1 Introduction
In colonial birds and mammals, recognition between mates and between parents and their offspring may be an essential condition for reproductive success since it allows them to find each other often among thousands of individuals [1,2]. Previous studies have shown that recognition processes are mostly dependent on acoustic signals [3–5]. Ecological constraints on such meetings are various and dependent on features of the reproductive systems. Among penguins, species without nests (penguins brooding their chick on their feet, King penguin Aptenodytes patagonica and emperor penguin A. forsteri), have a much more sophisticated system of vocal coding than other species of penguins having topographical cues to help to find mate and/or chick(s) (e.g., Gentoo penguin Pygoscelis papua, Adelie penguin P. adeliae, Rockhopper penguin Eudyptes chrysocome) (see [5] for a review). In nesting species, penguins perform individual recognition by analysing the spectral profile and pitch of calls (timbre analysis). In both non-nesting penguin species, individual vocal recognition is supported in the time domain by an amplitude/time analysis for the Emperor Penguin Aptenodytes forsteri, and by a frequency/time analysis for the King Penguin A. patagonicus [5–7]. Both non-nesting species have also a complementary coding system: the two-voice system. Birds have two syrinxes, each serving as a source of sound [8] and producing the two-voice phenomenon observed in flamingos [9,10] and in these two large penguins [11]. It was recently demonstrated experimentally in the field that the beat generated by two voices of non-nesting penguins is an identification code [12] and it was suggested that this special code is an acoustic adaptation in birds lacking topographical cues [5,13]. In support of behavioural studies, previous authors [14] have shown that the Potential of Individuality Coding (PIC), representing the ratio between within-individual variation and between-individual variation in call acoustic parameters, is higher for calls of non-nesting species than for calls of nesting ones.
To test this hypothetical relationship between structure of acoustic signals used for individual recognition, Potential of Individuality Coding (PIC) and nesting ecology, we compare two closely related species with contrasting manner of chick rearing: the nidicolous black-headed gull (Larus ridibundus), where chicks are reared in the nest and the nidifugous slender-billed gull (Larus genei), where chicks leave the nest early. We suppose that species with nidicolous chicks may face to a less difficult problem in parent-chick recognition than nidifugous species with chicks leaving the nest early to form crèches. In the first case, an adult coming back to its mate or progeny may rely first on topographical cues to localise its nest. On the contrary, a ‘non-nesting’ bird during the rearing stage must find chicks among numerous others without any topographical cues. Both species breed in dense colonies formed of thousands of pairs. The number of chicks bred simultaneously by an adult pair is 2–3 in both species. Black-headed gull's chicks stay in the nest until the age of 10 days and remain in the vicinity until independence at 35 days [15]. Whatever the chick's age, feeding always takes place at or near the nest. Slender-billed gull's chicks remain at the nest only for the first few days after hatching; they soon form mobile crèches gathering numerous individuals away from nesting area, near water [15]. In both species, offspring show the ability to recognize the voice of their parents against the background noise of the colony ([16] and Mathevon & Charrier, pers. obs.]. Both these species are morphologically similar. The black-headed gull is 10–15% smaller and more slender than the slender-billed gull (black-headed gull: 34–37 cm; slender-billed gull: 42–44 cm), but both species are quite similar in mass (black-headed gull: 227–350 g; slender-billed gull: 223–350 g) [15]. Both species have approximately the same size of syrinx and vocal tract and likely have the same acoustic abilities. Indeed, acoustic signal for individual identification by both species sounds quite similar to a human hear. The ‘long call’ is a multi-syllabic call emitted by adults when they return to feed chicks at the colony [15]. In this paper, we first compare the acoustic structure of ‘long calls’ of both species using signal analysis methods. We then determine acoustic parameters that likely carry information on individual identity, in order to assess the PIC of each species' ‘long call’.
