1 Introduction
Convergence between organisms is a widespread phenomenon that has been known for a long time [1,2]. It concerns populations as well as species that belong to different classes and orders. Over the last 20 years, numerous debates have arisen about the validity of molecular data for proving a common origin of taxa and for estimating the age at which they separated from one another [3]. However, a consensus exists concerning the fact that convergences are a priori the result of external constraints. When species occur on different continents, it must be supposed that the environmental conditions were comparable on these continents and have remained essentially unchanged until the present time. The convergence is clear when two species have acquired the same particularities that are infrequent or unique within their respective group. Likewise, a species can show characteristics that are infrequent or unique in its group but common in that of another species. Despite the fact that convergences constitute an important question in biology and evolution, few detailed studies have been undertaken on this subject. For example, authors have made comparisons between populations or communities of mammals based on apparent similarities in external features or diets [4,5]. Other authors have analysed convergences between clades or orders in morphology or metabolism, depending on the diet [6–9]. Fisher et al. [10] studied convergences in maternal care according to habitat characteristics. However, these studies were conducted at a general level and often limited to a simple statement concerning a few features. Overall, the most spectacular examples of convergences among mammals are to be found among aquatic, arboreal, gliding and subterranean species [5,11–13] and, to a much lesser degree, terrestrial species. This is due to the fact that changes are less obvious among the latter as a result of what are probably less restrictive living conditions.
From the end of the Eocene to the Pliocene (from 34 to 7 Myr ago), South America remained completely isolated from the rest of the world and evolved as an island. The nototrogomorph rodents present on this continent produced many cursorial species in the absence of other large terrestrial mammals [14]. Even now, a number of South American species are exceptional among rodents. A good example of this is the caviomorph lowland paca Cuniculus paca L., 1766 (Rodentia, Hystricomorpha), which inhabits the tropical forests of South America and appears to be very similar to the African tragulid water chevrotain Hyemoschus aquaticus Ogilby, 1841 (Cetartiodactyla, Ruminantia) [15] (Fig. 1). Both species are medium-sized terrestrial species, typical of their tropical mammal communities. Because of their great similitude, it seems particularly interesting to see how the different biological characteristics of the paca are comparable to those of the water chevrotain, all the more so since the Rodentia are thought to be separate from the Cetartiodactyla since the Cretaceous, ∼66–78 Myr ago [16,17]. This should provide useful information about the extent and the limits of the convergence between two species. To my knowledge, a detailed study has never been undertaken on this point.

The hystricomorph rodent Cuniculus paca (a) and the ruminant artiodactyl Hyemoschus aquaticus (b).
Over the last 40 years, several studies have focused on the diet, reproduction, ecology and behaviour of these two mammals. The goal of this work consists in comparing their different characteristics and in analysing the biological sectors where convergences appeared. Given the fact that these species live in a particular environment (lowland tropical rainforest), it might be expected that, above all else, many physical characteristics would be convergent. However, considering that the species must be adapted to their habitat, other categories of characteristics should also be concerned, contrary to what could be assumed (e.g., the ecology, as well as the behaviour, structure and organisation of the populations). Finally, this study should allow us to more precisely define the very particular niche of these two species.
2 Materials and methods
The comparison between the two species is carried out on the basis of 231 characteristics that are selected according to the current knowledge in different organismic sectors. We retain only characteristics known in both species. For this reason, physiology and molecular genetics are excluded. The 231 characteristics are grouped into five broad categories: external appearance of the body (31 characteristics), osteology s.l. (82 characteristics), anatomy (31 characteristics), ecology (28 characteristics) and behaviour (59 characteristics). External appearance encompasses all of the external body features: shape, weight, coat texture and colour, superficial glands, etc. Osteology s.l. covers descriptions and measurements of all parts of the skeleton: skull, dentition, vertebral column and limbs. Anatomy includes the characteristics of the encephalon and of the circulatory, respiratory, digestive and uro-genital systems, as well as reproductive characteristics directly dependent on the latter. Data concerning the geographical range, biotope, home range, diet, growth, reproductive and population dynamics constitute the ecological part. Lastly, the behavioural category includes all individual and social behaviours.
In each broad category thus defined, the rarity level of each characteristic is deduced from the available literature, as reported in Table 1, by assigning a value of 1 or 2 when the characteristic is common or infrequent/unique, respectively, in its own zoological group (rodents for the paca, ruminants for the chevrotain). A positive value indicates that the characteristic is present in the given species and a negative one when it is lacking.
