1 Introduction
As outlined in previous publications [1,2], the genus Pseudouroplectes, which is composed of soil-dwelling species, is extremely rare. In Madagascar, the other group of micro-scorpions, also composed of soil species, is represented by members of the endemic family Microcharmidae [3]. This group can be suggested to be better represented than Pseudouroplectes, and was better studied until now [3]. The first known species, Pseudouroplectes betschi Lourenço, 1995, was originally described based on two females collected with the use of extraction systems, in the dry southwestern spiny bush formation at Andramanoetse Be, Plateau Mahafaly [4]. Subsequently, a second species, Pseudouroplectes pidgeoni Lourenço & Goodman, 1999 was collected in the extreme southeastern dry forests of the “Parc national d’Andohahela” (parcel 2) [5]. This scorpion was collected in a soil litter sample from the spiny bush parcel of the reserve, within a few kilometers of the ecotone between dry and wet forest formations. Only several years later, additional material was obtained of the genus Pseudouroplectes. This led to the description of a third species, Pseudouroplectes maculatus Lourenço & Goodman, 2006 [1]. Subsequently, material collected in the dry forests of Ifaty in the Province of Toliara, revealed one more new species, Pseudouroplectes lalyae Lourenço & Ythier, 2010 [2] (Fig. 1).
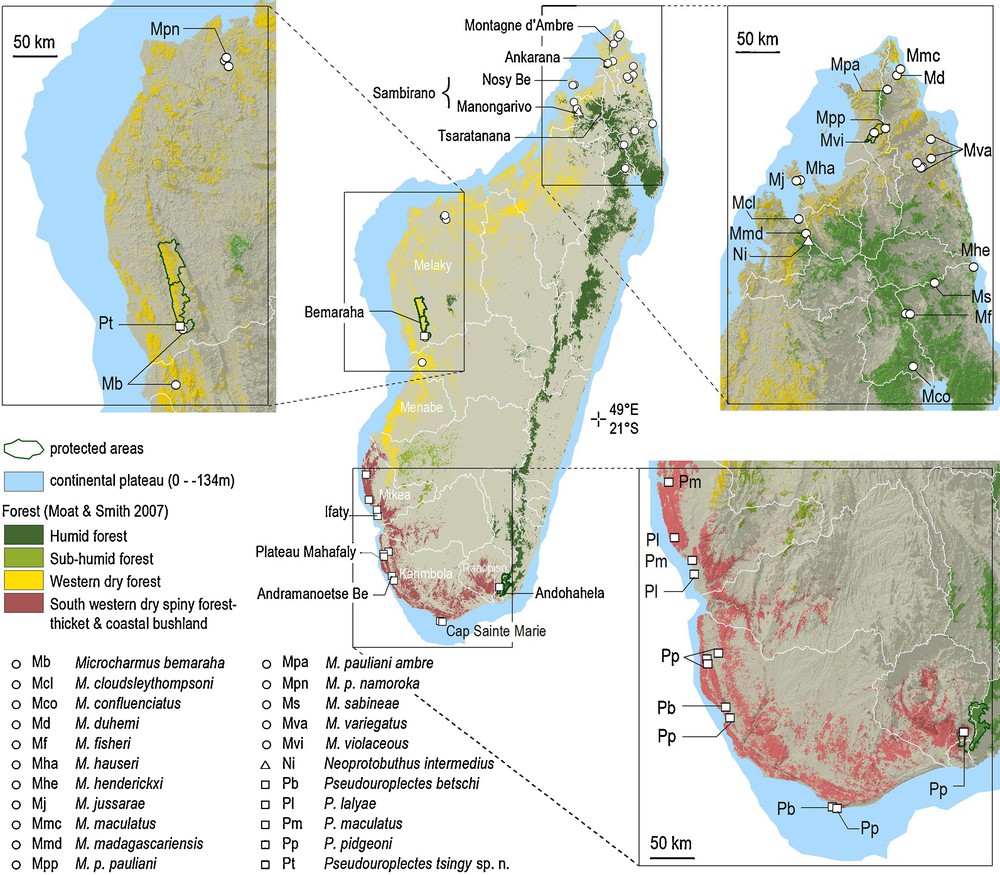
Collection localities of the humicolous micro-scorpions in the genera Microcharmus, Neoprotobuthus, and Pseudouroplectes, and forest types.
