1. Introduction
The small island developing states (SIDS) and member island countries in the Caribbean Sea have been recognized to share similar sustainable development challenges, as they often have limited resources and fragile economies [Robinson 2018; Thomas et al. 2020]. SIDS also correspond to hotspots of terrestrial and marine biodiversity with a number of endemic species that need protection [Maunder et al. 2008; Miloslavich et al. 2010; Bellard et al. 2014]. Unfortunately, the resilience of many fragile island ecosystems is threatened by global warming and its collateral effects, including sea level rise, coral bleaching, tropical cyclones, storm surges, and droughts, as well as invasive species and other forms of ecological interactions [Cohen et al. 2015; Benjamin and Thomas 2019; Fernandez-Palacios et al. 2021; Mycoo et al. 2022]. The SIDS, like other places in the world, are also facing numerous anthropic pressures from overfishing, pollution from the shore, development operations, mass tourism, urban development, etc. [Wolf et al. 2022].
Among the nine French outermost regions, Guadeloupe and Martinique (French West Indies) are exposed to the abovementioned threats, especially marine submersion (i.e., cyclones and tsunamis), coastal erosion and sea-level rise [Paris et al. 2021]. They are also exposed to several sources of pollution, such as polycyclic aromatic hydrocarbons (PAHs) in mangroves [Munoz et al. 1997; Ramdine et al. 2012], heavy metals in seagrass beds [Bouchon et al. 2016] and pesticides in soils and rivers [Crabit et al. 2016; Comte et al. 2022]. Dumping of solid and consumptive wastes by inhabitants and users has also been documented, particularly in mangrove ecosystems [Dahomé Di Ruggiero 2017]. Such improper solid waste deposition, associated with a lack of suitable waste treatment plants and inadequate pollutant storage areas, is one of the most severe threats to the sustainability of the natural resources of sensitive habitats and wildlife and of course to people. Despite these environmental concerns, these islands and archipelagos of the Caribbean are at the heart of important tourist flows, notably linked to cruises, because they offer all the amenities and facilities expected of a tropical destination. This tourist attraction is not devoid of environmental and social consequences [Miller and Auyong 1991; Wilkinson 2006; Scott et al. 2012; Wong 2015; Adams 2017; Mackay and Spencer 2017; Wilkinson et al. 2017; Wolf et al. 2022]. Consequently, the development of ecotourism, as a form of more sustainable tourism with lesser local impacts, corresponds to great challenges that will require the development of management and educational schemes and a better integration of historical contexts [Wood 2000; Dehoorne and Augier 2011; Nicolas-Bragance and Saffache 2016]. Probably, such development should not conceal a better regulation of the tourism flow and a reconsideration of the economic paradigm of tourism. In fact, even if the French islands present unique potential and assets, they have to adjust to their environmental context and to sociohistorical considerations if they want to respond adequately to both global and local challenges.
During the year 2016, the French National Center for Scientific Research (CNRS) had created one Human-Environment Observatories (OHM) which was named the OHM “Caribbean Coast”,1 to investigate the consequences of coastal landscape and resource transformations (i.e., artificialization, accelerating urban-port dynamics, use conflicts …) on the Guadeloupean socio-ecosystem. Even if the OHMs are in line with frameworks that have been developed to address complex human–environmental processes [Binder et al. 2013; Bourgeron et al. 2018], they are unique in the sense that they have defined their own socio-environmental context along with a disruptive event that is a human-based fact [Chenorkian 2020]. This disruptive event has its own ability to self-disperse into a plurality of positions, functions and implications. This concept of disruptive event is also considered as a benchmark or an interstice into which any OHM can dig through different approaches to study, at the local scale, socio-ecosystem trajectories (functions, communities, discourses, actors …). In the case of the OHM Caribbean Coast, the disruptive event corresponds to the modifications of urban-port dynamics in a perspective of sustainable development of the Guadeloupe archipelago. Through interdisciplinarity, integrative and multi-scalar approaches, the OHMs also aim to help stakeholders in environmental and social action/management planning, and to increase awareness of the population. As an illustration of the wide range of approaches that are needed to properly address the complexity and the plurality of environmental concerns, we present here several studies performed by the OHM Caribbean Coast on cross-contamination issues of the coastal environment in Guadeloupe. We show here that the environmental impact of pesticides that have emerged over time, and which can be linked to changes in agricultural practices and policies, are also having impacts on the Sargassum crisis.
2. Phytopharmaceutical products: use, diffusion and resurgence
2.1. A brief review of historical contamination by chlordecone
In the French outermost regions, there are important ongoing concerns related to the ban on the use of the organochlorine molecule chlordecone. This molecule, which belongs to the same chemical family as dichlorodiphenyltrichloroethane (DDT), was discovered in 1951 and produced in the United States in the early 1950s under the trade name Kepone® to combat leaf-eating insects, ants, roaches, and fly larvae. From 1966 to 1975, the company Allied Chemical, with contractor Life Sciences Products Company (LSP), produced Kepone at a small plant in Hopewell, Virginia, along the James River [Goldfarb 1978]. After a case report of employees suffering from shivering, studies demonstrated negative effects on human health [Cannon et al. 1978; Epstein 1978; Taylor 1982; Reich and Spong 1983]. Meanwhile, analyses showed that chlordecone was found throughout the James River, having spread downstream on suspended sediment and in fishes [Reich and Spong 1983]. Chlordecone was shown to have negative effects on marine unicellular algae [Walsh et al. 1977], estuarine animals [Schimmel and Wilson 1977] and fishes [Couch et al. 1977]. Even if there were debates on resistance forms, chlordecone and pentachlorophenol were also shown to inhibit the growth of estuarine bacteria [Orndorff and Colwell 1980; Mahaffey et al. 1982]. By the end of 1975, approximately half a year after the first employee case report, the production of Kepone was stopped in Hopewell, fishing was forbidden, and in 1977, all Kepone usage was banned. Allied, LSP, the city of Hopewell and six individuals were charged by the Federal court and fined [Goldfarb 1978]. This case serves as a model of prosecution for environmental pollution [Bacigal and Bacigal 1987]. Starting from these dates, several monitoring programs have shown that chlordecone can still be detected in the majority of white perch and striped bass samples even thirty years after the source of contamination was removed [Luellen et al. 2006]. Even partially, this information clearly reveals the large spectrum and long-term impacts of chlordecone, which was recognized as a persistent organic pollutant (POP) by the Stockholm Convention in 2009 [Madaj et al. 2018].
However, in the French West Indies, chlordecone (CLD) was used in intensive cropping of bananas between 1972 and 1993, even though a number of studies performed in the late seventies warned against the putative consequences, including the contamination and impacts on human health [Snegaroff 1977; Kermarrec 1980]. Currently, we know that a large portion of the population in Guadeloupe and Martinique is contaminated with chlordecone. A reference study, named KANNARI,2 which was performed from 2013–2014, revealed that a large proportion of the population, generally over 18 years old, has been contaminated by CLD, with differences depending on diet and place of residence. Persons living in contaminated zones consuming seafood or freshwater products from self-production of roots, tubers, poultry and eggs from contaminated zones presented a higher percentage above the internal chronic toxicological reference value (TRV) [Dereumeaux et al. 2020]. In fact, CLD has been described to alter body-weight homeostasis [Costet et al. 2022] and to be neurotoxic, an endocrine disruptor, reprotoxic and likely carcinogenic [Multigner et al. 2010, 2016].
