1 Introduction
During vertebrate embryogenesis, the epidermis derives from the ectoderm while the underlying tissue, the dermis, derives from the mesoderm. Both developing tissues closely interact at the molecular level: the instructive effects of mesenchymal factors on epidermal commitment correlates well with the induction role of mesodermal cells on ectodermal fate during embryonic skin development and in the adult [1].
Despite accumulating data on in vivo skin lineage differentiation, mainly through transgenic mice technology, the absence of an appropriate in vitro model system has hampered the study of early events responsible for epidermal and dermal commitment. Embryonic stem (ES) cells are derived from the pluripotent cells of the early mouse embryo. They can be expanded infinitely in vitro while maintaining their potential to differentiate spontaneously into any cell type of the three germ layers, including epidermal cells. ES cells are thus an effective tool to recapitulate in vitro the main steps of embryonic normal and pathological development. Accordingly, we recently found a synergic effect of mesenchymal acellular extracellular matrix and BMP-4 to induce efficiently mouse ES cells to differentiate into keratinocytes [2,3]. It thus demonstrates the ability of ES cells to recapitulate in vitro the reciprocal induction of ectodermal and mesodermal cells to produce both keratinocytes and fibroblasts [2,3]. We further show for the first time that a pluristratified epidermis could be generated in vitro from the committed ES cells, leading to great perspectives for the cellular and tissular therapeutic potential represented by ES cells (Fig. 1).
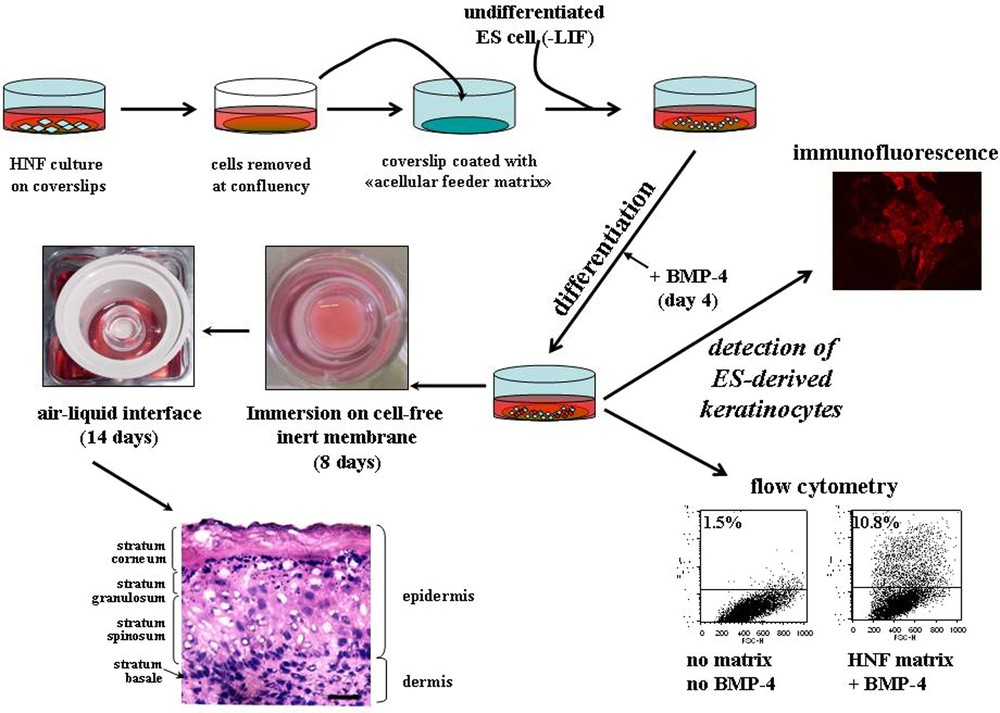
In vitro differentiation of ES cells as described in [3]. To prepare mesenchymal acellular extracellular matrix (ECM), human normal fibroblasts grown on glass coverslips are detached by EDTA/EGTA treatment at confluence. Undifferentiated ES cells are seeded on the ECM and induced to differentiate by removal of LIF from the medium, exposed at day 4 to 0.5 nM of BMP-4 for 3–10 additional days. Presence of ES-derived keratinocytes is scored using an antibody against the cytokeratin-14 intermediate filament. Then, the induced ES cells, harvested and inoculated onto cellulose ester membranes for an additional week, are mounted on stainless-steel grids to allow the surface of the cultures to be exposed to air. The cultures are incubated for an additional 14 days. Histological staining shows the different layers of epidermis, with a typical cuboidal cell-shape stratum basal, stratified suprabasal layers including a stratum spinosum, a stratum granulosum, and a stratum corneum at the top. An underlying dermal compartment is clearly identified below the basal layer. Bar: 11 μm.