Recent investigation on some non identified material, collected by the late J.-M. Betsch in Madagascar, and now deposited in the Museum in Paris, led to the location of one more specimen of Pseudouroplectes. The material was again collected in dry forests in a Tsingy formation of the P. N. Bemaraha. After detailed examination of the specimen we concluded for yet a new species. The number of species in the genus Pseudouroplectes is now raised to five.
2 Distributional pattern presented by the genus Pseudouroplectes
The distributional pattern of the genus Pseudouroplectes was already discussed in detail in the two previous publications dealing with this group [1,2]. In these publications, detailed lists of known localities for members of this genus are proposed. All the species of this genus, with the exception of the new species described here, are restricted to the extreme southern dry forest formations. Pseudouroplectes betschi and P. pidgeoni apparently present a parapatric or even a small sympatric zone of distribution in the southern portion of the island. A similar situation is observed between P. maculatus and P. lalyae, with the two species presenting, at least, a small zone of sympatry. This very restrict distribution could eventually be attributed to incomplete sampling collections, but a large portion of the southwestern region of the island was extensively prospected by several teams. Consequently, a more plausible explanation has been explored in the recent past of the genus’ evolution in respect to its modern distribution, but also considering the other species of humicolous micro-scorpions of the islands, more specifically the species in the endemic family Microcharmidae. The new species described here extends the distribution area of the genus into western Madagascar, and for the first time the distribution area of the genus Pseudouroplectes overlaps that of another micro-scorpion genus, Microcharmus Lourenço, 1995.
3 Methods
Illustrations and measurements were made with the aid of a Wild M5 stereo-microscope with a drawing tube (camera lucida) and an ocular micrometer. Measurements follow Stahnke [6] and are given in mm. Trichobothrial notations follow Vachon [7] while morphological terminology mostly follows Hjelle [8].
4 Taxonomic treatment
Family Buthidae C.L. Koch, 1837
Genus Pseudouroplectes Lourenço, 1995
Pseudouroplectes tsingy sp. n. (Figs. 2–3)
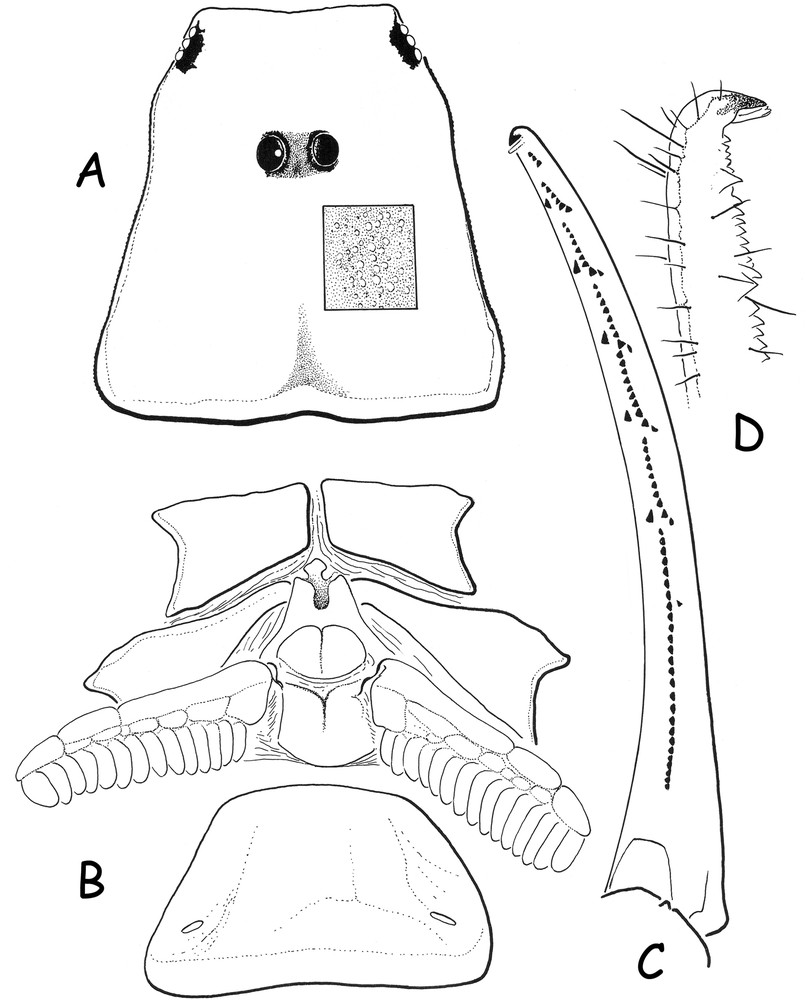
Pseudouroplectes tsingy sp. n. Female holotype. A. Carapace, dorsal aspect; the square zone shows the granulation pattern. B. Ventral aspect, showing coxapophysis, sternum, genital operculum, pectines and sternite III with spiracles. C. Disposition of granulations on the dentate margins of the pedipalp chela movable finger, dorsal aspect. D. Extremity of the finger, in detail, lateral aspect.