Contamination by CLD not only affects a large proportion of the human population but is also generalized to terrestrial, aquatic and likely atmospheric ecosystems. Similar to what has been shown in the USA, CLD has contaminated surface and ground water, and ineluctably through watershed hydrology, rain runoff and soil erosion [Bonan and Prime 2001; Mottes et al. 2016], the molecule has reached marine environments [Bertrand et al. 2010]. However, a limited number of studies have specifically focused on the transport of CLD, especially between terrestrial and marine ecosystems [Woignier et al. 2018; Dromard et al. 2022]. Currently, various coastal marine ecosystems, including mangroves, seagrass meadows and coral reefs, are known to be contaminated. Even if details on bioaccumulation processes are scarce, various organisms from biofilms to top predators, including farmed animals, present moderate to high concentrations of CLD [Coat et al. 2011; Dromard et al. 2016, 2018a, b; Mendez-Fernandez et al. 2018; Monti et al. 2020; Dromard et al. 2022; Hubas et al. 2022]. Chlordecone can be detected in cetaceans living within the AGOA sanctuary [Mendez-Fernandez et al. 2018]. Nevertheless, important debates still exist and further investigations have to address the actual stability of chlordecone in various terrestrial and aquatic environments [Saaidi et al. 2023].
2.2. Change in the use of pesticides
Since the end of the 1990s and the ban of CLD, several alternative herbicides have been used for weed management. One of the cross-cutting phytosanitary practices involves the use of glyphosate, the active ingredient of broad spectrum post emergent, and nonselective systemic herbicides [Li et al. 2016]. Glyphosate-based formulations have become the main herbicides used on a global scale [Cuhra et al. (2016]. Studies have revealed that glyphosate from both urban and agricultural uses occurs in soil, surface water, and groundwater, and residues such as aminomethylphosphonic acid (AMPA) are found at all levels of the food chain, such as in drinking water, plants, animals, and even humans [Aparicio et al. 2013; Maggi et al. 2020; Rivas-Garcia et al. 2022]. However, the recognition of pharmaceutical substances as being toxic, especially to the environment and humans, and the associated regulations on usage and accessibility in markets usually involve long processes that can vary depending on the state.
To control the use of glyphosate and glyphosate-based herbicides (GBHs), under the European initiative, the French government launched a program in 2008 that has since been revised twice. The program, named the Ecophyto plan, aims to reduce the uses, risks, and impacts of plant protection products. According to accessible data from the General Commission for Sustainable Development (GCSD), the glyphosate was over the 2009–2017 period, the country’s top-selling herbicide among the 119 herbicide active substances. For the French overseas territories, the NGO Générations Futures also reported the important use of glyphosate.3 Nevertheless, like other governments in the European Union, the French government has been committed to eliminate glyphosate use in cases for which alternatives already exist.
To our knowledge, only a few investigations on the presence of glyphosate and derivative products have been performed in the French West Indies. One report revealed that glyphosate is used year-round in all cropping systems in Martinique [Deffontaines et al. 2017]. The same authors also showed intensified detection during the wet season in the upstream part of the watershed, suggesting a seasonal intensification of treatments to compensate for increased grass pressure. In this particular study, it was shown that the metolachlor herbicide or probably its metabolites, was present in 50% of the samples, glyphosate was present in more than 20%, and AMPA—the main degradation product of glyphosate—was present in more than 90% of the samples [Deffontaines et al. 2017]. In addition, warnings on the use of the present pesticide applications and potential pollution of watershed outlets have been raised, especially in the case of their use in horticulture [Mottes et al. 2017].
2.3. Impact of agricultural uses within the coastal transition zone
Recently, the OHM Caribbean Coast co-funded two paleoecology projects on the consequences of agricultural uses of herbicides and pesticides. The projects examined core sediments in the transition zone from coastal areas with strict fishing prohibitions from Guadeloupe and Martinique. Although the two corresponding watersheds studied were different in terms of total cultivated area, other important characteristics were similar: soil cover, types of cultures, and climate. Using core sediment analyses, a drastic increase in soil erosion was demonstrated from the turn of the 21st century (Figure 2A), which corresponded to the beginning of the usage of glyphosate in banana fields [Sabatier et al. 2021]. Further analyses of the core sample fluxes helped define two periods: one period from 1950 to early 2000 characterized by a slow sedimentation rate (SSR) and a period from 2001 to 2017 characterized by a fast sedimentation rate (FSR). The two periods were also characterized by differences in fluxes of glyphosate and chlordecone, with virtually no glyphosate and low to moderate CLD flux found in marine sediments over the SSR period and increasing amounts of these pesticides found over the FSR period (Figure 2B–C).
These data allowed us to conclude that within the OHM studied areas and also in Martinique, the application of glyphosate, which is known to disrupted grass development, had a strong effect on soil erosion [Mickelson et al. 2001]. We showed that the use of glyphosate has induced an increase in CLD and its degradation products in coastal and marine areas [Sabatier et al. 2021]. Such an upsurge of pesticides trapped in soils some time ago by an actual application of glyphosate was also demonstrated for DDT in French vineyards [Sabatier et al. 2014]. These important results clearly demonstrate that it is crucial to further study the consequences of herbicide use regarding the mechanisms of remnant pesticide (i.e., CLD) mobility in various terrestrial and aquatic environments of the French West Indies [Mottes et al. 2021]. Further erosion and ecotoxicological risk assessments should also be conducted, and management practices should be adjusted to the local context. We believe that the presented data reinforce the need to develop nature-based solutions coupled with sustainable agriculture using fewer pesticides, particularly in insular territories that present limited agricultural lands that are often in close vicinity to residential areas [Deffontaines et al. 2020].
2.4. Impact of agricultural uses on biodiversity
The memory effect of agricultural uses can be investigated using sedimentary ancient DNA (sedaDNA), even if the processes that govern the production, transfer, incorporation, and preservation of sedaDNAs remain only partially understood. Indeed, DNA preservation depends on multiple abiotic and biotic processes [Dell’anno et al. 2002; Boere et al. 2011; Torti et al. 2015; Capo et al. 2016; Giguet-Covex et al. 2019; Capo et al. 2021]. As a complement to the abovementioned results on the impacts of glyphosate on the coastal transition zone in Guadeloupe, we explored sedaDNAs to evaluate the temporal impacts on biodiversity.
The sedaDNAs of the core samples were extracted, and then a DNA library was prepared by amplifying the V7 region of the 18S rRNA gene, a universal eukaryotic marker. After sequencing the barcodes and filtering processes, we obtained 41,081 eukaryotic operational taxonomic units (OTUs; cutoff 95%) that were taxonomically assigned [Hervé et al. 2023]. Regarding the alpha diversity (i.e., the species diversity in a site at a local scale), we found that both OTU richness and diversity (the Shannon index and inverse of the Simpson index) were significantly higher in the FSR samples than in the SSR samples. Looking at the beta diversity (i.e., the extent of change in community composition or degree of community dissimilarity between samples), nonmetric multidimensional scaling (NMDS) analyses revealed that the FSR and SSR eukaryotic communities were different (Figure 2D) and that this difference was also significant [Hervé et al. 2023]. Additionally, we found significant associations between the ordination axis scores and both the terrigenous and pesticide fluxes (Figure 2D).
We investigated in more detail variations in specific eukaryotic clades, with a focus on three eukaryotic groups among the coastal biomes that presented significant temporal patterns of variation and played different ecological roles. As representative members of the organic matter decomposer community, we chose Ascomycota (Opisthokonta, Fungi). Bacillariophyta (commonly known as diatoms) and Chloropicophyceae (Archaeplastida) were selected as members of the carbon-fixing organisms. For these three groups of organisms, we found no significant variation in their temporal relative abundance or diversity indices during the SSR period [Hervé et al. 2023]. However, during the FSR period, we found a significant decrease in Ascomycota abundance and a significant increase in relative abundance for both Bacillariophyta and Chloropicophyceae relative abundance (Figure 3). Altogether, our data demonstrate that changes in land uses alter the rates and patterns of sediment transport associated with increases in the flux of pesticides, which in turn modify biodiversity in the coastal transition zone, at least in one location of the Petit Cul-de-Sac Marin, Guadeloupe, French West Indies (Figure 1) is also experiencing important anthropogenic forcing (i.e., increases in marine traffic, coastal urbanization, etc.).