2 Neural commitment and BMP-4
Epidermal and neuronal precursors are supposed to derive from a common neuroectodermal precursor [4]. At the early gastrulation stage, the ventral ectodermal area differentiates into epidermis, while the dorsal zone eventually neuralizes. This separation is due to a gradient of both the ventral morphogen BMP-4 (TGF-β superfamily) and its dorsalizing antagonist factors (i.e. Noggin, Chordin, Follistatin). In Xenopus embryos, the embryonic epidermal commitment is induced by BMP4 in the ventral part of the egg, while its absence within the dorsal part leads to a ‘default neural’ differentiation process [5]. In Mammals, BMP-4 plays a similar critical role in epidermal commitment and displays an inhibitory effect on neural induction [6]. We recently clarified the role of BMP-4 in neural inhibition during embryonic epidermal commitment by showing that the Sox-1+ population derived from neural commitment of ES cells is the direct target of a BMP-4-induced apoptosis [7] (Fig. 2). Sox-1 is the most early marker specific of neural precursor (NP) populations along the developmental pathway [8–10]. These cells could be of particular interest to study neural cells in vitro and as an unlimited source for cellular therapy of neurodefective diseases. Since purification of such progenitor cells from adult or foetal tissues is hardly available, ES cells may represent an alternative source of NP cells for clinical use. Sox-1+ cells isolated from neural differentiated ES cells display neuroepithelial morphology [11], are able to differentiate into functional neurons in vitro [12] and into other neural cell types [13]. Therefore, Sox-1 has been considered as the most pertinent marker to characterize/identify the earliest multipotent neural precursor cells. Interestingly, ES-derived Sox-1− cells are also able to generate dopamine neurons [13], suggesting that this population may also contain neural precursors. Indeed, even if among the Sox-1− population, many cells express markers of undifferentiated cells (Oct 4, Nanog), as demonstrated by the production of teratoma after in vivo grafting, the resulting tumours are much smaller than those obtained from undifferentiated ES cells.
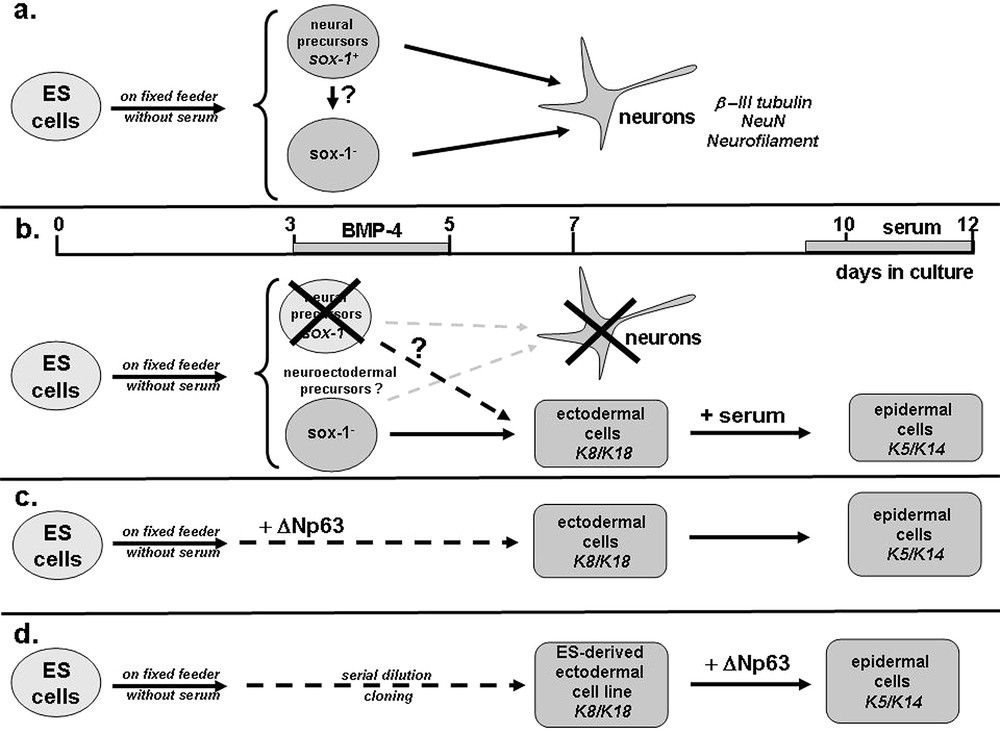
Schematic representation of ectodermal commitment and epidermal differentiation of ES cells. (a) ES cells cultured on fixed NIH-3T3 cells under serum-free conditions efficiently differentiate into neural precursors (day 3) and neurons (day 7) positive for Sox-1 and β-III tubulin/NeuN/Neurofilament, respectively. (b) When BMP-4 is added to the medium from day 3 to 5, neural commitment is totally prevented, while ectodermal cells (K8/K18+) are produced in large numbers, some of them become keratinocytes (K5/K14+) after addition of serum. This suggests that Sox-1− cells (and possibly a few remaining Sox-1+ cells, see broken arrow and question mark) are neuroectodermal precursors, which are able to differentiate into neuronal or epidermal cells in the absence or presence of BMP-4, respectively. (c) Exogenous expression of ΔNp63 is sufficient to mediate efficiently both ectodermal and epidermal commitments of ES cells. (d) ES-derived ectodermal cell lines (K8/K18+) were isolated by serial dilution cloning from committed ES cells. Exogenous expression of ΔNp63 is sufficient to mediate efficiently the differentiation of ectodermal precursors into keratinocytes.