2 Materials and methods
2.1 Recording procedure
Recordings at colony of 200 slender-billed gulls were made during the breeding season, in July 1999, at Salins-de-Giraud, Camargue, France. Great care was taken during recordings to avoid disturbing the colony. Recordings were made from a blind 2 m from birds with a Revox M 3500 microphone (frequency bandwidth: 150–18 000 Hz, ±1 dB) mounted on a 2–5-m telescopic boom and connected to a Sony TC-D5M audiotape recorder. Young of slender-billed gulls gather in a crèche that could move over several hundreds of meters from one day to the next. Moreover, parents would land at some distance from the crèche (5–15 m), call to their offspring, and then run away pursued by their young.
A week before our arrival to the colony, all the young birds had been banded with a unique combination of coloured and numbered bands. As some adults had been reared in this same colony during previous years, they also wore bands. The identity of an individual during a recording was given either directly by its band or indirectly, by band identity of the fed young. In this latter case, some confusion may occur between paired male and female, as both individuals may feed the same chick. Fortunately, some field plumage characteristics helped us to distinguish between them. Forty-eight calls from eight individuals were recorded.
Recordings of black-headed gulls were made at the largest colony of southern Europe (about 4000 pairs [17]) during the breeding season from May to mid-June 1999, at the ‘Étang de la Ronze’, ‘Plaine du Forez’, near Saint-Etienne, France. To approach birds without frightening them, we used a floating observation blind camouflaged with vegetation. We used the same recording procedures as those used for slender-billed gull; distance between the bird and the microphone was approximately 2 m. Individual identification was easy, since each adult pair was linked to a given nest; identification of male and female was made using size of head and form of cap. We made recordings of 37 long calls from eight individuals.
2.2 Sound analysis
Calls were digitised at a 22 050-Hz sampling frequency using a 16-bit Sound-Blaster acquisition board and examined with SYNTANA analytical package [18].
Different parameters in frequency and time were used to describe the characteristics of calls. One set of parameters described the frequency structure of each syllable in the calls. As bird vocalisations may present either one or two simultaneous fundamental frequencies (i.e., one or two voices), the number of fundamental frequency(ies) (nf) was assessed from spectrograms. Measurements of fundamental frequency(ies) (F1 in the case of only one fundamental frequency, F1 and F2 in the case of two) were done on the FFT spectra (power spectrum density). As a long call syllable may present a slight or sometimes a more pronounced frequency modulation, we calculated averaged spectrum given by FFT using a window enclosing most of the syllable duration. Frequency bandwidth (fb) was obtained by calculating the difference in Hz between the highest pitched harmonic measured on the FFT spectrum and the lowest pitched fundamental. Each fundamental frequency (F0) was associated with its corresponding harmonics, which were identified as H1(the lowest pitched harmonic) to Hn (the highest pitched one) for the 1 voice case, and H11 to Hn1 and H12 to Hn2 for the two-voice case. Indeed, in the last case, the spectrum could be divided into pairs of harmonics named H1=(H11,H12) to Hn=(Hn1,Hn2). To create a semi-quantitative picture of the energy distribution among the frequency spectrum, for each call we classified the five most emerging Hi harmonics (or Hi pairs of harmonics) as follows: E1, the most intense Hi harmonic; E2, the second most intense harmonic, and so on. For this analysis, we did not take into account the absolute value of the Hi harmonics, just their relative rank in the E1–E5 series. For each call, we obtained a set {E1;…;E5}, where E1 was the most intense among F0, H1, H2, H3, H4 and H5 (for example, if H2 was the harmonic – or pair of harmonics – with the greatest amount of energy, then E1=H2); E2 was the second most intense, and so on. This gave us a picture of the energy repartition among the frequency spectrum of each analysed call. For each bird species, we then pooled the results by taking into account all the analysed calls, obtaining then a semi-quantitative picture of the averaged distribution of energy among the frequency spectrum.
A second set of parameters was used to describe the temporal structure of calls. Number of syllables per call (nsyl), total duration of call (tdur), duration of each syllable (dsyll), duration of silent interval between syllables (dsile) were measured directly from oscillograms. Mean rhythm of amplitude modulation (rAM) of each syllable was assessed after calculation of the call envelope by means of the analytic signal concept [19]. The temporal evolution of the frequency was characterized by calculating the cepstrum of sounds to follow the frequency modulation of the fundamental [19]. The cepstrum is defined as the power spectrum of the logarithm of the power spectrum. The cepstrum has a strong peak corresponding to the fundamental frequency of the segment being analysed [20,21]. The maximum and the minimum values reached by the fundamental were then measured (in the case of two fundamentals, the cepstrum calculation isolates the most powerful one). We calculated the difference between the maximum and the minimum values reached by the fundamental (dFund) within the considered syllable.