Trichobothrial pattern of the pedipalp. A. Chela, patella nd femur PAR E. Chela, patella and femur. B. Patella, external aspect PAR F. Patella, external aspect.
Type-material, female holotype. Madagascar, Melaky Region, ex-Province Mahajanga, P. N. Bemaraha, dry forest in Tsingy, XI/1972 (J.-M. Betsch), extraction by Berlese.
Etymology: the specific name is a noun in apposition to the generic name and refers to the formation in which the new species was collected.
Diagnosis: small scorpions, when compared with the average size of most species of micro-buthid genera, and measuring 18.96 mm in total length (see morphometric values after the description). General coloration yellow marbled with light reddish-brown confluent spots over tergites; pedipalps, legs and metasomal carinae without spots. Carinae and granulations moderately to strongly marked; dorsal carinae on metasomal segments I to IV with conspicuous posterior spinoid granules. Metasomal segment I wider than long. Chela fingers with only 6–7 rows of granules. Pectines with 15–14 teeth and without fulcra. Trichobothriotaxy A-α (alpha), orthobothriotaxic.
Relationships: the new species can be readily distinguished from all the other species of the genus Pseudouroplectes and in particular from P. betschi by a number of features:
- • a general pale yellow coloration, but with pale confluent spots on tergites;
- • carapace and tergites with a intense granulation;
- • metasomal segment I wider than long;
- • chela fingers with 6–7 rows of granules;
- • dorsal carinae on metasomal segments I to IV with conspicuous posterior spinoid granules.
Description based on female holotype. Coloration yellow; only tergites are marbled with light reddish-brown confluent spots. Carapace yellow to slightly reddish-yellow; eyes blackish. Pedipalps and legs pale yellow without spots; rows of granules on chela fingers reddish. Metasomal segments yellow with carinae slightly reddish; telson yellow with aculeus reddish. Venter yellow; pectines and genital operculum pale yellow. Chelicerae yellow with finger's teeth reddish.
Morphology. Carapace with a moderately to strongly marked granulation; anterior margin almost straight. Carinae weak; furrows inconspicuous. Median ocular tubercle distinctly on the anterior third of the carapace; median eyes separated by less than one ocular diameter. Three pairs of lateral eyes. Sternum subpentagonal. Mesosoma: tergites moderately to strongly granular. Median carina moderate in all tergites. Tergite VII pentacarinate. Venter: genital operculum divided longitudinally, each plate having a more or less subtriangular shape. Pectines large: pectinal tooth count 15–14 in female holotype; basal middle lamellae of the pectines not dilated; fulcra absent. Sternites smooth with very short semi-slit-like spiracles; VII with minute granulations and two vestigial carinae. Metasoma: segments I to III with 10 carinae, moderately crenulate; IV with 8 carinae, crenulate; ventral carinae reduced or vestigial on segments I to IV; dorsal carinae with strongly marked posterior spinoid granules; intercarinal spaces weakly granular. Segment V rounded with vestigial carinae. Telson with a very elongated “pear-like” shape, smooth and with a strong setation; aculeus short, weakly curved; subaculear tooth absent. Cheliceral dentition characteristic of the family Buthidae [9]; fixed finger with two moderate basal teeth; movable finger with two very weak and almost fused basal teeth; ventral aspect of both finger and manus with dense, long setae. Pedipalps: femur pentacarinate; patella with vestigial carinae; internal face of patella with 4–5 spinoid granules; chela with vestigial carinae; all faces moderately granular. Fixed and movable fingers with 6–7 almost linear rows of granules; two accessory granules present at the base of each row; extremity of fixed and movable fingers with one long and sharp denticle. Trichobothriotaxy, A-α (alpha) orthobothriotaxic [7,10]. Legs: tarsus with very numerous fine median setae ventrally. Pedal spurs reduced; tibial spurs absent.