Level of specialisation of the paca and chevrotain and index of convergence/divergence between the two species for each characteristic.
| Characteristic | C. paca | H. aquaticus | Index |
| External appearance of the body [15,18–21,23,26,29,32,37–45] | |||
| Body red-brown | 1 | 1 | 1 |
| Light spots on back | –1 | 2 | –2 |
| Light spots on sides | 2 | 2 | 4 |
| Sparse coat | 2 | 1 | 2 |
| Thin skin | 2 | –2 | –4 |
| Young similar to adults | 1 | 1 | 1 |
| Medium weight: 6–12/7–15 kg | 2 | 2 | 4 |
| Body weight (kg)/height (cm): 0.34/0.30 | 2 | 2 | 4 |
| Anterior part of body lower | 2 | 2 | 4 |
| Enlarged head at the zygomatic arches | 2 | –2 | –4 |
| Head without appendages | 1 | 2 | 2 |
| Small ears | 2 | 2 | 4 |
| Short neck | 2 | 2 | 4 |
| Short tail | 2 | 1 | 2 |
| Male smaller than female | –1 | 2 | –2 |
| Sexual dimorphism of the head | 2 | 1 | 2 |
| Hairy muzzle | 1 | –1 | –1 |
| Interramal vibrissae present | 1 | 1 | 1 |
| Mystacial/superciliary vibrissae present | 1 | –1 | –1 |
| Preorbital glands missing | 1 | 2 | 2 |
| Sebaceous chin gland present | 2 | 2 | 4 |
| Interdigital glands missing | 1 | 2 | 2 |
| Preputial gland present | –1 | 2 | –2 |
| Perineal glands present | 1 | 2 | 2 |
| Number of toes on hind foot: 5/4 | 1 | –1 | –1 |
| Feet plantigrade | 1 | –1 | –1 |
| Feet equipped with claws | 1 | –1 | –1 |
| Axillary nipples present | 1 | –1 | –1 |
| Inguinal nipples present | 1 | 1 | 1 |
| Ventral nipples missing | 2 | 1 | 2 |
| External testes | –2 | 2 | –4 |
| Osteology s.l. [26,27,32,39,41,43,46–48] | |||
| Sagittal crest present | 1 | 2 | 2 |
| Occipital crest present | 1 | 1 | 1 |
| Orbit angle to the horizontal: 50/55̊ | 2 | 2 | 4 |
| % of length of muzzle/total skull: 50/48 | 1 | 2 | 2 |
| Skull basis flat | 1 | 2 | 2 |
| Broad zygomatic arch | 2 | –1 | –2 |
| Orbital ring incomplete | 1 | –1 | –1 |
| Inflated tympanic bullae | 1 | 1 | 1 |
| Rostral bone present | –1 | 2 | –2 |
| Narrow palate | 1 | –1 | –1 |
| Bony palate extended beyond jugal teeth | –1 | 1 | –1 |
| Straight mandibular arch | 1 | 2 | 2 |
| Anterior crest at humerus | 1 | –1 | –1 |
| % of length of humerus/foreleg: 49/47 | 1 | 2 | 2 |
| Complete ulna | 1 | 2 | 2 |
| Flat ulna | –1 | 1 | –1 |
| % of length of olecranon/ulna: 23/17 | 1 | 1 | 1 |
| Ulna stronger than radius | 1 | –1 | –1 |
| Ulna motionless | 1 | 1 | 1 |
| Ulna fused with radius by its whole proximal surface | –1 | 1 | –1 |
| % of length of metacarpals/forelimb: 14/21 | 2 | 2 | 4 |
| Paraxonic forelimb | 2 | 1 | 2 |
| Taxeopody of carpals and tarsals | 1 | –1 | –1 |
| Upper jugal teeth oriented outward | 1 | –1 | –1 |
| Upper incisors present | 1 | –1 | –1 |
| Upper canines present | –1 | 2 | –2 |
| Number of jugal teeth: 4/6 | 1 | –1 | –1 |
| High-crowned jugal teeth | 1 | –2 | –2 |
| Jugal teeth with transverse cusps | 1 | –1 | –1 |
| Low coronoid process of mandible | 1 | 2 | 2 |
| Mandibular condyle longitudinal | 1 | –1 | –1 |
| Mandibular plateau | 1 | –1 | –1 |
| Mandibular diastema present | 1 | 1 | 1 |
| Lower canines present | –1 | 1 | –1 |
| % of length of jugal teeth/mandible: 36/51 | 1 | –2 | –2 |
| % of length/width of atlas: 47/50 | 1 | 2 | 2 |
| % of width/length of odontoid process: 22/25 | 1 | 2 | 2 |