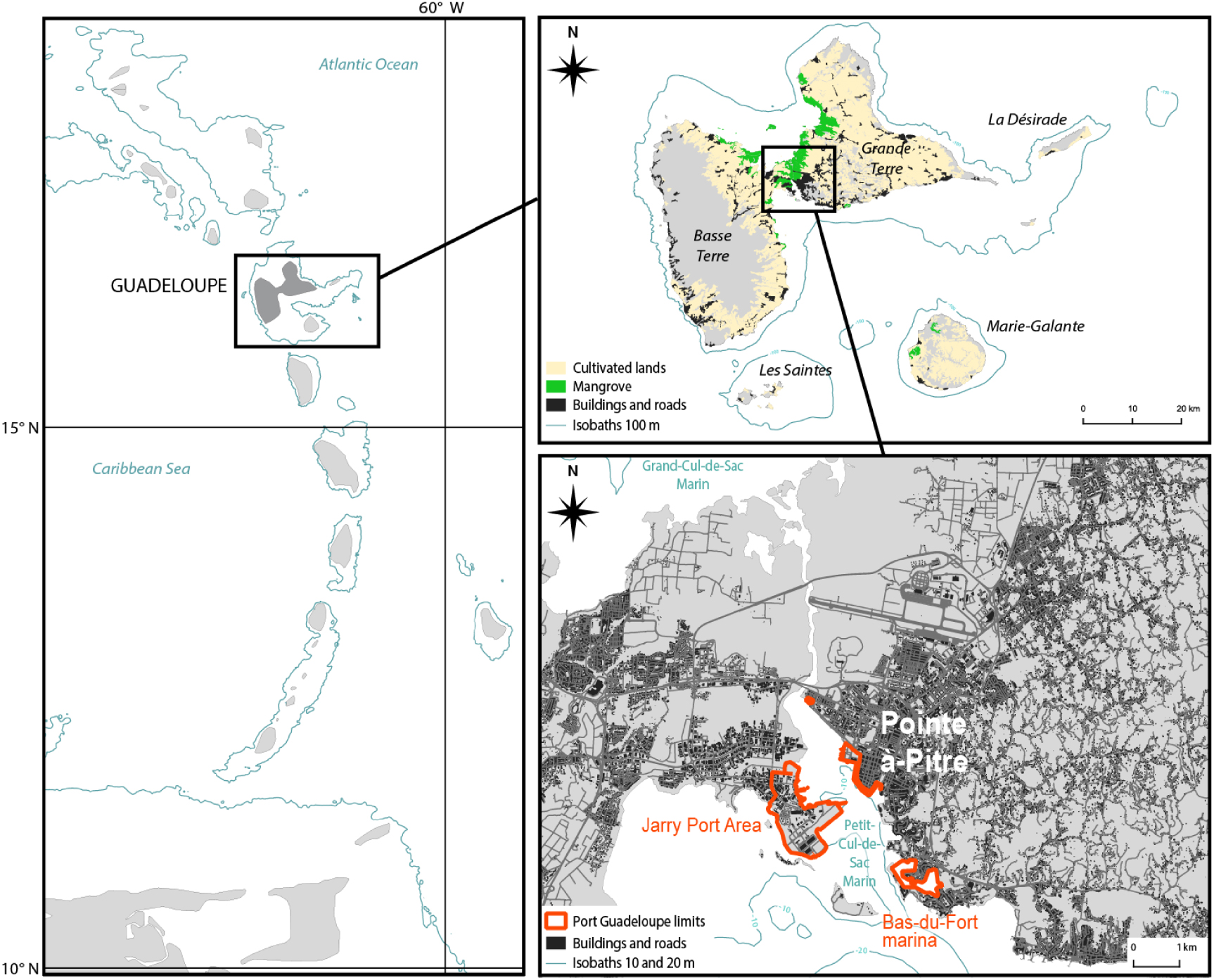
Maps of Guadeloupe. (Left) Location of Guadeloupe in the Lesser Antilles. (Top right) The Guadeloupe Archipelago. (Bottom right) Enlargement of Petit Cul-de-Sac Marin revealing the highly densified area and location of Guadeloupe Port Caraïbes. (BD Carto IGN, 2019; BD Topo IGN, 2019; GEBCO, 2020; TM World Borders, 2021.)
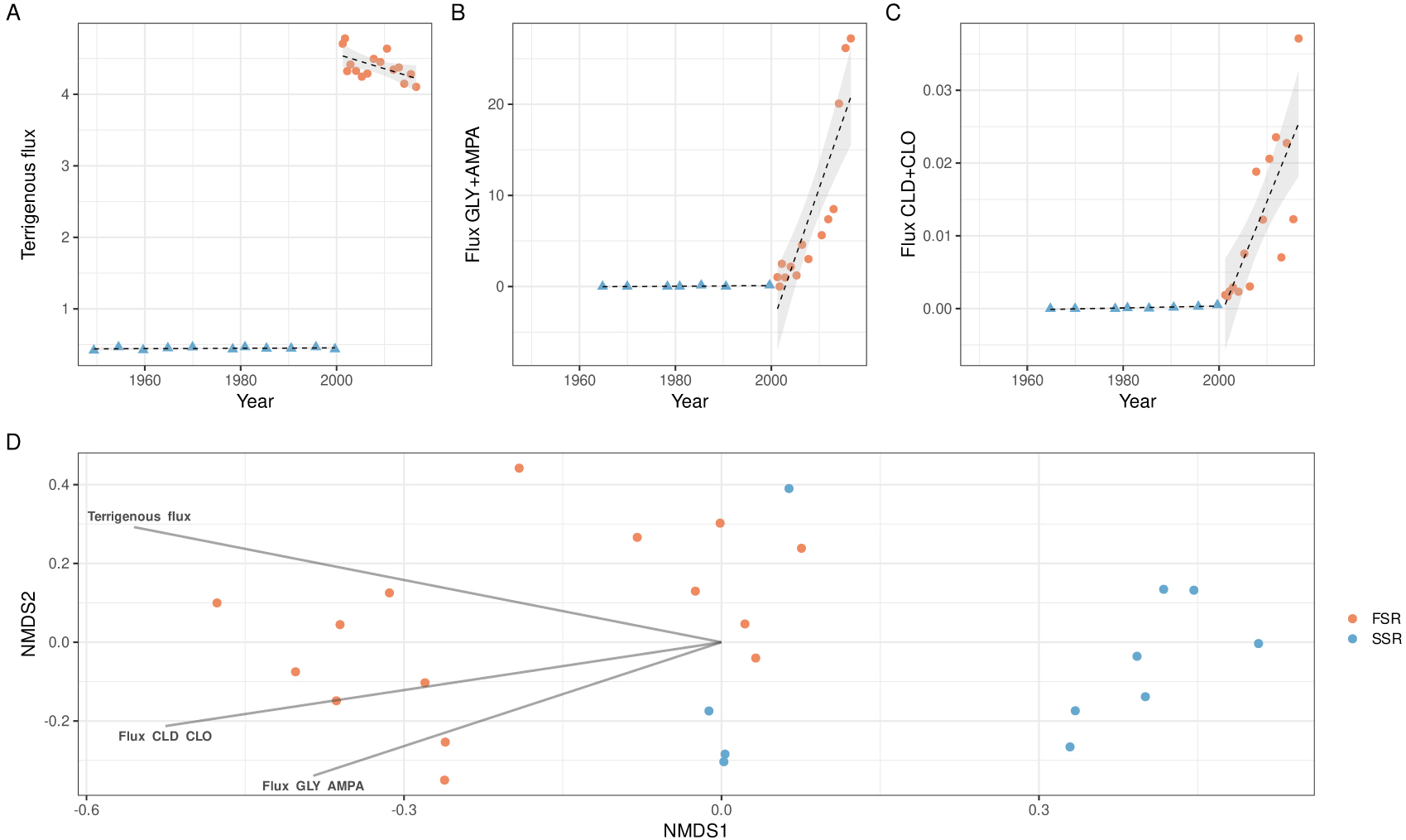
Changes in sedimentological data, pesticide flux and eukaryotic community composition in a core archive from a marine transition zone of Guadeloupe. (A) Terrigenous flux, (B) glyphosate (GLY) + aminomethyl-phosphonic acid (AMPA, a glyphosate primary degradation product) flux (in mol⋅cm−1⋅yr−1), and (C) chlordecone (CLD) + chlordecol (CLO, a chlordecone transformation product) flux (in mol⋅cm−1⋅yr−1). (D) Nonmetric multidimensional scaling (NMDS) ordination of the eukaryotic community composition based on Bray–Curtis distances. Arrows indicate significant correlations (p < 0.05) with environmental variables.
