1 Introduction
In social insects nest site selection is intimately related to fitness due to its obvious impact on colony survival and offspring production. Selection of adequate sites is particularly critical in swarming species where the fate of the entire swarm is at stake, as the swarm is constituted by a part of the colony or even the entire colony. The choice of a new nest site, made by scouts, corresponds to a combination of data gathering and assessment of adequate sites, followed by self-organizing patterns arising from simple positive feedback and attrition interactions among the scouts [1].
In Neotropical social wasps, nest site choice, as well as nest architecture, has evolved under the selection pressure of weather and strong predation by vertebrates and ants. Consequently, the choice of habitat characteristics permitting protection from adverse weather and concealment from predators has presumably been paramount, so that selected nest sites are thought to be related to colony success [2–4]. In the paper wasp tribe Epiponini numerous species find protection from inclement weather by building their nests under shelter such as entrances of caves, overhanging rocks, human constructions and, especially, under the large leaves of certain plants [3–5]. In French Guiana, this is particularly the case for Clusia grandiflora (Clusiaceae) treelets that very frequently shelter wasp nests under their large and rigid leaves [4].
In Neotropical swarm-founding social wasps one can distinguish reproductive swarming accompanied by colony fission from absconding swarming with no fission in the adult population. For example, when a colony loses its nest due to predation or accident the adult wasps escape and emigrate as an absconding swarm, then renest elsewhere. This is accompanied by losses of adults during the emigration itself and by a lack of replacement of individuals during the subsequent preemergence growth period in the new nest [2,6]. Furthermore, it is known that in reaction to climatic variation some swarm-founding social wasp populations vary from the dry to the rainy season [7,8], colonies being obliged to abscond due to heavy rainfall [9,10]. In such a context, shelters offered by large leaves can be favorable as wasp nesting sites depending on characteristics of the plant species such as the large size and the rigidity of the leaves [3,11].
In this study we hypothesized that a behavioral adaptation can permit colonies of the most abundant social wasp species in French Guiana, Polybia bistriata (F.) (Vespidae: Polistinae: Epiponini), to overcome significant climatic changes. We therefore followed the forecast in order to conduct experiments during highly disparate periods and chose the 2005 dry season (July–November) and the rainy season that followed. We verified how P. bistriata colonies reacted to climatic variations by studying colonies situated under leaves of C. grandiflora.
2 Materials and methods
2.1 Site and the climate of French Guiana
This study was conducted in 2005 and 2006, in Sinnamary, French Guiana, near the Laboratoire Environnement at Petit Saut (5° 03’ 39” N; 53° 02’ 36” W).
Situated between 2°N and 6°N, French Guiana has an equatorial climate with regular winds and temperatures over the year. The amount of rainfall is related to the movements of the Atlantic intertropical convergence zone (ITCZ), which is displaced north of the Equator in the Atlantic as a result of the asymmetry in continental geometry and air-sea interactions. During its oscillations the ITCZ reaches Guianian coastal areas twice each year, delineating a seasonal cycle with four unequal periods. From July to November, the ITCZ lies north of French Guiana, which corresponds to the dry season. While moving southward the ITCZ is over French Guiana from December to February, corresponding to the “short rainy season”; when it reaches its extreme position during March precipitation levels decrease (petit été de Mars). Then the ITCZ slowly moves northward and a new period of heavy rains occurs from April to June, corresponding to the major rainy season [12].
In addition, the northeastern Amazon and French Guiana are affected by El Niño Southern Oscillation (ENSO) due to a chain of correlated events. Like northeastern Amazon, during El Niños the Guianian climate is drier, while La Niñas are correlated with increased precipitation [13,14].