| Cervical vertebrae slightly opisthocelous | 1 | 2 | 2 |
| Praeclavium present | 1 | –1 | –1 |
| Number of rib pairs: 13/14 | 1 | 1 | 1 |
| Flat ribs | –1 | 1 | –1 |
| Neural spine directed forwards at 13th/12th thoracic vertebra | 1 | 1 | 1 |
| Number of lumbar vertebrae: 6/6 | 1 | 1 | 1 |
| Thin transverse processes | 1 | –1 | –1 |
| Number of sacral vertebrae: 5/5 | 2 | 1 | 2 |
| Long acromion | 1 | –1 | –1 |
| Short coracoid process | 1 | 1 | 1 |
| Semilunar scapula | 1 | –1 | –1 |
| Suprascapula present | –1 | 1 | –1 |
| Small supraspinatus fossa of the scapula | –1 | 1 | –1 |
| Clavicle present | 1 | –1 | –1 |
| Olecranon foramen at humerus present | 1 | –1 | –1 |
| 2 proximal carpal bones fused | 1 | –1 | –1 |
| Central bone missing | 2 | 1 | 2 |
| Unfused metacarpals | 1 | 2 | 2 |
| Metacarpus 3>4 | 1 | 1 | 1 |
| Anterodistal extremity of metapods smooth | 1 | 2 | 2 |
| Complete lateral toes at forelimb | 1 | 2 | 2 |
| Pelvic girdle parallel to vertebral column | –1 | 1 | –1 |
| Pelvic girdle fused with 2 vertebrae | –1 | 1 | –1 |
| Ilium-sacrum angle: 35/43̊ | 1 | 1 | 1 |
| Ratio of length of ilium/ischium: 1.5/1.3 | 1 | –1 | –1 |
| Ilium and ischium thin | 1 | –1 | –1 |
| Flared ilium | 1 | –1 | –1 |
| Tuberosity at ischium present | 1 | –2 | –2 |
| Large ischiopubic part | –1 | 1 | –1 |
| Long ischiopubic symphysis | 1 | 1 | 1 |
| Thin ischiopubic symphysis | 1 | –1 | –1 |
| Trochanter fossa of humerus present | 1 | 1 | 1 |
| Rounded head of femur | 1 | –1 | –1 |
| % of length of femur/hindlimb: 45/39 | 1 | 2 | 2 |
| Complete fibula | 1 | –1 | –1 |
| % of length of metatarsals/hindlimb: 14/21 | 2 | 2 | 4 |
| Number of tarsal bones: 5/1 | 1 | –2 | –2 |
| 1 trochlea at upper side of astragalus | 1 | –1 | –1 |
| 2 facets at lower side of astragalus | 1 | –1 | –1 |
| Astragalus angle: 24/24̊ | 1 | 2 | 2 |
| Unfused metatarsals | 1 | –1 | –1 |
| Mesaxonic hindlimb | 1 | –1 | –1 |
| Metatarsus 3=4 | –1 | 1 | –1 |
| Toe 3>4 at hindlimb | 1 | –2 | –2 |
| Tiny 2nd toe at hindlimb | –1 | 1 | –1 |
| Anatomy [19,21,24,26,42–58] | |||
| Elongated pupil | 1 | –1 | –1 |
| Broad encephalon | 2 | –2 | –4 |
| Dubois index: 0.25/0.14 | 2 | –2 | –4 |
| Cerebral cortex few folded | 1 | 2 | 2 |
| Large thyroid cartilage present | –1 | 2 | –2 |
| Organ amplifier of sounds present | 2 | 2 | 4 |
| Os cordis present | –1 | 1 | –1 |
| Number of lung lobes: 5/2 | 1 | –2 | –2 |
| Number of liver lobes: 6/3 | 1 | –1 | –1 |
| Tiny red blood cells | –1 | 2 | –2 |
| Gall bladder present | 2 | 1 | 2 |
| Undivided stomach | 1 | –1 | –1 |
| % of length of intestine/body: 16/11 | 2 | –2 | –4 |
| Cecotrophy present | 1 | –1 | –1 |
| Penis situated in cloaca | 1 | –1 | –1 |
| Appendix at glans penis | 1 | 2 | 2 |
| Os penis present | 1 | –1 | –1 |
| Cowper's glands present | 1 | 1 | 1 |
| Bicornuate uterus | 1 | –1 | –1 |
| Urethra opening in clitoris | 1 | –1 | –1 |
| Discoidal placenta | 1 | –2 | –2 |
| Spontaneous ovulation | 1 | 1 | 1 |
| Number of follicles left side = right side | 1 | 1 | 1 |
| Vaginal plug present | 1 | 2 | 2 |
| Vaginal membrane present | 1 | –1 | –1 |
| Oestrous cycle length: 31/26 days | 2 | 1 | 2 |
| Short copulation | 1 | –2 | –2 |
| Insemination in vagina | 1 | –2 | –2 |
| Gestation period: 115–118/180–240 days | 2 | 1 | 2 |