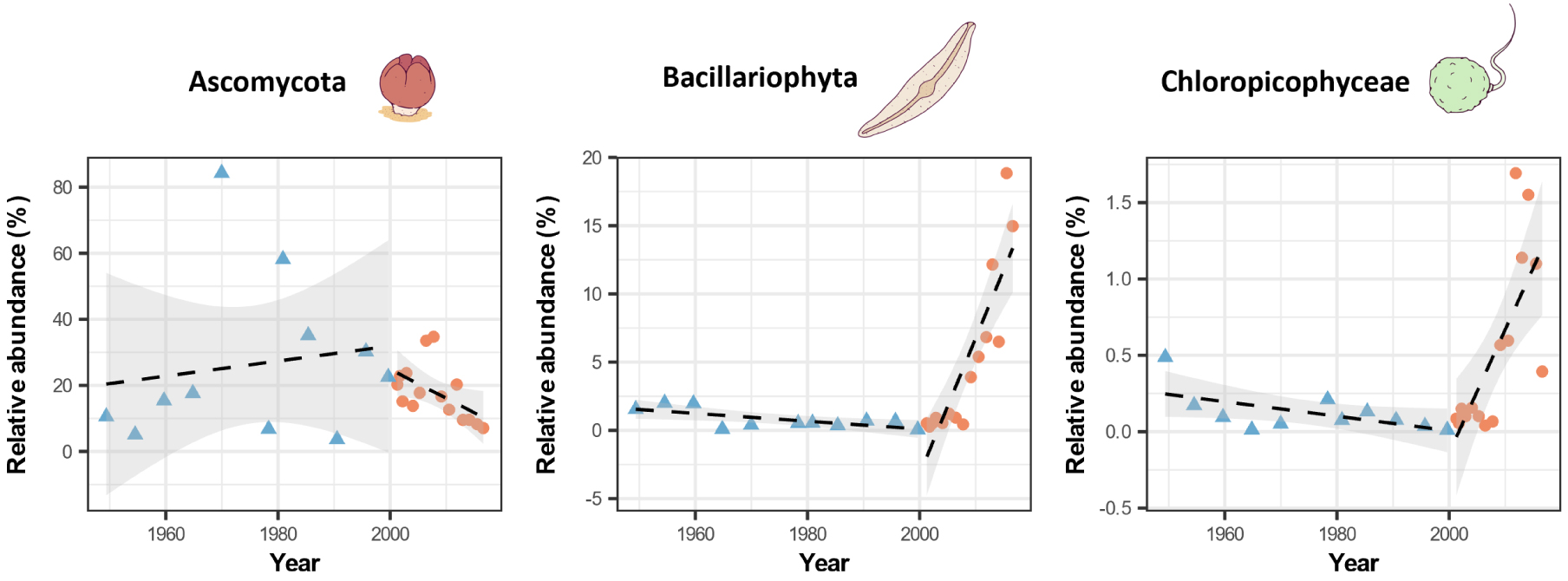
Temporal evolution of the relative abundance of some important eukaryotic clades over the SSR and FSR periods.
For many kinds of aquatic organisms [Tresnakova et al. 2021], it is known that exposure to glyphosate can alter the diversity and abundance of fungal [Druille et al. 2013; Van Bruggen et al. 2021; Vazquez et al. 2021] or diatom communities [Debenest et al. 2010; Vera et al. 2010; Larras et al. 2013; Corrales et al. 2021]. Interestingly, it was also shown that glyphosate can differentially affect the growth of marine diatom species, with some species being capable of using glyphosate as the sole phosphorous source [Wang et al. 2016]. Moreover, the impacts of glyphosate and its uptake and degradation processes by both prokaryotic and eukaryotic photosynthetic organisms are known to depend on physicochemical conditions and nutrient concentrations [Drzyzga and Lipok 2018; Lozano et al. 2018; Mesquita et al. 2021; Wang et al. 2022; Zabaloy et al. 2022]. Combined with our results, the latest information suggests that glyphosate and chlordecone can affect the composition and structure of coastal marine microorganism communities. Since photosynthetic organisms are directly involved in primary productivity and microbial decomposers in carbon and nutrient cycling, any alteration in their composition could potentially have long-term consequences on ecosystem functioning and stability as a result of changes in both green and brown food webs [Zou et al. 2016; Mougi 2020].
Interestingly, analyses of the diversity present in the core sample also revealed the presence of OTUs assigned to the brown algae Sargassum. Even if precise taxonomic assignation was rather difficult for these seaweeds because of the short length of the 18S rRNA gene amplified here and probably also because this genetic marker cannot resolve differences at the species level, we found a tendency toward an increase in their relative abundance over the most recent years. Over the period from 2012 to 2017, which corresponds to the appearance of stranding, the relative abundance of Sargassum OTUs tended to increase (Figure 4A). In fact, this relative abundance was significantly higher during the FSR than during the SSR period (Figure 4A). Additionally, among these OTUs, we detected 7 abundant OTUs that were specific either to the SSR period or the FSR period (Figure 4B). Further studies will be necessary to show whether it is possible to analyze the temporal evolution of Sargassum stranding based on sedaDNAs. However, such analyses will have to consider the potential contribution of the increase in the colonization of numerous coral reefs by algae, among which Sargassum can be an important actor [Thabard et al. 2011; Loffler and Hoey 2018]. Finally, there is also great interest in defining an appropriate molecular marker to resolve the Sargassum phylogeny, especially for the three pelagic Sargassum morphospecies, Sargassum fluitans III, S. natans I and S. natans VIII [Parr 1939; Schell et al. 2015; Amaral-Zettler et al. 2017; Martin et al. 2021], that are spreading in the Caribbean region.