Morphometric values (in mm) of female holotype. Total length (including telson), 18.96. Carapace: length, 2.40; anterior width, 1.40; posterior width, 2.54. Mesosoma length, 6.07. Metasomal segments. I: length, 1.14; width, 1.20. II: length, 1.34; width, 1.14. III: length, 1.47; width, 1.07. IV: length, 1.80; width, 1.00. V: length, 2.47; width, 0.97; depth, 0.93. Telson: length, 2.27. Vesicle: width, 0.67; depth, 0.67. Pedipalp: femur length, 1.77, width, 0.74; patella length, 2.47, width, 0.94; chela length, 3.50, width, 0.67, depth, 0.74; movable finger length, 2.34.
5 Key to the known species of Pseudouroplectes
| 1. Pale scorpions, yellowish to reddish-yellow, with or without spots_______________2 |
| (1). Dark scorpions with confluent blackish spots over the body and appendages.................P. maculatus |
| 2. Coloration yellowish without any spots; pectinal tooth count 18–20.................P. betschi |
| (2). Coloration yellowish with light reddish-brown confluent spots or two to four longitudinal reddish-brown stripes over the tergites; pectinal tooth count 14–16.................3 |
| 3. Coloration yellow with light reddish-brown confluent spots over the tergites; granulations strongly marked on carapace and tergites................P. tsingy sp. n. |
| (3) Two to four longitudinal reddish-brown stripes over the tergites; granulations moderately to strongly marked on carapace and tergites................4 |
| 4. Two longitudinal brownish stripes over the tergites; carapace, pedipalps and metasomal segments without spots................P. pidgeoni |
| (4). Four longitudinal brownish stripes over the tergites; carapace, pedipalps and metasomal segments strongly spotted................P. lalyae |
6 Biogeography of the Madagascar humiculous micro-scorpions: Current distribution
The Microcharmidae family has its modern area of distribution centered in northern Madagascar and is currently represented by 16 taxa, including 14 species with only two species occurring in the west (Fig. 1). With the exception of M. pauliani occurring from the humid forest of Montagne d’Ambre and in the dry and subhumid forests of Ankarana in the north, and in the dry forest in the northwest, with three localized subspecies, the other 13 species show small areas of distribution (Fig. 1) [11]. In northern Madagascar, the Microcharmus species are encountered in every type of vegetation, including the humid forest of the northeast and Montagne d’Ambre, the dry forest in the north and northwest, and also in the almost subarid types of vegetation encountered near the northern tip of the island (Fig. 1). As exemplified in niche conservatism [12,13], sister species inhabit environments that bear some similarities. Hence, the ancestor clade of the current Microcharmus spp. lived in all types of habitat.
Neoprotobuthus is only know from the type species N. intermedius, documented by two specimens collected at an altitude of 1240 m a.s.l. in the Sambirano region in March 1999 [14].