| Post-partum estrus present | 1 | –1 | –1 |
| Corpora lutea persist during gestation | 1 | –2 | –2 |
| Ecology [18–25,29,30,32,34,42,45,56,59–64] | |||
| Number of species in the genus: 2/1 | 2 | 2 | 4 |
| Geographical range: 6800/5400 km | 2 | 2 | 4 |
| Lowland forests | 1 | 1 | 1 |
| Terra firma near water | 2 | 2 | 4 |
| density: 60-90/8-28 ind./km2 | 1 | –1 | –1 |
| Use of hollow trees | 1 | 2 | 2 |
| Digging capacities | 1 | –1 | –1 |
| Use of nest | 1 | –1 | –1 |
| Exclusively nocturnal | 1 | 2 | 2 |
| Male area overlaps that of only 1 female | 2 | –1 | –2 |
| Male areas not overlapping | 1 | 1 | 1 |
| Female areas overlapping | 1 | –2 | –2 |
| Home range area: 2–3/13–23 ha | 2 | –1 | –2 |
| % of fruits in diet: 84/69 | 1 | 1 | 1 |
| % of leaves and stems in diet: 15/30 | 2 | 1 | 2 |
| % of invertebrates in diet: 0.04/0.14 | –1 | 1 | –1 |
| Aseasonal reproduction | 1 | 1 | 1 |
| 1 young/litter | 2 | 1 | 2 |
| Precocial newborn | 2 | 1 | 2 |
| Average % of pregnant females: 61/82 | 1 | 1 | 1 |
| Suckling period: 3/5 months | 2 | 1 | 2 |
| Age at weaning: 9/12 months | 1 | 1 | 1 |
| Age at sexual maturity: 1/1.5 years | 2 | 2 | 4 |
| Birth sex ratio M/F: 1:1/1:1 | 1 | 1 | 1 |
| Adult sex ratio M/F: 1:1/1:2 | 2 | –1 | –2 |
| % of mortality at 1 year of age: 51/49 | 1 | 1 | 1 |
| % of individuals older than 8 years: 8/23 | 1 | –1 | –1 |
| Longevity: 16/13 years | 2 | 1 | 2 |
| Behaviour [18–20,24,26,32,38,42,45,47,52,63] | |||
| Solitary life | 2 | 2 | 4 |
| Monogamy present | 2 | –1 | –2 |
| Hierarchy missing | 1 | 2 | 2 |
| Slow gait | 1 | 2 | 2 |
| Diagonal gait | 1 | 1 | 1 |
| Running with leaps | 1 | 1 | 1 |
| Rolling on the ground | 1 | –1 | –1 |
| Climbing capacity | 1 | –1 | –1 |
| Standing up on hindlegs | 1 | –1 | –1 |
| Smelling with raised head | 1 | 1 | 1 |
| Stopping with a raised foreleg | 1 | 1 | 1 |
| Lying on side or back | 1 | –1 | –1 |
| Sitting on hindlimbs | 1 | 2 | 2 |
| Alarm bark present | 2 | 1 | 2 |
| Bristled coat in alarm | 1 | –1 | –1 |
| Foot stamping in alarm | 1 | –2 | –2 |
| Teeth grinding in threat | 1 | 2 | 2 |
| Marking with urine | 2 | 2 | 4 |
| Marking with faeces | –1 | 2 | –2 |
| Frequent defecation in water | 2 | 2 | 4 |
| Micturition position slightly crouched | 1 | 2 | 2 |
| Food held with forelegs | 1 | –1 | –1 |
| Food carrying | 1 | –1 | –1 |
| Frequent body smelling of partners | 1 | –2 | –2 |
| Body contact with partners | 1 | –2 | –2 |
| Marking of partner with chin gland | 1 | 2 | 2 |
| Submission display present | 1 | –2 | –2 |
| Submission cry missing | 2 | 2 | 4 |
| Cries rare | 2 | 2 | 4 |
| Poor vision | 1 | 2 | 2 |
| Visual displays rare | 2 | 2 | 4 |
| Escape in water | 2 | 2 | 4 |
| Food reserves missing | 2 | 1 | 2 |
| Territorial behaviour present | 2 | –1 | –2 |
| Marking of vegetation with chin gland | 2 | 2 | 4 |
| Chin-laying of male on female | 1 | 1 | 1 |
| Marking of ground with anal glands | 1 | –1 | –1 |
| Cleaning of muzzle with hands | 1 | –1 | –1 |
| Rubbing against surfaces | 1 | –1 | –1 |
| Allogrooming present | 1 | –2 | –2 |
| Urine spraying on sexual partner | 1 | –1 | –1 |
| Fighting with teeth | 1 | 2 | 2 |
| No kick during fight | 2 | 1 | 2 |
| Ritualised fighting with body pushes | 2 | –2 | –4 |
| Flehmen present | –1 | 1 | –1 |