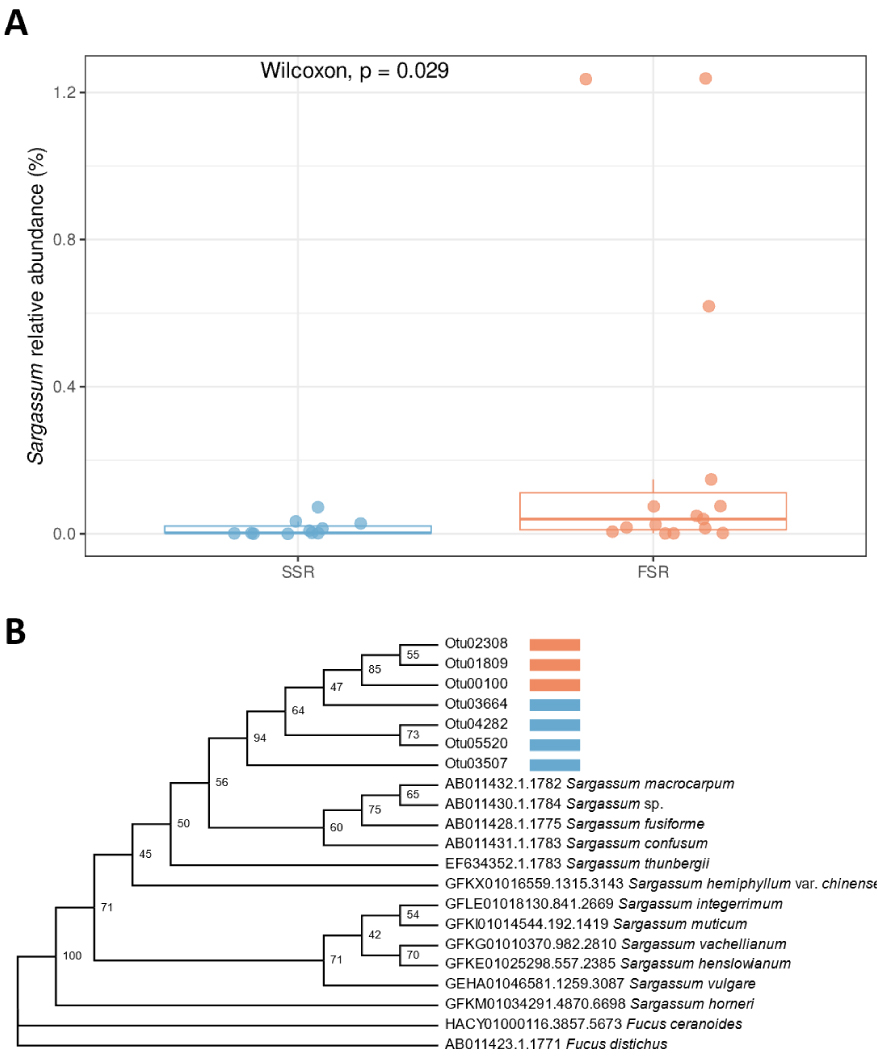
Abundance and diversity of Sargassum-related OTUs in sediment samples from the coastal transition zone in Guadeloupe. (A) Significant difference in the relative abundance of Sargassum between the SSR and FSR periods. (B) Maximum-likelihood tree showing the relationships between the Sargassum-related OTUs recovered from the Guadeloupe sediment (FSR period in orange and SSR period in blue) and other publicly available reference sequences of Sargassum species. This phylogenetic tree was inferred with IQ-TREE version 2.2.0 [Minh et al. 2020]. Evaluation of branch supports was computed using SH-aLRT (1000 replicates). Fucus species were used as outgroups. Masquer
Abundance and diversity of Sargassum-related OTUs in sediment samples from the coastal transition zone in Guadeloupe. (A) Significant difference in the relative abundance of Sargassum between the SSR and FSR periods. (B) Maximum-likelihood tree showing the relationships between the ... Lire la suite
3. The golden-brown crisis and emerging concerns
Since 2011, the Caribbean and West Africa coastlines have been subject to massive and episodic influxes of floating Sargassum seaweed [Wang et al. 2019]. The large amounts of seaweed biomass washed up along the coastlines have direct and indirect consequences for beaches [Williams and Feagin 2010; Innocenti et al. 2018; Rutten et al. 2021] and for the functioning and resilience of nearshore ecosystems [Van Tussenbroek et al. 2017; Cabanillas-Teran et al. 2019; Rodriguez-Martinez et al. 2019]. The mass stranding of these brown algae (Figure 5) also has significant negative impacts on livelihoods, public health, tourism, fisheries and the coastal economy [Resiere et al. 2018; Schuhmann et al. 2022].

Photographs of Sargassum along the coasts or at storage sites in Guadeloupe and Martinique. (A) Baie du Marigot (Les Saintes, Guadeloupe), (B) Sargassum spp., (C) Le Diamant (Martinique), (D) Le Marigot (Martinique), (E) Saint Félix (Guadeloupe), (F) Capesterre (Marie-Galante, Guadeloupe), (G) storage site of Pointe Faula (Martinique), and (H) storage site Capesterre, (Marie-Galante, Guadeloupe).
Members of the OHM Caribbean Coast associated with other colleagues from the University of Antilles were involved in an interdisciplinary project co-funded by the French agency for ecological transition (ADEME in French) (see the complete report),4 and dedicated to chemical and biological contamination issues related to Sargassum and to degrees of acceptability of their use in organic amendments. The aims were (i) to determine levels of contamination by heavy metals and CLD in Sargassum seaweeds, (ii) describe the eukaryotic and prokaryotic biodiversity associated with Sargassum spp., (iii) test the potential use of these algae in organic amendments, and (iv) reveal collective representations of social actors and of the population through diachronic and prospective approaches. Hereafter, we focus on contamination and biodiversity-related issues.
3.1. Heavy metals in Sargassum
The accumulation of heavy metals by Sargassum spp. has created concerns that the inland storage and disposal of these seaweeds might also impair high value subsidiaries such as agribusiness opportunities (i.e., fertilizer in crop production or food supplements for livestock production).5 Our project was carried out in both Guadeloupe and Martinique with sampling campaigns during 2018 and 2019, which correspond to periods with high levels of Sargassum stranding [Wang et al. 2019]. In 2018, we collected approximately 100 samples corresponding to Sargassum spp. from shore zones in Guadeloupe and Martinique, offshore sites and storage sites in Martinique (Figure 6A). Among the heavy metals and metalloids tested, we found that even if arsenic is only present at very low concentration in marine waters, it is bioaccumulated by Sargassum with average value of 78 mg/kg dry wet (Table 1). In fact, bioaccumulation of arsenic by pelagic Sargassum has also been demonstrated in other Caribbean regions and territories [Florez et al. 2017; Rodriguez-Martinez et al. 2020; Cipolloni et al. 2022; Devault et al. 2022; Ortega-Flores et al. 2022] and for various Sargassum species [Devault et al. 2021].