Pseudouroplectes tsingy sp. n. has been collected in the dry forest of the Bemaraha protected area. The species previously described in the genus have been collected in the subarid region along the southern coast of the island, including in the driest environment on the Mahafaly plateau and in the Mikea Forest where mean annual rainfall is below 500 mm (Fig. 1) [15,16]. The genus Pseudouroplectes’ known distribution was circumscribed in the driest centers of endemism of Ranopiso, Karimbola and Mikea. The new species expands the range of the genera to the Melaky center of endemism, but without encompassing the Menabe [17,18]. The four species previously described have a large range, as in the case of P. pidgeoni, or medium ranges with few records in the Karimbola center of endemism (P. betschi) or limited ranges as in the case of P. maculatus and P. lalyae both endemic to the Mikea center of endemism, sensu Wilmé et al. [18]. P. pidgeoni has been collected in the dry spiny forest-thicket of the southeast, at only some 12 km from the humid forest where it has not been recorded (Fig. 1) [5].
7 Some explanations on the humicolous micro-scorpion distribution
Since the Cretaceous and until the Eocene (56.0–33.9 million years ago), Madagascar was positioned on the high-pressure desert belt and was mainly arid, from north to south. Wells [19] proposed that the oldest biome is the one encountered in the driest part of the island, in southern Madagascar and near the extreme northern tip of the island. The known species of humicolous scorpions in the Microcharmidae family or the genus Pseudouroplectes have an extremely old history going back at least to the Miocene (23.03–5.333 million years ago). They have retained a basal behavior in living in the upper humid soil surface, in the humus where moisture is retained. In order to explore and better understand the current distribution of these species, we will limit our biogeographical considerations to the last million years circumscribed within the Plio-Quaternary (5.333 million years ago); this also allows the comparison with Neogrosphus spp. [20].
8 “Neogrosphus rule”
It has been shown that northern Madagascar has experienced rapid changes over the past million years, with new geographic barriers explaining allopatric speciation in some groups (e.g., Neogrosphus [20]). As other primitive micro-scorpions, the Microcharmidae and Pseudouroplectes spp. are humicolous micro-scorpions, mostly limiting their “open air excursion” for foraging and reproduction [21–23]. Given their small size coupled with humicolous/cryptozoic behavior, species in the family Microcharmidae and in the genus Pseudouroplectes have extremely limited dispersal abilities.
If species diversity in any given area is caused by speciation, extinction and dispersal [24], there are only two processes driving the scorpion diversity: speciation and extinction [20,21]. As a global rule for the scorpions, and in particular for the humicolous micro-scorpions: the lower the species’ dispersal ability and the greater the niche breadth of the ancestor taxon, the higher the species richness in a changing environment producing geographical barriers, and vice-versa. This translates into the following formula:
The genus Microcharmus represents a typical case-1 taxon (Table 1), i.e., high species diversity in a rapidly changing environment of a taxon with an ancestor with great niche breath. Northern Madagascar has experienced volcanism at various ages, including during the Quaternary in Nosy Be and Montagne d’Ambre (Fig. 1). The region has entered the intense monsoonic system during the Pliocene with increased rain in the Sambirano region due to the local topography (Fig. 4) [19].
The “Neogrosphus rule” in a changing environment producing geographical barriers, the lower the species’ dispersal ability and the greater the niche breadth of the ancestor taxon, the higher the species richness, and vice-versa is equivalent to its contrapositive: the higher the species’ dispersal ability or the smaller the niche breadth of the ancestor taxon, the lower the species richness, and vice-versa.
| Case | A | B | C | A | ∧ | B | ↔ | C | |
| 1 | True | True | True | Low dispersal | AND | Great niche breadth | EQV | High spp richness | True |
| 2 | True | False | False | Low dispersal | AND | Small niche breadth | EQV | Low spp richness | True |
| 3 | False | True | False | High dispersal | AND | Great niche breadth | EQV | Low spp richness | True |
| 4 | False | False | False | High dispersal | AND | Small niche breadth | EQV | Low spp richness | True |
| 5 | True | False | True | Low dispersal | AND | Small niche breadth | EQV | High spp richness | False |
| 6 | False | True | True | High dispersal | AND | Great niche breadth | EQV | High spp richness | False |
| 7 | False | False | True | High dispersal | AND | Small niche breadth | EQV | High spp richness | False |
| 8 | True | True | False | Low dispersal | AND | Great niche breadth | EQV | Low spp richness | False |

Shortest distance to coast and to the outer limit of the continental plateau for every collection locality of humicolous micro-scorpions (see Fig. 1 map), and altitude of the collection localities (anti clockwise from northeast to southeast).