| Male striking the female during courtship | 1 | –2 | –2 |
| Female standing still for copulation | –1 | 2 | –2 |
| Lying copulation | 1 | –1 | –1 |
| Standing copulation | 2 | 1 | 2 |
| Copulation dance | 1 | –1 | –1 |
| Lying parturition | 1 | –1 | –1 |
| Young licked by mother | 1 | 1 | 1 |
| Aggressive new mother | 1 | –1 | –1 |
| New mother seeking isolation | 1 | 1 | 1 |
| Suckling lying | 1 | –1 | –1 |
| Suckling standing | 2 | 1 | 2 |
| Mother calling young | 1 | 1 | 1 |
| Solitary play | 1 | 2 | 2 |
| Social play | 1 | –2 | –2 |
For each characteristic, we calculate a convergence or divergence index between the two species as the product of the value given to each species, as mentioned above (Table 1). One or the two species can be specialised or not specialised in a given characteristic, compared to other members of its respective own zoological group. Consequently, positive indices of level 4 correspond to a real specialisation of both species in the same direction, with the presence of the same infrequent characteristic. Positive indices of level 2 mean a specialisation of only one species in the same direction as the other species, which has the same characteristic but which is not specialised on this point, and positive indices of level 1 imply no specialisation in any species. Inversely, negative indices of level 4 indicate that the two species are both specialised but in opposite directions, since the corresponding characteristic is present in one species and missing in the other. Negative indices of levels 2 or 1 reflect a specialisation of only one species or no specialisation in any species, respectively, with, once again, the presence of the characteristic in one species and the absence in the other. Thus, positive indices indicate that the characteristics are convergent and negative ones that they are divergent. We have chosen the product because it best represents the degree of similarity/dissimilarity of the species, owing to the rarity level of the presence/absence of each characteristic in each zoological group.
Because data are distributed among classes, the species and characteristic categories are compared using the χ2 test. An average convergence or divergence index is calculated for all characteristics and for each category. Averages are expressed with their standard deviations and compared with the t-test.
3 Results
3.1 Overall similarity of the species
Table 1 lists the characteristics taken into account, according to the categories identified.
Although the number of convergent and divergent characteristics is nearly the same (112 vs. 119, respectively), the average resemblance of the two species is greater than their average dissimilarity (Table 2). The distribution of the characteristics according to the different convergent and divergent indices is also different (, P < 0.001; Fig. 2). The relative importance of the indices progressively decreases with their level. Level-1 indices represent 49.78% of all characteristics (115/231) vs. 37.23% (86/231) for the level-2 indices, and only 12.99% (30/231) for those of level 4.
Mean absolute value (± SD) of convergent and divergent characteristics between the paca and the chevrotain, according to the biological categories.