2.2 Known elements of the biology of Polybia bistriata
For such a common species, relatively little has been published about the biology of P. bistriata. Colonies tend to be small; as summarized by Jeanne [2], ranging from 26–129 adults, but multiple-comb nests contained over 200 adults in Brazil [15], and up to 358 adults in French Guiana (BC, pers. obs.). Colonies are founded by small swarms of older queens and younger workers [15], and the population is small in the “preemergence” stage (prior to the emergence of workers). As colonies increase in size during the stage of worker production, queens and workers of all ages occur. During the stage of gyne production, most queens again are older, and are both fewer in number and larger than during worker production [15]. Both males and future queens are produced during the stage of gyne production, but mating has not been observed. The length of time typically spent in each stage is not known, but the colony cycle is asynchronous, in common with other epiponines. There is no published information on colony longevity, but AD has observed one colony persist at least two years. Swarming wasps install their nests under large leaves of young palm trees (Ericaceae), Heliconia (Musaceae), Bromeliads, and particularly Clusia (Clusiaceae) [3,4,15].
2.3 Seasonal variation in the position of Polybia bistriata nests
Like other epiponines [2,6], P. bistriata colonies frequently abscond during the beginning of the rainy season (AD, pers. obs.). In order to analyze this phenomenon in depth, we decided to conduct this study during a period when the contrast between the dry and the rainy seasons was particularly strong. Because the 2005 forecast predicted a light La Niña event [16], we expected particularly heavy rainfall during the short rainy season. This was indeed the case, the December 2005–February 2006 period being the wettest recorded since 1981, while the dry season that preceded was the driest noted since the same date, resulting in the greatest contrast noted since 1981 (Méteo France; pers. comm.).
Firstly, we conducted a survey of 341 C. grandiflora treelets growing along the road leading to the Petit Saut dam. We noted the number of treelets sheltering a P. bistriata nest during the first week of July and the last week of November 2005 (the end of rainy and dry seasons, respectively), and then in February 2006 (after two months of heavy rainfall). The results were compared using Fisher's exact test and the sequential Bonferroni correction (multiple comparisons). Furthermore, seven areas also situated along the road leading to the Petit Saut dam were monitored during the first week of July 2005, 2006 and 2007 (the end of the rainy seasons; 70 treelets from each area resulting in a total of 490 treelets). We compared the number of C. grandiflora treelets sheltering a P. bistriata nest using the Friedman test (repeated measure test) and Dunn's test for pairwise comparisons.
Second, in the same areas cited above, we counted the number of leaves and noted the position of the wasp nest (see Fig. 1) for 40 C. grandiflora sheltering a P. bistriata nest eight times between June 2005 and February 2006. Variation in the position (height) of the nests on the C. grandiflora leaves – counted from the youngest leaf to avoid bias due to tree growth – was analyzed using a generalized linear model [17] with a binomial error (proportion data; F-test) and the total number of C. grandiflora leaves as denominator vector. The 8-pronged factor “month” was fitted to the data (full model). Afterwards, simplified models grouping certain months were adjusted to the data and only those that were not significantly different from the main model were retained (simplified models; F-test).
