1 Introduction
Attempts to predict the response of the biota to projected future climate change can be aided by study of past analogues. The most recent period of dramatic climatic fluctuation, with net global warming, was the transition from the last glaciation to the present interglacial (LGIT), between approximately 25,000–10,000 calendar years ago and corresponding to the later part of Marine Isotope Stage (MIS) 2 and the early part of MIS 1 (Fig. 1; [24]). The period encompasses the generally very cold Greenland stadials 3 & 2, ca. 22.5–14.7 ka, including the poorly-defined ‘Last Glacial Maximum’; the warm Bølling/Allerød (Greenland interstadial 1) ca. 14.7–12.6 ka; the cold Younger Dryas (Greenland stadial 1) ca. 12.6–11.5 ka; and the Early Holocene from ca. 11.5 ka.
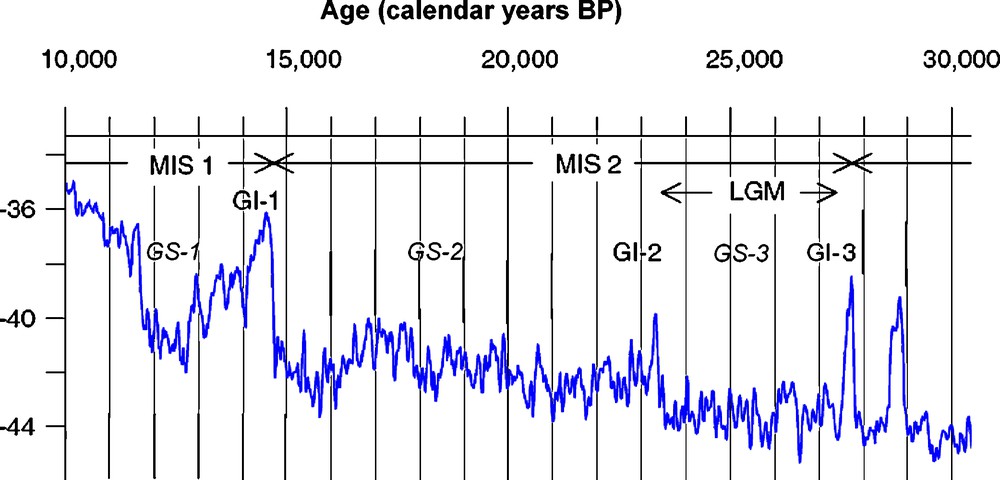
Climate curve for the interval 30–10,000 years ago. The curve shows the δ18O record for the NorthGRIP on the GICC05 timescale (modified after Svensson et al. [24]). Vertical axis = δ18O in ‰. MIS = Marine Isotope Stage; GS = Greenland stadial; GI = Greenland interstadial; LGM = Last Glacial Maximum. GS-1 = Younger Dryas; GI-1 = Bølling + Allerød. The LGM has traditionally been defined as the time of maximum globally integrated extent of ice-sheets, but is now generally restricted to Greenland Stadial 3 [24].
Fig. 1. Courbe de climat pour l’intervalle de temps 30–10 000 ans. La courbe montre l’enregistrement δ18O pour le NorthGRIP sur l’échelle de temps GICC05 (modifié d’après [24]). Axe vertical = δ18O, en ‰. MIS = stade isotopique marin ; GS = stade Groenland ; GI = interstade Groenland ; LGM = Dernier Maximum Glaciaire ; GS-1 = Dryas récent ; GI-1 Bølling + Allerød. Le dernier maximum glaciaire a été traditionnellement défini comme l’époque de l’extension maximum globalement intégrée de la couche de glace, mais est à présent en général réduite au stade Groenland 3 [24].
The changes in terrestrial ecosystems through this time period are becoming increasingly well known in many parts of the world. This paper focuses on northern Eurasia, and in particular on the response of large mammals to climate change, mediated to a significant degree by changes in regional vegetation.
The mosaic steppe-tundra vegetation of northern Eurasia during much of MIS 4–2 was replaced, during the LGIT, by tundra in the far north and forest further south, although the process was a complex one. The effects on large mammal species were dramatic: some (such as red deer Cervus elaphus,) expanded in range; others (such as reindeer, Rangifer tarandus) shifted their range to areas that were in part previously uninhabitable; many (such as horse Equus ferus) saw a massive contraction of their range, but survived; and a final category went extinct either during the LGIT (e.g., woolly rhinoceros Coelodonta antiquitatis) or during the Holocene (e.g., woolly mammoth Mammuthus primigenius and giant deer Megaloceros giganteus). In our NERC-funded project on Late Quaternary extinctions in northern Eurasia, we focus on large-mammal species of northern Eurasia that became extinct either globally (e.g. mammoth, woolly rhinoceros), or in the northern Eurasian realm (e.g. lion). Patterns of range change are reconstructed by mapping radiocarbon dates made directly on remains of the target species from across their range. We incorporate both published dates, carefully audited for accuracy of determination and reliability of dating; and many new AMS dates undertaken at the Oxford radiocarbon Accelerator Unit as part of our project.