2.3 Statistical analysis and data interpretation
Statistical analysis was made with StatGraphics Plus software. The comparison between long calls of both species was done in two steps. First, we focused on the acoustical structure of the calls by comparing each measured parameter between slender-billed gull and black-headed gull calls. Second, we assessed the potentiality of individual identity coding by each species' long calls. Kruskal–Wallis test allowed determination of which parameters may bring individual identity information. These last parameters were the only ones used for subsequent analysis process. We measured within-individual and between-individual variations of each variable by calculating within-individual (CVi) and between-individual (CVb) coefficients of variation according to the formula: CV=100×(1+1/4n)×SD/X [22]. Within each species and for each variable, we calculated mean CVi by averaging CVi of the eight individuals. The CVb/CVi ratio indicates how great the between-individual variation was relative to the within-individual variation. The CVb/CVi ratio has been called the Potential of Individuality Coding (PIC) [23,24]. Total PIC in the temporal domain was calculated by adding PICs of each temporal feature; adding PICs of frequency parameters gives a global PIC in the frequency domain.
3 Results
3.1 Acoustic structure of the long calls
Both species share the same basic structure for their long call, i.e., a repetition of stereotyped complex sound syllables separated by intervals of silence (Fig. 1). Number of syllables per call differs significantly between both species (nsyl in Table 1). Most slender-billed gull calls contains more than seven repeated syllables while most of black-headed gull calls comprise four to six syllables. Total duration (correlated with number of syllables) of the slender-billed gull call was longer than the black-headed gull one (tdur in Table 1). Mean duration of black-headed gull syllables was not different than that of slender-billed gull; duration of the black-headed gull between-syllable silences was slightly less but the difference remains non significant (respectively dsyll and dsile in Table 1). Calls of both species were modulated in amplitude, with black-headed gull call presenting the slower rhythm for amplitude modulation (rAM in Table 1). In the slender-billed gull call, the sound constituting a syllable presents two fundamental voices and their respective harmonic series; within a syllable, the two voices follow similar frequency and amplitude modulations (Fig. 1b). The black-headed gull syllable presents only one fundamental frequency with its associated harmonic series (Fig. 1a). Frequency bandwidth of slender-billed gull sound was narrower than that of black-headed gull call (fb in Table 1). The mean numeric values of fundamentals do not differ significantly between both species (F1, F2 in Table 1). The repartition of energy within harmonics differs greatly between the two species (Table 2). In the slender-billed gull's call, most energy (E1 and E2) was concentrated in the two first harmonics of each harmonic series: H1 (i.e. H11 and H12) and H2 (i.e., H21 and H22) were always the most powerful frequencies of the spectrum. The two fundamentals are less powerful, but still represent an important proportion of spectrum energy; the upper harmonics (H3 to Hn) were very weak. In the black-headed gull's call, the spectrum was wider and energy was spread over a greater number of harmonics. The most powerful harmonics may be either H1, H2, or H3 for E1, or H3, H4, or H5 for E2. The fundamental brings a very small part of the spectrum energy. Within a syllable, variation of the fundamental frequency was more pronounced in the black-headed gull's call, but the difference between the two species was not significant (dFund in Table 1).