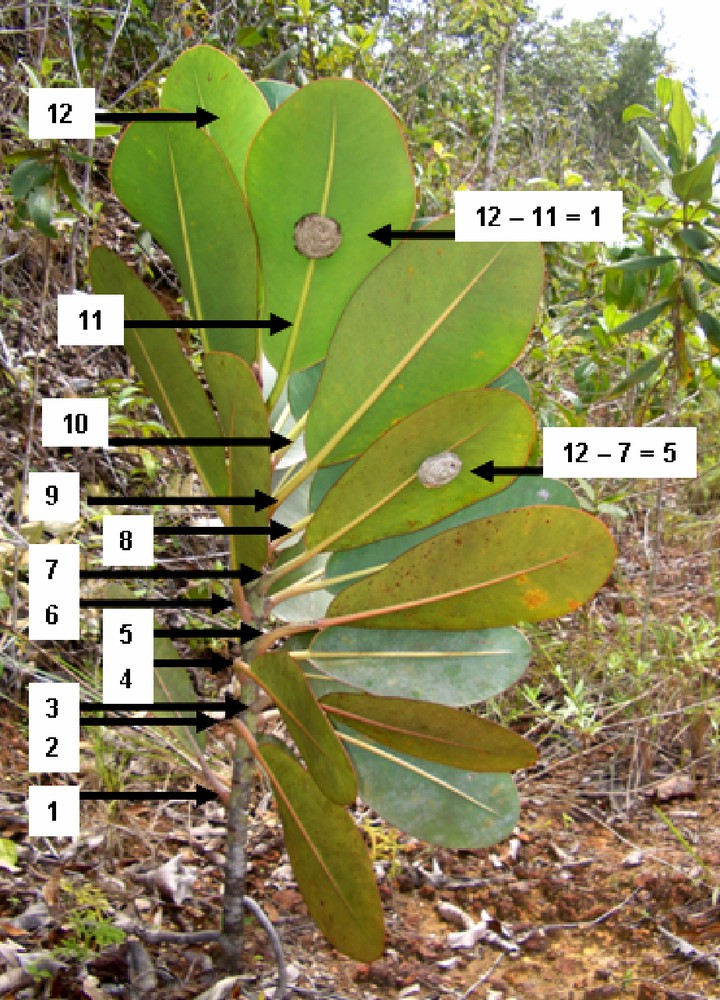
Illustration of the position of Polybia bistriata nests. In this case, the colony was in the process of moving. The new nest (wasps visible) is attached under the central vein of the 11th leaf; the old nest, under the seventh leaf. For the leaf positions, the leaves were counted starting from the youngest leaf to avoid bias due to plant growth (illustration to the right of the picture). The trade winds blow from the back of the picture, so that the nests are protected from the rain by the large leaves.
Third, from December 2005 to mid-January 2006 (heavy rainfall) we noted on 30 occasions a wasp colony in the process of moving its nest from one leaf to another on the same tree (see Fig. 1). We compared the new and the former positions of the wasp nests using the Wilcoxon paired test. Statistical comparisons were performed using Statistica 5.5 software.
3 Results
From the same 341 C. grandiflora treelets we noted that the dry season corresponded to an increase in P. bistriata nests (from 23 to 66 wasp nests in July and November 2005, respectively; 65.1% increase), while the short rainy season, December 2005–February 2006, with heavy rainfall, was accompanied by the disappearance of nests (from 66 to 26 nests; 60.6% decrease). The difference was significant between the end of the dry season (November 2005) and the two other cases (P < 0.001), but was not between July 2005 (end of the previous major rainy season) and February 2006 (short rainy season) (Fisher's exact-tests and sequential Bonferroni correction).
During the study concerning the 490 C. grandiflora treelets monitored during the first week of July 2005, 2006 and 2007 (i. e., just after the end of the rainy season), we noted significantly more individuals bearing a wasp nest in 2005 (37 wasp nests) than in 2006 (20 wasp nests) or even in 2007 (21 wasp nests; Friedman test: P = 0.0027; Dunn's multiple comparison test: 2005 vs 2006: P < 0.01; 2005 vs. 2007: P < 0.05; 2006 vs 2007: NS). During this study we noted only one (or no) P. bistriata nest at a time on the C. grandiflora treelets, but a treelet can shelter two or more wasp nests particularly when the colony is in the process of moving (see Fig. 1).
The height (leaf positions) at which the wasp nests were found varied progressively through the dry season, from June to end of November 2005 (Fig. 2). This was followed by a rapid change occurring with the first heavy rains of the rainy season between the end of November and the first 10 days of December 2005. The position of wasp nests remained at the upper parts of the trees during the entire rainy season (data of June 2005, then December 2005 to February 2006). Also, the wasp colonies that moved their nests from one leaf to another on the same C. grandiflora individual during the onset of the heavy rains installed their new nests higher than they were before (median and range: 2nd, 1st–4th leaves versus 8th, 1st–14th leaves; N = 30 treelets; Wilcoxon paired test: Z = 4.65; P < 0.001).