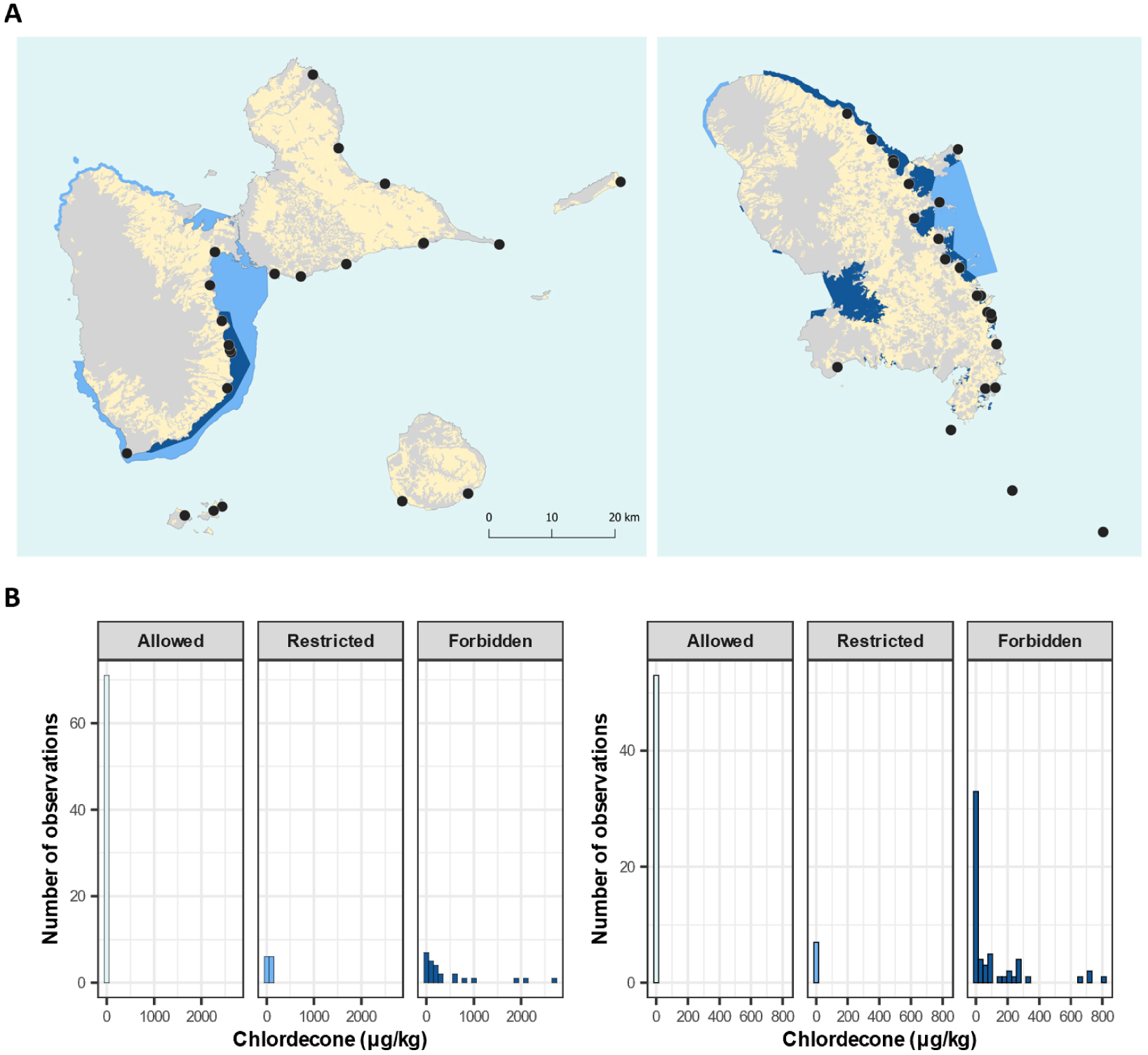
Maps showing the Sargassum sampling sites and chlordecone concentrations. (A) Some sampling sites from campaigns 2018 and 2019 overlapped. The Guadeloupe Archipelago (left) and Martinique Island (right). The color code corresponds to (yellow) cultivated lands, marine zones with no restrictions on fishing (light blue), marine zones with restrictions on fishing (medium blue), and marine zones where fishing is forbidden (dark blue) (BD Carto IGN, 2019). (B) Distribution of the chlordecone concentration (μg/kg) in Sargassum samples according to fishing area regulations, with the Guadeloupe Archipelago (left) and Martinique Island (right). Masquer
Maps showing the Sargassum sampling sites and chlordecone concentrations. (A) Some sampling sites from campaigns 2018 and 2019 overlapped. The Guadeloupe Archipelago (left) and Martinique Island (right). The color code corresponds to (yellow) cultivated lands, marine zones with no ... Lire la suite
Metal’s composition in Sargassum spp. collected in 2018 from Guadeloupe and Martinique
| Localization | na | Cd | Cr | Co | Cu | Hg | Ni | Znb | Pb | As | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coastal | Shore zone | 97 | 0.47 ± 0.19 | 11.6 ± 23.7 | 1.13 ± 1.44 | 4.20 ± 7.31 | <LOD | 3.91 ± 1.11 | 7.66 ± 7.45 | 0.51 ± 0.71 | 71.9 ± 28.4 |
| Offshore | 3 | 0.43 ± 0.05 | 4.04 ± 0.84 | 0.44 ± 0.07 | 3.73 ± 0.31 | <LOD | 4.91 ± 0.16 | 2.20 ± 0.40 | 0.13 ± 0.02 | 100.5 ± 4.9 | |
| Inland | Storage sites | 9 | 0.28 ± 0.07 | 11.5 ± 1.2 | 0.98 ± 0.12 | 5.24 ± 1.58 | <LOD | 2.54 ± 1.58 | 6.31 ± 1.77 | 1.11 ± 0.43 | 25.6 ± 15.9 |
The concentrations correspond to mg of metal per kg of dried Sargassum. Values are mean ± standard deviations.
a Number of samples analyzed.
b The results of the coastal shore zone for the Zinc correspond to 96 and not 97 samples since one of them was below the limit of detection (LOD).
To obtain further information on the putative effects of landing and drying processes, we performed analyses of arsenic species (i.e., inorganic and methylated forms) in Sargassum spp. but after the separation of seaweeds by location, with seaweeds from the shore, recently landed and dried (i.e., with a brownish color). We found a significant decrease in total arsenic concentration upon drying (Table 2). The decrease in total arsenic concentration was also significant for As(V) and MMA (Table 2). Even if we do not know the precise mechanisms underlying the decrease in Sargassum-associated arsenic concentration (i.e., direct or indirect leaching by rainfall or due to seaweed drying, etc.), our results warn us against the potential release of high concentrations of harmful arsenic species. Leaching and transformation into various compounds that might depend on complex physical, chemical, and biological processes can probably occur along the usage chain. These data reveal the need to further investigate the consequences of arsenic accumulated by Sargassum on coastal and terrestrial ecosystems, but also on the putative health concerns for populations. In addition, the presence of various arsenic forms in Sargassum spp. might also hinder several downstream uses [Devault et al. 2021].
Arsenic concentration in Sargassum spp. collected in 2019 from Guadeloupe and Martinique
| Localization | na | As total | As(III) | As(V) | MMA | DMA | AsBet | AsC | |
|---|---|---|---|---|---|---|---|---|---|
| Marine water | 59 | 96.1 ±24.8 | 1.00 ±2.87 | 65.2 ±23.6 | 16.0 ±12.8 | 5.56 ±7.16 | 4.59 ±3.63 | 0.47 ±0.73 | |
| Shore | Wet | 31 | 96.8 ±23.5 | 1.99 ±6.29 | 64.7 ±23.3 | 13.9 ±12.3 | 6.75 ±8.39 | 4.13 ±3.17 | 0.61 ±0.97 |
| Dry | 28 | 54.4 ±26.8 | 0.39 ±0.46 | 34.3 ±22.5 | 7.6 ±10.2 | 5.72 ±5.62 | 2.54 ±2.20 | 0.70 ±1.10 | |
The concentrations correspond to mg of metal per kg of dried Sargassum. Values are mean ± standard deviations.
a Number of samples analyzed.
The forms of arsenic are: arsenite (As(III)), arsenate (As(V)), monomethylarsonate (MMA), dimethylarsinate (DMA), arsenobetaine (AsBet), and arsenochlorine (AsC).
3.2. Pesticide contamination
Because of the presence of chlordecone in various compartments of the French West Indies, questions about the accumulation of this pesticide in Sargassum were raised in 2015. The first study performed at that time in Martinique demonstrated its presence in Sargassum samples.6
We further investigated contamination levels by CLD in Sargassum collected from coastal areas without or with restricted uses (Figure 6A). Indeed, since 2005, several prefectural decrees have led to the delimitation of coastal areas that are strictly prohibited from fishing a list of targeted fish, crustacean, mollusk and echinoderm species and others with partial protection. In both Guadeloupe and Martinique, CLD was found in samples from the watersheds of land used for banana cultivation (Figure 6B). In Guadeloupe, the highest concentrations were detected in sites that belong to the municipality Capesterre–Belle–Eau, where soils and the Pérou River are highly contaminated with chlordecone. In Martinique, the highest concentrations were found at “Quartier bac”, which belongs to the municipality La Trinité. Because of observed interannual variabilities, we propose that the authorities should implement more systematic analyses of CLD concentrations in Sargassum, at least for a number of local sites.