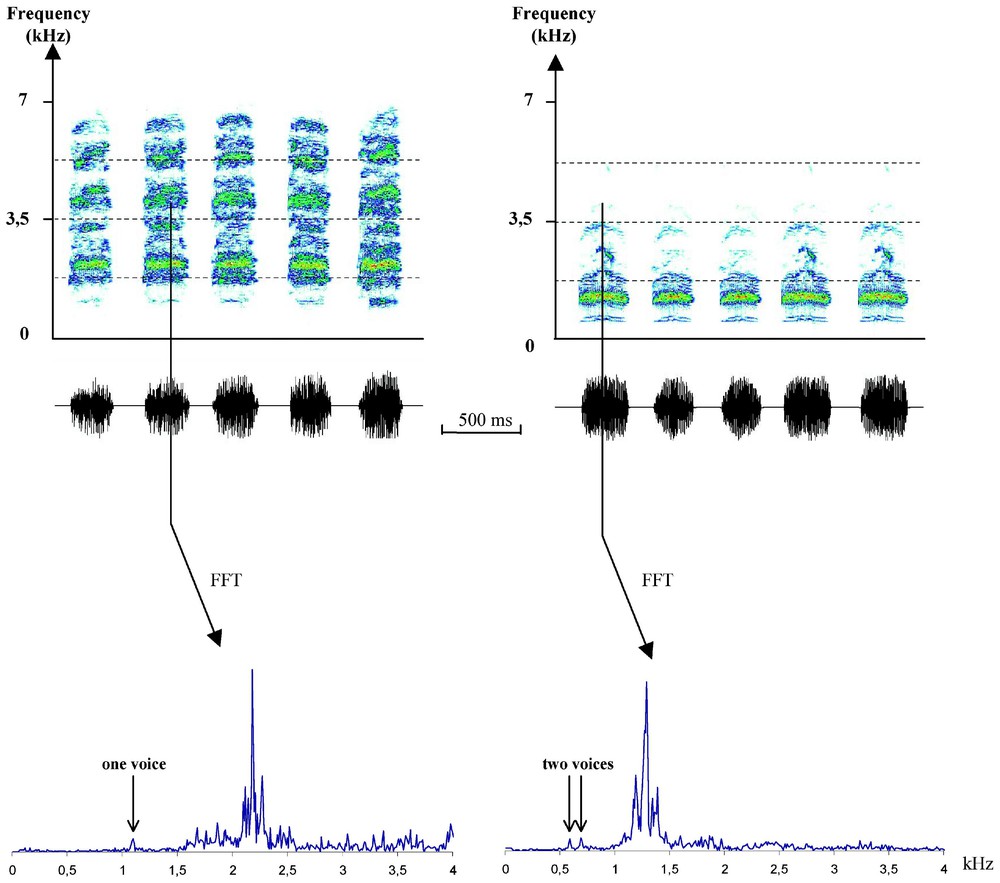
Sonagraphic (above) and spectral (below) representations of long calls: left, black-headed gull, right: slender-billed gull (sonagraph: window size=1024; FFT: window size=2048).
Comparison of the acoustic parameters of both gulls species with Kolmogorov–Smirnov two-sample test
| Variable | Black-headed gull | Slender-billed gull | |
| (n=8) | (n=8) | ||
| nsyl | 5.5±1.7 | 7.5±2.1 | ∗ |
| tdur (s) | 1.68±0.31 | 2.39±0.76 | ∗ |
| dsyll (ms) | 247±41 | 225±55 | ns |
| dsile (ms) | 168±64 | 136±18 | ns |
| rAM (Hz) | 72.6±21.9 | 89±8.0 | ∗ |
| nf | 1 | 2 | ∗ |
| fb (Hz) | 7764±162 | 4026±877 | ∗ |
| F1 (Hz) | 562±122 | 592±73 | ns |
| F2 (Hz) | – | 660±79 | |
| dFund (Hz) | 155.7±91.7 | 111±34 | ns |
∗ P<0.05.