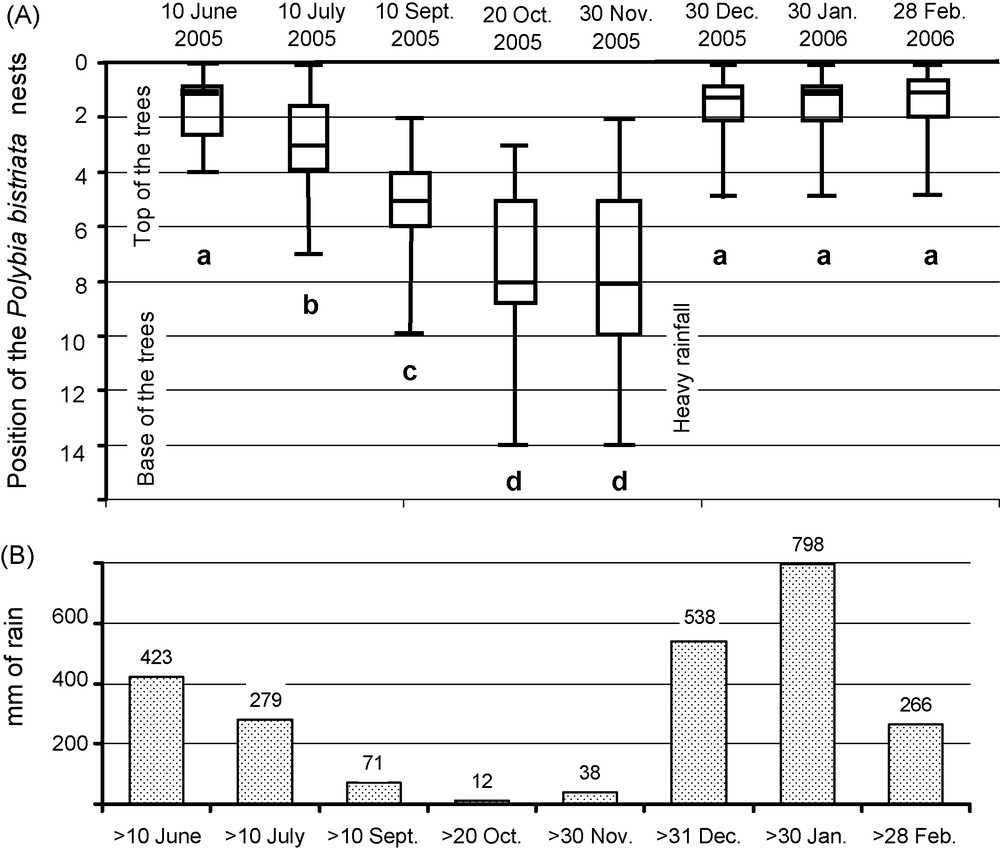
A. Seasonal variation in the position of the Polybia bistriata nests according to the position of the sheltering Clusia grandifolia leaves (end of the rainy season during the first week of July; November = end of the dry season; February = end of the short rainy season) (40 cases each). The box plots indicate the median (large horizontal bars), the 25th and 75th percentiles (white squares), and the minimum and maximum values (whiskers). Statistical comparison (generalized linear model): full model (F7,312 = 724.5, P < 10−5; R2 = 66.9%); simplified model (F3,316 = 723; P < 10−5; R2 = 66.9%) with four groups (see letters on the graph); model difference NS (F4,312 = 0.326; P = 0.68). Different letters in the figure indicate significant differences at P < 0.01. B- Rainfall during the 30 days preceding each data record from Fig. A.
4 Discussion
The sharp change in rainfall and subsequent humidity from the preceding dry season is an obstacle the wasps have to overcome at the onset of each short rainy season. Indeed, the number of Neotropical social wasp nests decreases each year during the beginning of the rainy season, and then returns to the same level after swarming occurs at the beginning of the dry season [7,8]. Nevertheless, with 60.6% P. bistriata nests disappearing during the particularly heavy rainfall during the 2005–2006 short rainy season, a threshold for rainfall and humidity above which most wasp colonies cannot survive was probably reached, while the rest of that rainy season also received heavy rainfall. As a comparison, 1602 mm of rainfall between December 2005 and February 2006 (see Fig. 2), short rainy season, corresponds to more than the average annual precipitation of London: 593 mm, Paris: 642 mm and Seattle: 990 mm. The effect was so strong that it was prolonged at least 18 months (see values for July 2005, 2006 and 2007), illustrating the existence of strong abiotic pressure on social wasps, particularly species whose nest is covered by an envelope.