| Convergent characteristics | Divergent characteristics | Difference | |||
| n | Mean ± SD | n | Mean ± SD | P | |
| External appearance | 19 | 2.526 ± 1.219 | 12 | 2.000 ± 1.279 | t = 1.14, df = 29, NS |
| Osteology | 34 | 1.794 ± 0.845 | 48 | 1.167 ± 0.377 | t = 4.05, df = 80, P < 0.001 |
| Anatomy | 10 | 1.900 ± 0.876 | 21 | 1.762 ± 1.044 | t = 0.38, df = 29, NS |
| Ecology | 19 | 2.000 ± 1.155 | 9 | 1.444 ± 0.527 | t = 1.75, df = 26, NS |
| Behaviour | 30 | 2.267 ± 1.143 | 29 | 1.483 ± 0.688 | t = 3.20, df = 57, P < 0.01 |
| Total | 112 | 2.081 ± 1.070 | 119 | 1.454 ± 0.778 | t = 43.59, df = 229, P < 0.001 |
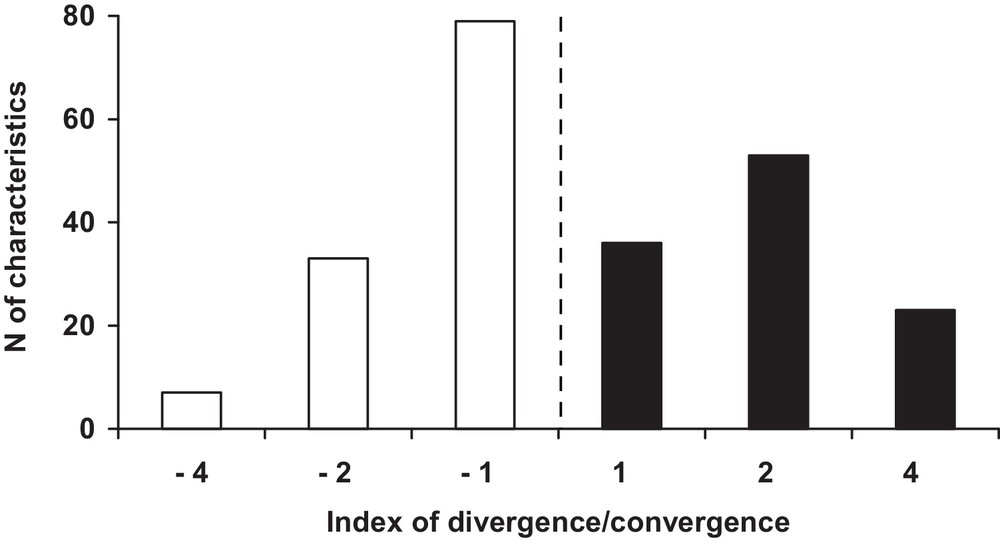
Distribution of the characteristics according to the divergence (white bars) and convergence (black bars) indices between the paca and the chevrotain. The vertical line indicates the separation between divergent and convergent characteristics.
The proportion of convergent or divergent characteristics also varies a great deal according to the indices. The divergence index –1 is twice as frequent as the convergence index 1 (79 vs. 36 characteristics: , P < 0.01). On the contrary, the convergence index 2 is 1.6 times more frequent than the divergence index –2 (53 vs. 33 characteristics: , NS), and the characteristics of convergence index 4 are 3.3 times more numerous than those of divergence index –4 (23 vs. 7: , P < 0.05).
3.2 Similarity of the species according to the characteristic categories
There are more convergent than divergent characteristics in the external appearance of the body (19 vs. 12) and in ecology (19 vs. 9). On the contrary, the divergent characteristics are more numerous than the convergent ones in osteology (48 vs. 34) and in anatomy (21 vs. 10). Finally, convergent and divergent characteristics are equivalent in terms of behaviour (30 vs. 29, respectively). The average convergence of characteristics is greater than the average divergence in all of the categories, although significantly only in osteology and behaviour (Table 2). The average convergence is the highest in external appearance and the lowest in osteology. The difference between these two groups of characteristics is significant (t = 2.32, df = 51, P < 0.05).
Characteristics with a value of 1 are generally divergent in all categories (especially in osteology and anatomy), except in ecology (Fig. 3). The characteristics with a value of 2 generally become convergent, except in anatomy, which is also the case for the characteristics with a value of 4. Thus, the distribution of convergent and divergent characteristics significantly varies depending on their value in osteology (, P < 0.001) and behaviour (, P < 0.02).
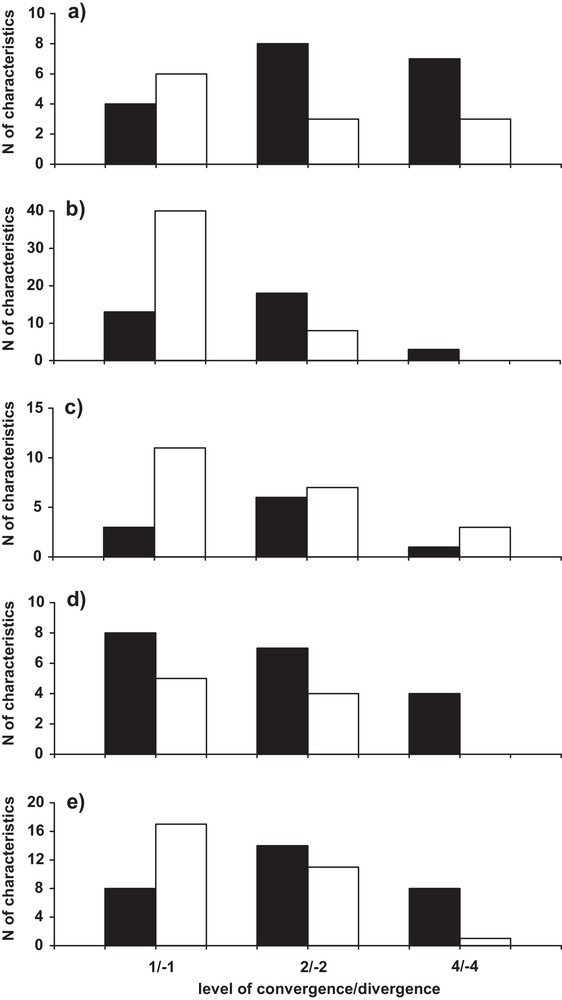
Percentage of convergent (black bars) and divergent (white bars) characteristics between the paca and the chevrotain, according to index levels in external appearance (a), osteology (b), anatomy (c), ecology (d), and behaviour (e).