Energy distribution among the frequency spectrum in % (see text for explanations)
| F0 | H1 | H2 | H3 | H4 | H5 | |
| Black-headed gull | ||||||
| E1 | 0 | 33 | 29.2 | 29.2 | 4 | 4 |
| E2 | 0 | 10 | 2.1 | 29.2 | 27.1 | 31.3 |
| E3 | 2.1 | 8.3 | 27.1 | 25 | 31.3 | 6.3 |
| E4 | 4.2 | 4.2 | 41.7 | 16.7 | 31.3 | 2.1 |
| E5 | 50 | 43.4 | 0 | 0 | 6.3 | 0 |
| Slender-billed gull | ||||||
| E1 | 0 | 46.7 | 53.3 | 0 | 0 | 0 |
| E2 | 0 | 66.7 | 33.3 | 0 | 0 | 0 |
| E3 | 40 | 33.3 | 26.7 | 0 | 0 | 0 |
| E4 | 13.3 | 53.3 | 26.7 | 0 | 0 | 0 |
| E5 | 50 | 43.4 | 0 | 0 | 0 | 0 |
3.2 Potentiality of individuality coding
In both species, results of Kruskal–Wallis indicate that the most individualized parameters were the values of fundamental frequencies (F1, F2 in Table 3). Features that seem not to be linked to individual identity are number of syllables per call, duration of the call, frequency bandwidth, and for the slender-billed gull, silence duration (Table 3). As the number of fundamental voices was constant within species (one for the black-headed gull and two for the slender-billed gull), no analysis was needed: of course, this parameter was species-specific and cannot bring any information about individual identity.
Kruskall–Wallis two-sample test on the acoustic parameters of the long calls within each gull species
| Black-headed gull | Slender-billed gull | |
| Variable | H | H |
| nsyl | 1.02 | 1.2 |
| tdur | 1.11 | 1.03 |
| dsyll | 35.5∗ | 37.14∗ |
| dsile | 33.1∗ | 1.01 |
| rAM | 29.2∗ | 14.3∗ |
| fb | 1.01 | 1.02 |
| F1 | 41.0∗ | 43.3∗ |
| F2 | – | 38.6∗ |
| E1 | 35.1∗ | 37.97∗ |
| E2 | 31.1∗ | 36.3∗ |
| E3 | 17.2∗ | 21.3∗ |
| E4 | 15.7∗ | 11.02∗ |
| E5 | 14.5∗ | 18.2∗ |
| dFund | 30.75∗ | 35.58∗ |
∗ P<0.05.
The fundamental voice of the black-headed gull call showed greatest Potential of Individuality Coding (PICF1=4.7 in Table 4) and similar to the PIC for first fundamental voice of the slender-billed gull (PICF1=4.9). The fundamental of the second voice of slender-billed gull also had a high PIC value (PICF2=3.7). The PICs of the harmonics of the slender-billed gull call were higher than those of the black-headed gull vocalization (Table 4), which may indicate that the distribution of energy among harmonics were more constant within calls of slender-billed gulls than within calls of black-headed gulls. In both species, PIC values decreased from E1 to E5, with only two exceptions: for the slender-billed gull, the PIC of E5 was a bit higher than the PIC of E4, and for the black-headed gull, the PIC of E4 was slightly higher than the PICs of E3 and E5. In both species, PIC of frequency modulation was quite high while the PIC of amplitude modulation remains low (respectively dFund and rAM in Table 4). In both species, PIC of duration of syllables, as well as of duration of silences for black-headed gull, present a relatively large value (respectively dsyll and dsile in Table 4). Summation of time related PICs was similar in both species; summation of frequency related PICs was greater for slender-billed gull calls (Table 4).
Assessment of the potential of individuality coding of the acoustic parameters of both species long calls
| Variable | Black-headed gull | Slender-billed gull | ||||
| Mean CVi | CVb | PIC | Mean CVi | CVb | PIC | |
| Temporal parameters | ||||||
| dsyll | 7.3 | 16.8 | 2.3 | 7.9 | 26.1 | 3.3 |
| dsile | 22.2 | 49 | 2.2 | 13.0 | 13.0 | 1.0 |
| rAM | 9.6 | 17.3 | 1.8 | 5.8 | 7.0 | 1.2 |
| dFund | 27.9 | 72.5 | 2.6 | 11.9 | 38.1 | 3.2 |
| Frequency parameters | ||||||
| F1 | 3.6 | 16.9 | 4.7 | 2.3 | 11.3 | 4.9 |
| F2 | – | – | – | 2.4 | 8.9 | 3.7 |
| E1 | 17.7 | 42.5 | 2.4 | 3.9 | 12.1 | 3.1 |
| E2 | 18.5 | 37 | 2.0 | 6.3 | 18.9 | 3.0 |
| E3 | 28.2 | 33.8 | 1.2 | 20.5 | 47.2 | 2.3 |
| E4 | 29.4 | 38.2 | 1.3 | 34.3 | 41.2 | 1.2 |
| E5 | 14.6 | 16.06 | 1.1 | 29.8 | 56.6 | 1.9 |
| Summation of PICs in the temporal domain | 8.9 | 8.7 | ||||
| Summation of PICs in the frequency domain | 12.7 | 20.1 |
4 Discussion
This paper describes the acoustic structure of long calls of two species of gulls, focusing on the capability to encode information about individual identity. Long calls of both species consist of repeated syllables separated by silences. The slender-billed gull's call has more syllables than that of the black-headed gull. The basic structure of syllables is a broadband complex sound. The fact that both species are very close phylogenetically and morphologically could explain why the values of the fundamentals calls of both are similar. However, the acoustic structure of the slender-billed gull call looks more complex due to the presence of two voices. In both species, information about individual identity appears to be encoded in the acoustic structure of each syllable of a given call rather than in repetition of the syllables or the duration of the call. Syllabic repetition constitutes a redundancy of information, which is more pronounced in the slender-billed gull's long calls.