Contamination of Sargassum by other organic pollutants has been shown in the Caribbean with the presence of hydrocarbon compounds due to liquid oil spilling during the Deepwater Horizon disaster [Torralba et al. 2017]. Based on our findings, we suggest a more systematic investigation of the presence of labile, persistent organic pollutants (POPs) and the so-called contaminants of emerging concern (i.e., pharmaceutical and personal care products, etc.) in these seaweeds. Chemical contaminants of edible and nonedible seaweeds are becoming a general concern [Ma et al. 2018; Cherry et al. 2019; Anbazhagan et al. 2021; Hossain et al. 2022] that will also require the adjustment of legislation and development of relevant guidelines for their handling, storage, use and disposal.
3.3. Sargassum-associated biodiversity
Pelagic Sargassum constitutes a floating ecosystem hosting various marine species, such as sea turtles, seabirds, fishes and even invertebrates [Parr 1939; Weis 1968; Fine 1970; Dooley 1972; Ryland 1974; Haney 1986; Jobe and Brooks 2009; Ballard and Rakocinski 2012; Witherington et al. 2012; Jacobucci and Leite 2014; Susilowati et al. 2015; Monroy-Velazquez et al. 2019], with a few being endemic species. Concerning the less described microbial (i.e., archaea, bacteria and small eukaryotes) composition, it is likely that they also contribute to Sargassum growth, nutrient uptake, macroalgae spore release and germination, defense against pathogens or reproduction [Egan et al. 2013; Florez et al. 2017; Van Der Loos et al. 2019]. Exchanges between the Sargassum holobiont and the surrounding environment might have reciprocal impacts and might occur depending on the environmental conditions; for example, microorganisms initially involved in algae growth, raft formation and maintenance or algae sinking might later contribute to algae biodegradation upon beaching [De Fouw et al. 2016]. In addition, microbial communities associated with the seaweeds can proliferate in new habitats, as postulated for the export of surface-dwelling fauna associated with Sargassum from the surface to the seafloor.
At the molecular level, the investigation of the richness of microbial communities was mainly performed from surface drifting algae species [Torralba et al. 2017; Michotey et al. 2020]. We investigated for the first time the communities associated with Sargassum at beaches and storage sites [Hervé et al. 2021]. For this purpose, we amplified regions corresponding to the 16S rRNA gene for prokaryotes and to 18S rRNA for eukaryotes from 100 samples collected in 2018 and corresponding to seawater surrounding the seaweeds, Sargassum from beaches (e.g., along the shores and landed) or storage sites. Concerning microbial richness, we identified 22,214 prokaryotic operational taxonomic units (OTUs) and 17,679 eukaryotic OTUs. Among them, we found that a large number of both prokaryotic and eukaryotic OTUs were shared between these three compartments (Figure 7A–B). Nevertheless, statistical analyses demonstrated that these three communities were different [Hervé et al. 2021].
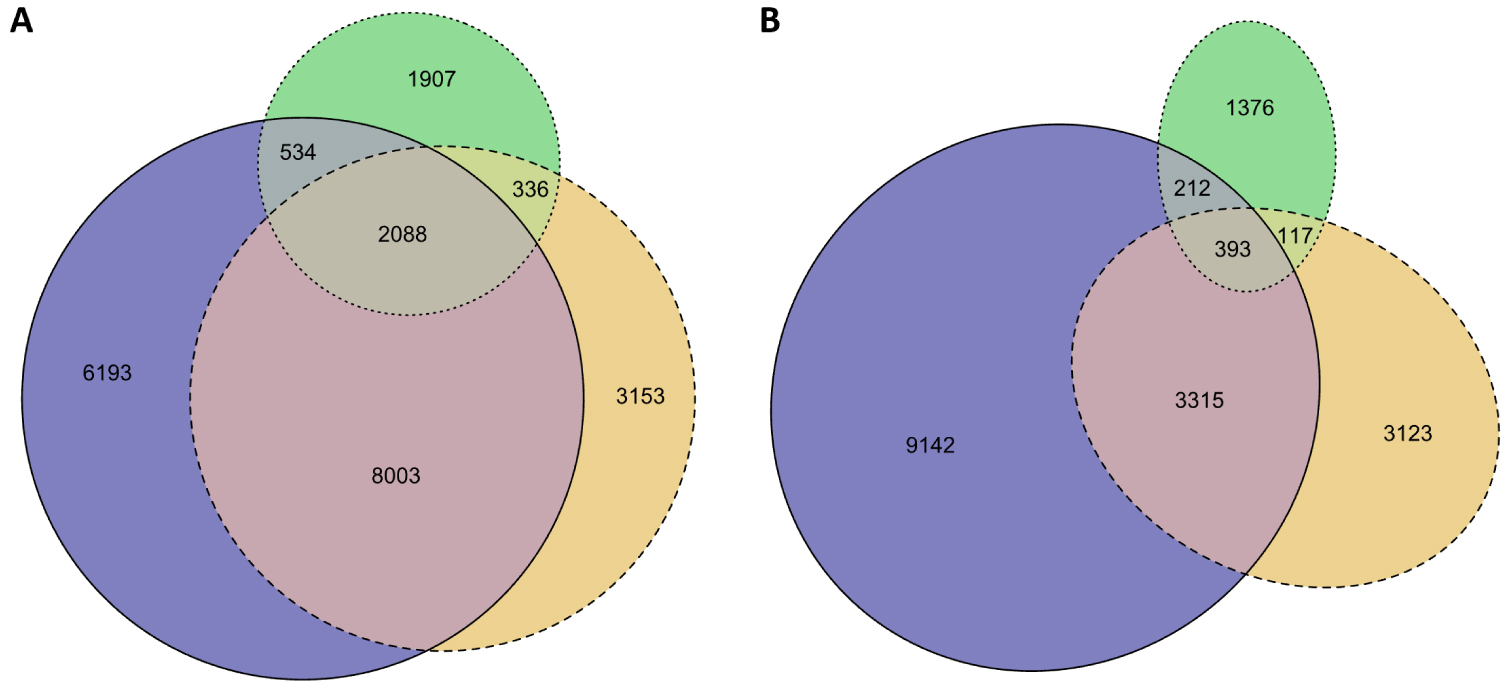
Venn diagrams of Sargassum-associated prokaryotic or eukaryotic OTUs. (A) Prokaryotes and (B) Eukaryotes. The color code corresponds to seawater surrounding Sargassum (blue), stranding Sargassum on the shore (orange), and Sargassum from storage sites (green).
Interestingly, among the prokaryotes, we found a large number of bacterial OTUs that are probably involved in sulfur metabolism. From inland storage sites, we detected methanogenic archaea, which suggests that a portion of the carbohydrates from the seaweed alga is probably degraded and used as a potential energy source by these prokaryotes [Hervé et al. 2021]. Further specific investigations of this microbial diversity could probably facilitate the development of biogas production and energy conversion procedures. We also found that the Sargassum-associated microbiota might contain potentially harmful and nonnative species that are probably co-transported by Sargassum. If confirmed, such results may have direct implications for risk assessment and reinforce the need to develop further studies on Sargassum holobionts, including those focused on biosafety and biosecurity.