3.3 Differences between the two species
On the whole, the paca shows less infrequent or unique characteristics (value 2) compared to other members of its zoological group than the chevrotain compared to its own (56 vs. 90: , P < 0.001). It is clearly less specialised than the chevrotain in osteology (7 vs. 25 characteristics), anatomy (7 vs. 14) and behaviour (16 vs. 27), but quite similar in external appearance (14 vs. 17). On the contrary, it is more specialised than the chevrotain in ecology (12 vs. 7 characteristics).
4 Discussion
Although the dissimilar characteristics are as numerous as the similar ones, the two species globally resemble each other. However, the characteristics with a value of 1, which correspond to the fundamental symplesiomorphies of the taxa to which the paca and the chevrotain belong, represent nearly half of all the characteristics analysed, and are two times more dissimilar than similar (79 vs. 36) in all categories (especially osteology and anatomy), with the exception of ecology. This indicates that the two taxa are very different from each other in their basic composition, which is in accordance with their early separation from each other since the Cretaceous [16,17].
Convergences of indices 2 and 4 together represent 32.9% of all characteristics, whereas the divergences of the same levels only reach 17.3%. This proportion (76 vs. 40 characteristics) is exactly the reverse of the precedent. The percentage of these convergent characteristics notably increases from anatomy and osteology (22.6 and 25.6%, respectively) through behaviour and ecology (37.3 and 39.3%) to external appearance (48.4%). This is directly linked to the adaptation of the animals to their physical environment. On this point, it is not surprising that anatomy and osteology are the least concerned. This suggests a strong influence of phylogenetic history, as reported by Jones [7] concerning the convergences between marsupial carnivores and placental carnivores. Therefore, the convergences can be considered as responses to external constraints, as assumed earlier. Since they are twice as frequent as the divergences, there is a real specialisation of one or both species in the same direction.
However, convergences are not limited to an adaptation of animals to their physical environment since ecology and behaviour are also concerned. This is true in other groups. Thus, convergences appear in morphological particularities linked to diets between Xenarthra and Pholidota, two orders of ant-eating mammals [6], and between marsupial carnivores and placental carnivores [7,8]. Likewise, convergences in maternal care strategies between macropods and ungulates are linked to habitat characteristics [10].
On the whole, the paca shows less infrequent or unique characteristics compared to other members of its zoological group than the chevrotain, particularly in osteology, anatomy, and behaviour. On the one hand, infrequent or unique characteristics are more numerous in the paca in ecology. This is consistent with the special status of the two species within their zoological group. The paca appears to be a particular species among the rodents by its spatial location and organisation, diet composition, behaviour, reproductive characteristics, and population dynamics [18–25]. On the other hand, the chevrotain has been known for many years to be the living intermediary between the non-ruminant and the ruminant artiodactyls on the basis of many characteristics, e.g., skull osteology, metapod structure, marking of the terrain, pacific and aggressive interactions, reproductive and social behaviours [19,25–28]. Moreover, it does not notably differ from other forest ruminants in terms of its ecological characteristics and diet [29,30].
Other South American hystricomorphs are also similar to African artiodactyls [15]. However, the paca and the chevrotain differ from other forest species by their location near water. Water is important because it is the preferred refuge in the case of danger: individuals leap directly into it, sometimes from a height of five or more metres, and submerge themselves [29]. Indeed, they are not true runners but rather slow walkers, unlike the other species [31]. This characteristic is observable in several convergent characteristics (low anterior part of the body, unfused metacarpals, smooth anterodistal extremity of metapods, astragalus angle, escape in water, slow gait, etc.), and in the scarcity of myoglobin in the muscles. This inability to run for any length of time is obviously due in part to the elevated weight/height ratio of their body, since the other sympatric terrestrials show much lower values (on average 0.06–0.17 compared to 0.30–0.34; pers. data). For example, the male chevrotain has a lower weight/height ratio than the female (0.27 vs. 0.32) and remains farther from the water during the day (184 m vs. 100 m) [29]. Thus, the inability to run for an extended period and a habitat near water are closely related. The paca is the only terrestrial mammal in South American forests to possess this trait (the capybara, Hydrochaeris hydrochaeris, is a species of open areas). The other hystricomorphs (Dasyprocta spp. and Myoprocta spp.) are of a lighter build and are good runners [31], as are the two sympatric cervids (Mazama spp.). The same is true for the chevrotain compared to the other ruminants of African forests (Cephalophus spp.). However, it appears that living near water constitutes a possible niche for other tropical forest mammals. For each of the three other genera, each belonging to a separate order and each comprising four-six species in the same African region, there is one species that has also specialised in life near watercourses [32].