Maybe the more striking result of this study is the association between number of voices and degree of difficulty to find chicks: two voices in calls of the nidifugous slender-billed gull, that forms crèches, and one voice in the nidicolous black-headed gull's call, which does not form crèches. The fine acoustic structure of syllables differs between both species because of the presence of two fundamentals in the former species versus only one in the later one. For individual recognition, a two-voice signal may constitute an advantage, since receiving birds can rely upon two harmonics series instead of one to identify an individual. Moreover, the potential number of different, individualized, signals, given by the combination of two fundamental frequencies is highly superior than the potential number offered by use of a single fundamental voice. The PICs of harmonics are consequently more important for the slender-billed gull call. Distribution of energy is stable within individuals in this species (among calls of a given individual, the most powerful harmonic is always the same, i.e. E1 keeps the same frequency value, and this is also true for E2, E3, and, to a lesser extent for E4 and E5). In the black-headed gull's call, distribution of energy among the spectrum is more variable.
These results suggest that the slender-billed gull's long call appears more fitted to encode and transmit information about individual identity than the black-headed gull's call. Nevertheless black-headed gull chicks face only small difficulties to recognize its parents, since the nest is used as a meeting place. Adult birds emit their long calls while approaching the nest. When a chick hears one of its parents, it immediately checks for the presence of a flying adult by looking upward. If it does not see a bird approaching the nest, it immediately stops looking for its parent. In contrast, the slender-billed gull chicks are in a very constraining situation. A parent approaching the colony lands at some distance from the crèche and emits some long calls. Its young has first to recognize the parent and then runs to it, calling. Young chicks cannot first make visual checks since, at any time, numerous adults are landing near the crèche, calling to their own young. Acoustic recognition has to be reliable: a chick that tries to get food from an adult that is not one of its parents will always be rejected and sometimes hurt [15]. This accuracy in the recognition process is certainly allowed by the special acoustic characteristics of the slender-billed gull's long call, i.e. two-voices, stability of the energy distribution among harmonics and important redundancy of syllables. Differences between both types of long call may then rely upon the differential constraints imposed by the nesting habits during the rearing stage.
As assumed previously, presence of two voices in some gulls and penguins seems to be related to a particular breeding system, characterised by the absence of a fixed nest and the occurrence of crèches. The present study reinforces the hypothesis that calls used for individual recognition have similar evolutionary constraints in colonial birds. Use of two voices, in both gulls and penguins, constitutes a striking example of an adaptive convergence of an acoustic signal under similar nesting ecologies.
Acknowledgements
Many thanks to the La Tour du Vallat Foundation, which welcomed us to make the recordings of the slender-billed gulls in Camargue. We are especially grateful to Nicolas Sadoul for his precious and kind advice, as to Jean-Dominique Lebreton for welcoming us to work at the La Ronze black-headed gull colony. We appreciate the improvements in English usage made by Peter Lowther through the Association of Field Ornithologists' program of editorial assistance. We are grateful to the referees for their kind advices and remarks.