4. Observation for knowledge and awareness
An important role of the OHM Caribbean Coast is also to promote and disseminate research findings to local stakeholders and the population, including for the teaching sphere and the younger generations. Indeed, we believe that even if there is probably in Guadeloupe, following the suggestions of Trevethan [Trevethan 2017], a continuum on environmental concerns from general awareness knowledge (GAK) to detailed and specific knowledge (DSK), there might be considerable personal heterogeneity in awareness within the population that must be analyzed and documented for both the consequences of pesticide use and Sargassum influxes. In addition to scientists, social networks and local media play important roles in the construction of GAK. In a first step to analyze the putative differences between scientific information and public media, we compared here the number of scientific publications mentioning the words “biodiversity”, “arsenic”, “glyphosate”, “Sargassum” or “chlordecone” to the French equivalent from the local media of France Antilles (we pooled both the Martinique and Guadeloupe editions). Using the SCOPUS database and searches of article titles, abstracts and keywords, we found an increasing number of publications (Figures 8A–C) except for chlordecone, which presented a more erratic pattern of publication with peaks in the mid-seventies corresponding to contamination issues at Hopewell and since 2010 due to issues in the French West Indies (Figure 8D). In contrast, in the local media, the interest was rather different, with a high number of publications focusing on Sargassum (Figure 8G) and chlordecone (Figure 8H) and barely any reports on arsenic or glyphosate (Figure 8F). For Sargassum, we found two peaks of publication in 2015 and 2018 corresponding to years with high levels of stranding, and potentially to the presence of an international conference on Sargassum that occurred in Guadeloupe during the year 2018. However, it will be interesting to study in more detail the link between press release on environmental concerns and other social issues such as political events.
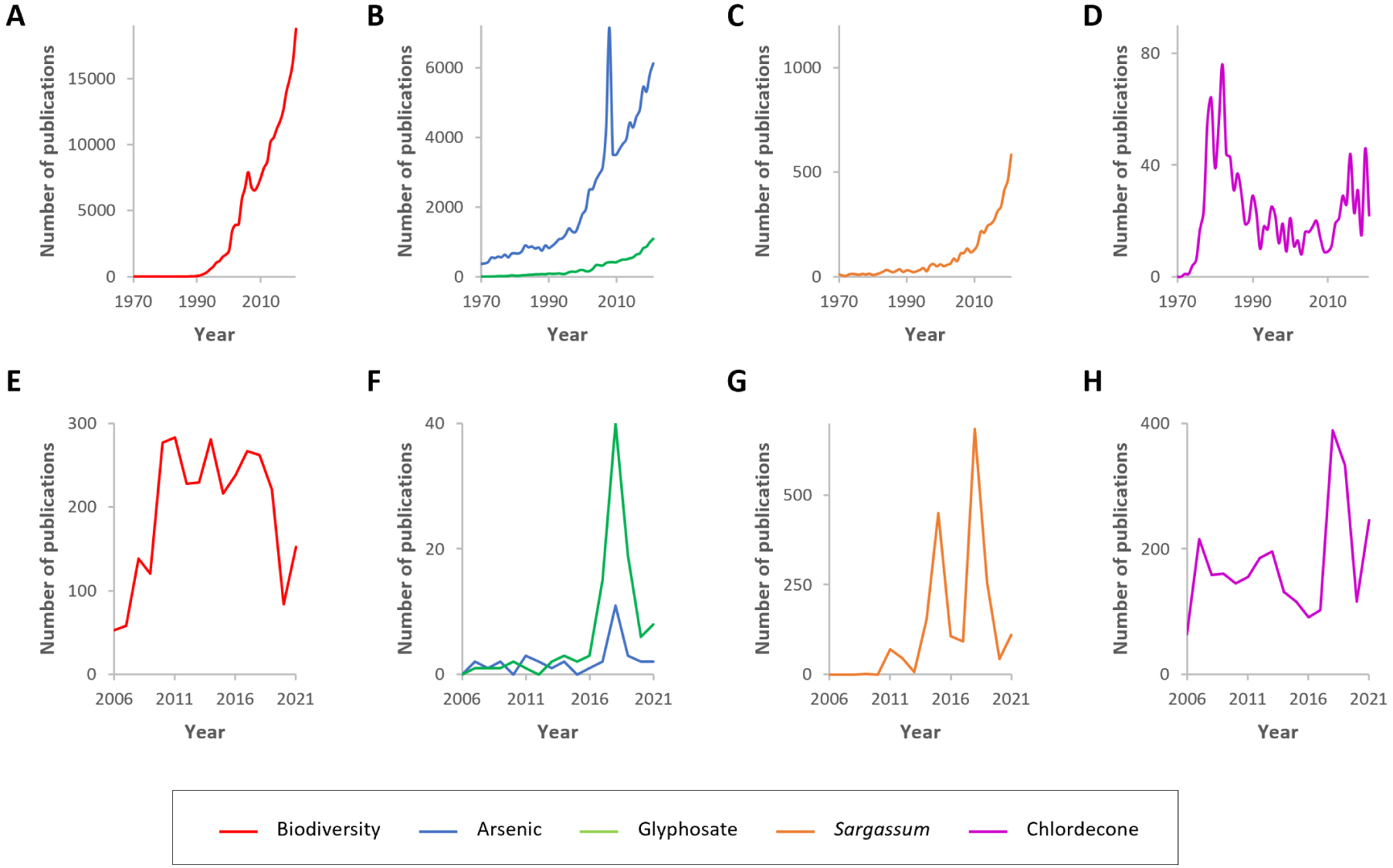
Environmental concern reports in the scientific literature and media of France Antilles (Guadeloupe and Martinique). Graphs correspond to searches of the SCOPUS database for 1970–2021 (A–D) or the France Antilles database for 2006 to 2021 (E–H). For arsenic, the exceptionally high number of publications from 2008 is likely related to the Second International Congress titled “Arsenic from Nature to Humans” of 2008, which accounted for 1492 conference papers published during this year. This survey was performed on October 2022.
Even if these analyses are still very partial, they illustrate the differences between scientific publications, local media and most likely general public awareness. Such an observation reinforces our motivation as an observatory to further develop actions to diffuse knowledge and scientific information on contaminants along the land-ocean continuum. In the future, we hope to further analyze public awareness, concerns and priorities by comparing various publication supports completed through surveys and interviews. We therefore hope to be able to gain insight into the sensitivity of the local populations and to draw comparisons to people from other European nations in regard to, for example, marine environmental concerns [Gelcich et al. 2014].
5. Conclusions
The most recent “Sargassum crisis” that started with the first stranding of Sargassum in 2011 has created new cross-contamination issues between seaweed, the coastal zone and inland storage sites by heavy metals (i.e., arsenic) and organic pollutants (i.e., chlordecone). Analyses of other pollution drivers within the coastal transition zone have revealed dependencies not only between past and current agricultural uses but also with soil erosion and the composition of coastal eukaryotic assemblages. We should also develop more theoretical approaches to evaluate social concerns, as proposed by Fitzgerald [Fitzgerald 2019], to properly address the multiple causalities, causal asymmetries, and most likely equifinality of environmental pollution concerns. We hope that the OHM Caribbean Coast, which uses a long-term interdisciplinary framework, will properly investigate social environmental trajectories, envision integrated solutions and bring awareness to local populations.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Acknowledgments
We would like to thank France-Antilles for allowing access to their publication database. We would like to thank the Labex DRIIHM; the French program “Investissements d’Avenir” (ANR-11-LABX-0010), which is managed by the ANR. This work was supported in part by the Agence de l’environnement et de la maîtrise de l’énergie (ADEME). The authors thank the anonymous referees for their helpful comments that improved the quality of the manuscript. We are also grateful to our colleagues working within the OHM Caribbean Coast, and to Oscar for his support.
1 See the OHM “Littoral Caraïbe” at https://ohm-littoral-caraibe.in2p3.fr/.
2 This study is currently being extended to better evaluate the impregnation levels of the populations (https://www.chlordecone-infos.fr/sites/default/files/documents/impregnation-chlordecone-kannari.pdf).
3 https://www.generations-futures.fr/cartes/carte-ventes-de-glyphosate-ha-de-sau-departement-2017/.
4 Eco3Sar: Ecologie, Ecotoxicologie et Economie des Sargasses. https://librairie.ademe.fr/recherche-et-innovation/4039-eco3sar-ecologie-ecotoxicologie-et-economie-des-sargasses.html.
5 https://guadeloupe.ademe.fr/sites/default/files/guide-valorisation-sargasses.pdf.





 CC-BY 4.0
CC-BY 4.0