According to its function during embryogenesis, we further demonstrate that BMP-4 is a potent epidermal inducer of ES cells [2,7]. While inducing apoptosis of Sox-1-positive neural precursors, BMP-4 promotes epidermal commitment of surviving cells, as evaluated by a large increase in K8/K18+ ectodermal cells. Addition of serum to the culture, five days after BMP-4 treatment, leads to the production of K5/K14+ epidermal cells [2,7]. Altogether, these results suggest the exciting existence of bipotent ES-derived neuroectodermal precursors able to become either neurons or epidermal cells depending on the presence or the absence of BMP-4 activity, respectively (Fig. 2).
3 Epidermal differentiation and role of p63
p63, a member of p53 family, plays a pivotal role in epidermal development [14,15]. The p63 gene encodes for six distinct isoforms, due to two alternative promoters (TA and ΔN) and alternative splicing. Mutations on the p63 gene are responsible for severe human genodermatoses with abnormalities in ectodermal structures (including skin) and limb development [16]. Accordingly, p63-deficient mice display absent limbs along with a highly disorganized skin and other stratified squamous epithelia that lead to newborn death because of dehydration [14,15]. While the epidermal phenotype of the two p63-deficient mice was identical, divergent observations of the respective animals led each group to suggest a distinct function for p63. During vertebrate embryogenesis, the developing epidermis derives from the ectoderm, which gives rise to the single-layer ectodermal cells expressing the cytokeratin K8 and K18 until day 8.5. The day after, the ectodermal cells are committed to stratification, event marked by the onset expression of cytokeratin K5 and K14. In D. Roop's KO mice, the p63-deficient epidermal cells still expressed K8/K18 instead of K5/K14, strongly suggesting impairment on epidermal commitment and stratification [14]. In the second study, the group of F. McKeon observed sparse patches of pluristratified epidermis positive for late differentiation markers like involucrin and filaggrin. This observation strongly suggests that, in the absence of p63, stratification and epidermal differentiation can proceed for limited rounds because of a defect in the regeneration of the epidermis [15,17]. The authors thus hypothesize that p63 must be necessary for the stem cell maintenance and that its absence impoverishes the epidermal stem cell pool. The two hypotheses, as well as the nature of the p63 isoforms involved in these critical embryonic steps, remain however highly controversial.