Our dataset permits us to demonstrate that the wasps relocate their colonies according to the seasons. This process always exists, but was highlighted due to greatly contrasting abiotic conditions during the selected period of study. A progressive change during the dry season involving reproductive swarming (occurring mostly during the dry season; pers. obs. during this study) is followed by absconding during the first days of heavy rainfall, the colonies losing their nests and their brood. Not only did the wasps move to the shelter of the plants’ upper leaves with the onset of the rainy season, but in all cases these leaves were situated on the side of the plants opposite the trade winds and so best protected from the rain and likely to dry the fastest after a shower (pers. obs.).
The reasons for these colony relocations are multiple. First, to bring the level of humidity down in the nest: at the onset of the short rainy season in December 2005 and January 2006 numerous P. bistriata individuals per nest were observed licking the moisture soaking the carton of their nests almost constantly, and discarding drops of water. Despite this behavior they were unable to dry their nests completely. Second, we noted several times that the combs situated at the base of large nests of P. bistriata, as well as the species P. occidentalis, P. rejecta and P. sericea, were so soaked that they came loose and dropped off with a part of the brood; soon after the colonies absconded. Third, workers continued to hunt, storing their prey – mostly winged termites that swarm with the first rains – in the upper parts of their nests or even in any available space, so that we regularly counted several hundred to ca. 1500 winged termite prey in P. bistriata nests and in several other species of Epiponini. During the first weeks of 2006 the prey quickly decayed in the humid nests, forcing the wasps to abscond several times (the same was noted for P. occidentalis in Brazil [18]). Although the new nests were increasingly smaller due to successive absconding swarms associated each time with loss of the nest and brood, the problem remained the same because the wasps continued to hunt. For instance, we noted 55 termites in a small (3 cm in diameter) P. scrobalis surinama nest. Fourth, during the 2005 dry season (the driest ever recorded in French Guiana) wasp colonies progressively moved into the lowest parts of the trees.
These results are reminiscent of studies conducted on variation of the population of Darwin's ground finches in the Galapagos after a drought (in 1977) and a flood (1983 El Niño event) [19], but other reasons can explain the sharp decrease in the number of P. bistriata nests during the 2005–2006 La Niña year. For instance, climatic change may favor the development of a pathogen, as was noted for amphibians [20]. Neotropical wasps are known to be sensitive to gregarines (Apicomplexa) when humidity increases [10,21], although we did not detect Apicomplexa during a preliminary screening under a microscope. Sequencing of wasp extracts allowed DNA amplification from a Wolbachia (bacteria) a coccidian (apicomplexa protozoa), a microsporidia (fungi) and three unidentified fungi (Claire Tirard, pers. comm.). However, the presence of each of these potential pathogens was noted only in one or a few samples, which is not consistent with an epidemic.
5 Conclusion
We have presented data that contribute greatly to our knowledge of colony relocation in Neotropical swarm-founding wasps. Studying this phenomenon during a period of particularly heavy rainfall during the 2006 La Niña year highlighted the fact that inclement weather can strongly affect social wasp populations. The selective pressure resulting from heavy rainfall, particularly those rains falling during the first weeks of the short rainy season, can be overcome through the rapidity with which colonies of most epiponine wasp species move to an adequate nest site from the very first days of rain. The capacity to move to an alternative site with the onset of the short rainy season, while leaving a minimum of brood or no brood at all, may represent an adaptive trait that is crucial for epiponine wasps in exceptionally wet years. This is of particular importance for the survival of these wasps because above average rains have been frequent in recent years, while climatologists predict drier and longer dry seasons followed by increased rainfall during the rainy season in the northeastern Amazonia and French Guiana [22,23]. These results can serve as a foundation for future studies of the specific mechanisms and selective pressures that social wasp populations have to assume in a general context of climate changes.
Acknowledgements
We are grateful to Cécile Reynouard (Laboratoire environnement de Petit Saut) and Damien Bonal (INRA-Guyane) for furnishing us with data on the pluviometry of French Guiana. This work was supported by the Programme Amazonie (projects BIG and 2ID) of the French Centre national de la recherche scientifique and by the Programme Convergence 2007–2013 Région Guyane (project DEGA) from the European Community.