Other convergent characteristics could be related to nocturnal life: light spots on the sides of the body, orbit angle/horizontal, organ amplifier of sounds, solitary lifestyle, poor vision, rare visual displays, solitary play, etc. Two characteristics are also constituents of the niche particular to these two species: medium body weight and one precocial newborn. First, the fact that both species are of medium weight deserves special attention because it corresponds to an inverse position of the two species on the weight scale of their respective zoological group. The paca clearly belongs to the heaviest rodents, contrary to the chevrotain, which is a small ruminant, although it is the largest actual tragulid. Thus, weight markedly differentiates each of these species from all other sympatric terrestrials. Secondly, although precocial young are the rule among the hystricomorphs, it is surprising that the paca produces only one young per litter, since most other terrestrial hystricomorphs are polytocous [33]. One reason for this similarity in litter size between the paca and the chevrotain could be their body characteristics. The weight/height ratio is higher in the paca than in the chevrotain (Table 1). Furthermore, it is higher in females than in males in both species (0.36 vs. 0.32 in the paca; 0.32 vs. 0.27 in the chevrotain). With a single full-grown fetus, the female ratio becomes 0.39 in the paca vs. 0.35 in the chevrotain (8.3% higher than in non-pregnant females vs. 9.4%, respectively). With twins, it would be nearly 0.42 in the paca, i.e. 16.7% higher than in non-pregnant females, which could be incompatible with the safety and survival of females.
5 Conclusion
As expected, the apparently spectacular resemblance between the paca and the chevrotain is especially notable in the characteristics that favour a physical adaptation of the species to their spatio-temporal environment in tropical forests. However, convergences are not at all limited to external features since they also occur in ecological and behavioural characteristics. Only the osteology and the anatomy of the two species are little concerned.
It could be expected that the species resemble each other in population dynamics and social organisation. This is not the case. Thus, we must admit that the convergence concerns the characteristics of individuals per se and not those of their populations. This is difficult to explain. However, there are interesting differences between the two mammal communities. The species diversity is lower in South American than in African forests: only eight plant-eating terrestrial mammals > 1 kg vs. usually 15/16 species, respectively (pers. data). As a possible consequence, the paca occurs at much higher densities than the chevrotain. This could explain why many discrepancies are recorded between the two species in terms of population dynamics and social organisation, e.g., lower female fecundity, lower percentage of old individuals, smaller but more overlapping home ranges, higher sex ratio, monogamy, and numerous inter-individual behaviours in the paca compared to the chevrotain.
It is difficult to estimate the constraints exerted by a forest environment on terrestrial animals. They are probably predominantly of a physical nature. One of them is obviously the difficulty for the animals to move about since their physical characteristics are in relation to vegetation density [34,35]. However, the safety needs of such prey species appears the be of utmost importance, given that many of the convergences correspond to an adaptation of the individuals in this sense: body size, homochromy with the environment during the day, lack of signals indicating presence, and possibilities for rapid escape to a safe refuge.
This niche is apparently well defined since only one or two species exist in each genus over a large geographical range (5–7000 km) [33]. However, it occurs only on the American and African continents. In Asian regions, the smaller tragulids are good runners on dry land [31], as are the cervids, and there is no terrestrial mammal like the paca or chevrotain [33]. Even the Chinese water deer that frequents reeds and grassy areas near water is mainly diurnal and feeds primarily on grasses [36]. The reason for this absence remains unknown.
Disclosure of interest
The author declares that he has no competing interest.
Acknowledgements
This work was supported by the ‘Muséum national d’histoire naturelle’ and the ‘Centre national de la recherche scientifique’. Personal data on pacas and chevrotains were obtained from numerous visits to French Guiana and Gabon. I am grateful to Drs J.-P. Gasc, S. Sen and G. Vannier, and two anonymous referees for their helpful comments.


