlets, because the formers are more resistant against various environmental stresses, possess higher chlorophyll retaining capacity and have better viability [21–23].
Applications of different chemicals elicitors for alteration of plant secondary metabolism offer a novel approach to induce some beneficial changes in the production of photochemical, which can be exploited commercially. Effect of plant growth retardants on plant metabolism and physiology is very much important to achieve a specific outcome. Application of CCC in MS medium had a positive impact on the secondary metabolism of plantlets, which was manifested by elevated stevioside production. This result is almost similar with the effect of triazole, another growth retardant, that altered caftaric acid and cichoric acid content in Echniacea purpurea L. grown in vitro [24]. The CCC-induced higher stevioside production as discussed in this paper could be attributed to the direct triggering of secondary metabolism. The observations imply that priming of microshoots with CCC-altered gibberelic acid biosynthesis, increased the number of chloroplasts, promoted the chlorophyll concentration and stimulated secondary metabolism. These changes were associated with shorter shoots, increased leaf sizes, higher chlorophyll retaining capacity, increase in fresh weights and ultimately larger stevioside content. No sign of environmental shock and associated wilting was found on CCC treated S. rebaudiana plantlets during transplantation to soilrite-soil mixture (Figs. 11 and 12). This was clearly reflected by their better survival percentage (92.3 ± 3.05% in MS with 3 mg/l IBA and 3 mg/l CCC, 83 ± 2% in MS 3 mg/l CCC) and vigour after final transplantation to soil in comparison to untreated sets (50.66 ± 3.51% in MS with 3 mg/l IBA) (Table 3). These data vindicate the fact that CCC substantially increases the stress resistance capacity of in vitro grown Stevia rebaudiana, as in the case of potato [25]. However, the negative effects associated with the application of higher concentrations of plant growth retardant require meticulous study. The overall positive impact of CCC application in micropropagation of S. rebaudiana and particularly in stevioside content is a novel approach and has a promising future, especially in the pharmaceutical industry.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgement
Authors gratefully acknowledge their respected supervisors for valuable and constructive suggestions. We are also thankful to the Head, Department of Botany, The University of Burdwan for providing laboratory facilities. Financial assistance from University Grant Commission, New Delhi is duly acknowledged.