Within the humicolous micro-scorpions, Neoprotobuthus intermedius is the largest species (females up to 20 mm vs 13–18 for the females Microcharmus) [3,14]. Neoprotobuthus has the characteristics of a relict species with one species only occurring at high elevation, above 1200 m, on an old and eroded volcano (Manongarivo) in an area which has experienced major changes in recent times from which many groups have been extirpated (Sambirano). It has been shown that the Sambirano region (Fig. 1) has acted as a major barrier for scorpion species in several groups, including for species in the Grosphus or Neogrosphus genera, but also primates in the Indriidae family, or birds in the endemic order of the Mesithorniformes. Neoprotobuthus intermedius could be an extreme case 2 (box in the top right corner of Fig. 1), with a monotypic genus, therefore pointing towards a specialized ancestor.
The genus Pseudouroplectes occurring in the oldest biome of Madagascar [19] has lived in a “stable” environment, as compared to the members of the Microcharmidae in the north. The absence of the genus in subhumid or humid vegetation types (Fig. 1), may be explained by the limited niche breath of the ancestor taxa, only able to cope with arid to dry conditions. The humicolous behavior is considered as basal, dating back to the “out of aquatic environments” event of the ancient lineages of scorpions, sometimes between the Carboniferous and the Triassic ([358.9 ± 0.4]–[201.3 ± 0.3] million years ago), at a time when scorpions were not adapted to the dry epigenous environments [21–23]. The four species occurring in southern Madagascar have large ranges as compared to the micro-endemic Microcharmus species. Given the collection effort in southern Madagascar, Pseudouroplectes scorpions are extremely rare or totally absent from most places through their range. Their extant coastal low altitudinal distribution (Fig. 4) may reflect an ancient extended range over the current continental plateau which has been emerged as recently as ca. 20,000 years ago during the Last Glacial Maximum. The southern extend of this continental plateau could have acted as a bridge between the populations of P. pidgeoni or P. betschi (Fig. 1). This may also explain why P. tsingy sp. n. is isolated; it could be a relict population which has survived in refugia within the former range of the species. The limestone tsingy formation found in the Bemaraha is a karstic residual landform where water is absent on the surface but can be maintained in caves or avens [25]. The lithology, tectonic and geomorphological evolution have sculpted the local landscape, allowing a diversity of micro-climates and the establishment of a variety of vegetation types. The modern large but patchy and coastal distribution of the members of the genus Pseudouroplectes, a typical class-2 taxon (Table 1), may constitute another argument to explain a reduced niche breath of the Pseudouroplectes.
9 Conclusion
The three groups of endemic humicolous micro-scorpions show distinct biogeographical features related to the pace and extent of environmental changes. With their extremely limited dispersal abilities at the time scale of scorpion evolution, the genus Microcharmus shows high species richness in the rapidly changing environment in northern Madagascar, while the monotypic Neoprotobuthus illustrates a relict population in the family Microcharmidae. The Pseudouroplectes have a littoral distribution along the driest region of southern Madagascar, with a relict population in the western Bemaraha limestone formation which is described here as P. tsingy sp. n. The extended distribution of the species in the genus Microcharmus in the most humid types of vegetation in northern Madagascar, together with the total absence of the genus Pseudouroplectes from the humid types of forest in southeastern Madagascar exemplifies the specialized features of the ancestor Pseudouroplectes vs plasticity of the Microcharmus ancestor. This may be an explanation for the absence of humicolous micro-scorpions in the eastern humid forests. The Microcharmidae, as well as the genus Pseudouroplectes follow the “Neogrosphus rule”; with a species richness related to dispersal ability and niche breadth of the ancestral taxa in a changing environment. The “Neogrosphus rule” does apply to scorpions but also to mobile species and does allow for biogeographic understanding at taxonomic higher levels.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We are most grateful to Bernard Duhem (MNHN, Paris) for his contribution to the preparation of the drawings, and we would like to acknowledge constructive discussions and exchanges with Joerg Ganzhorn (University of Hamburg) and Jean-Luc Mercier (University of Strasbourg).