During ES differentiation, we show that the ectodermal transition induced by BMP-4 occurs through the activation of ΔNp63, whereas TAp63 is never activated [2]. This is in accordance with in situ hybridization studies made on mouse embryonic skin, which demonstrate that ΔNp63, but not TAp63, is expressed in ectodermal and epidermal cells [18]. BMP-7 and FGFR2b have been recently identified as target genes of ΔNp63 during mouse embryogenesis by the same group [18] and, accordingly, we observe their activation in differentiated ES cells following BMP-4 treatment. Interestingly, the ΔNp63 gene activation occurs after ectodermal commitment of ES cells and only a fraction of the K8/K18 positive ectodermal cells produces endogenous ΔNp63. In the course of ES-induced epidermal differentiation and after serum addition, part of K8/K18 ectodermal cells eventually becomes keratinocytes (K5/K14), all positive for ΔNp63 (Fig. 2). To better define the precise role of p63 in commitment and differentiation, we isolated by serial dilution cloning ES-derived ectodermal cell lines (K8/18+). Transcriptome analysis reveals that these ES-derived cell lines are potential precursors of epidermal cells and not simple epithelia cells, which are also positive for K8/K18 in adult. While these cell lines remain phenotypically stable, few of them become spontaneous epidermal cells (K5/14+) at every passage. Furthermore, exogenous expression of ΔNp63 into these ectodermal cells efficiently differentiates them into keratinocytes (Fig. 2). Moreover, the forced expression of ΔNp63 efficiently enhances ES-derived ectodermal cell proliferation and cell survival. These data clearly demonstrate that ΔNp63 is not required for ectodermal fate, but necessary for either epidermal cell differentiation or proliferation [2]. This observation seems in contradiction with D. Roop's group results; these authors reported that TAp63 is able to force K8/K18 simple epithelia cell lines to express de novo the keratinocyte-specific K14 cytokeratin. They concluded that TAp63 must be required for the epidermal commitment of ectodermal cells [19]. We observed that the exogenous expression of TAp63 is also able to force epidermal commitment of ES-derived ectodermal cells, but to a much lesser extent than ΔNp63. Since TAp63 is not expressed during the early steps of epidermal formation in vitro (unpublished data) and in vivo [18], this feature, which would be in accordance with Roop's data, might be not physiological. Recent studies on transgenic mice expressing either TA or ΔNp63 within a p63–/– background further confirm that ΔNp63, but not TAp63, partly restores a stratified embryonic epidermis [20] and is able to activate directly the K14 promoter. Moreover, in vivo inhibition of ΔNp63 in zebrafish embryos disturbs skin formation and AER maintenance [21,22]; on the contrary, its overexpression is sufficient to block anterior neural specification while promoting early steps of epidermal specification, even in zebrafish embryos lacking BMP-4 signalling [21,22]. These two studies suggest that ΔNp63 plays a dual role in the early steps of zebrafish epidermis formation: it acts as both an epidermal inducer and an inhibitor of neuroectodermal formation [21,22], and later on, it is required for epidermal cell proliferation (Fig. 2). In our ES cellular model, ΔNp63 activation was not responsible for the BMP-4-induced apoptosis of the Sox-1+ neural precursor cells. It will be thus interesting to define whether ΔNp63 may have an inhibitory effect on more committed ES-derived neural cells. However, since no neural defect has been apparently described in p63-deficient mice, it might reveal differences in ΔNp63 role in zebrafish and mammals.
Many experiments remain to define the functions of each p63 isoform in embryonic skin formation. It remains to clarify whether ΔNp63 is directly involved in epidermal commitment or in the proliferation and cell survival of committed epidermal cells. We believe that ES cells, along with functional genomic technology including siRNA, will be a powerful tool to identify the function of each wild type and human ectodermal dysplasia-derived mutated p63 isoforms in both commitment and proliferation/self-renewal of epidermal stem cells.
The epidermis is in constant renewal due to the presence of epidermal (keratinocyte) stem cells that reconstitute not only the epidermis, but also the cutaneous appendages (hair follicles, sebaceous and sweat glands) [23]. The skin is the target of many severe hereditary and acquired pathologies, including genodermatosis, deep burns, skin carcinoma, alopecia, and acne. Despite intense efforts, we still know very little about epidermal stem cell fates and multipotency. The characterization of epidermal stem cell markers and the identification of molecules that allow their maintenance and guide their differentiation into one lineage (epidermis, skin appendages) are of critical importance for the implementation and improvement of novel strategies for cutaneous therapies. The ability to maintain epidermal stem cells in vitro has allowed engraftment of cultured skin onto burned patients. This technology have saved hundreds of severely burned patients around the world, but is very expensive, time consuming, and skin appendages are not formed, while their presence is needed to improve the functionality of the reconstituted skin in terms of plasticity, thermoregulation, and long-term graft survival. Therefore, the need for alternate sources of epidermal stem cells must be addressed. In this perspective, the development of human ES cell-derived epidermal tissues could be a major breakthrough for clinical application. Furthermore, in cosmetology, in vitro production of human follicular keratinocytes and sebocytes, derived from human ES cells, would provide unique available models for the treatment of acne vulgaris and alopecia. These cellular models would provide alternatives to animal testing of drugs and toxins in the pharmaceutical and cosmetological industries.
Acknowledgements
The work described here has been partially supported by the Sixth EEC Framework Program under the EPISTEM project, INSERM and the ‘Institut national contre le cancer’ (INCa), France.