2 Range shifts in response to climate change
In earlier papers [22,23] we highlighted two taxa, woolly mammoth Mammuthus primigenius and giant deer Megaloceros giganteus, illustrating some features of the response of mammalian taxa to rapidly fluctuating climate change, and the complex distributional changes leading ultimately to extinction (Fig. 2). Woolly mammoth, common across much of Europe and northern Asia during MIS3, retreated during the interval 21–19 ka, surviving (probably in reduced numbers) in northern Siberia, and in Europe only in the western Central Russian Plain. Although this interval is within the very cold Greenland stadial 2, the factors reducing the range of mammoths in this particular 2000-year interval are unclear. The giant deer, although the data are less abundant, appears to have vacated Europe for a much longer period – between about 28–15 ka. Its refugial location during this period is unclear, and is the subject of current investigation. However, as a more temperate-adapted animal, stadial conditions clearly affected it to a greater extent. The earlier return of mammoth to Europe during the still cold and open conditions around 19 ka, in particular, contrasts with the giant deer which did not return until the warming at the onset of the Late Glacial interstadial (GI-1) starting around 15 ka.
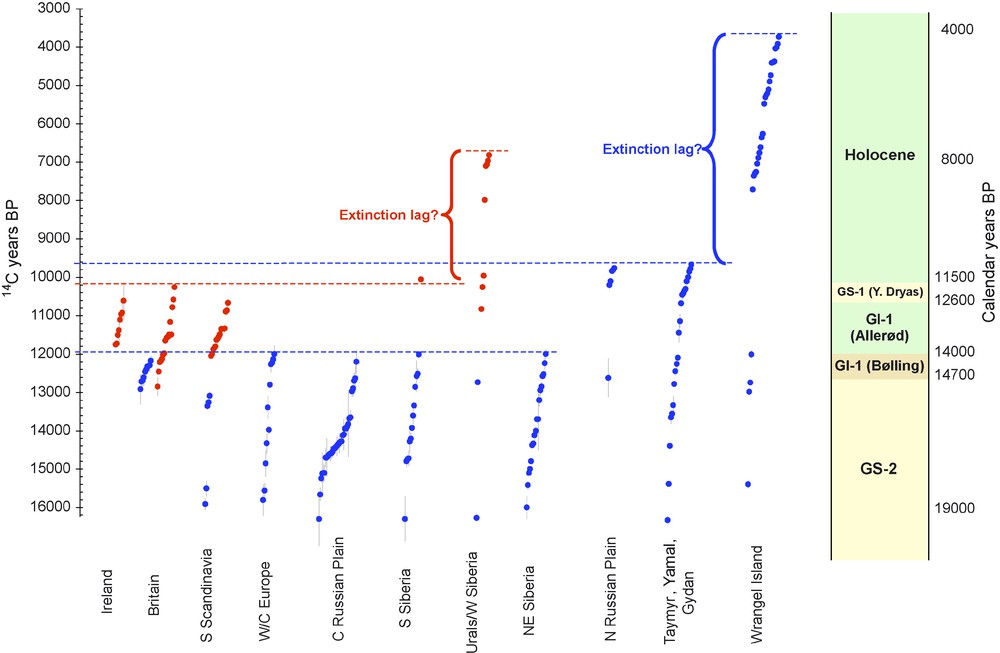
Radiocarbon record of Eurasian Mammuthus primigenius and Megaloceros giganteus for the interval 19–4 ka, modified from Stuart et al. [23]. Red dots = radiocarbon dates + /1 s.e. for M. giganteus; blue dots = the same for M. primigenius. Points are plotted uncalibrated on radiocarbon timescale (left axis), but an approximate timescale in calendar years is shown, based on IntCal04 (right axis). M. giganteus shows contraction of range within the Younger Dryas (GS-1) to currently only known Holocene refugium in Urals/W. Siberia until extinction ca. 8 ka; M. primigenius shows two-stage contraction at ca. 14 ka and ca. 11 ka to refugium on Wrangel island until ca. 4 ka.
Fig. 2. Enregistrement radiocarbone des Mammuthus primigenius et Megaloceros giganteus eurasiens pour l’intervalle de temps 19–4 ka, modifié d’après [23]. Taches rouges = datations radiocarbone + /1 s.e. pour M. giganteus ; taches bleues = la même chose pour M. primigenius. Les points sont réunis non calibrés sur l’échelle de temps radiocarbone (axe gauche), mais une échelle de temps en années calendaires est présentée, basée sur IntCal04 (axe droit). M. giganteus montre une réduction de son aire géographique, au cours de Dryas récent (GS-1), au seul refuge connu de l’Holocène dans l’Oural/Sibérie occidentale, jusqu’à son extinction à 8 ka ca ; M. primigenius montre une réduction de son aire en deux étapes à 14 et 11 ka environ, jusqu’au refuge de l’île Wrangel à 14 ka environ.
The subsequent history of these two species in Europe further highlights how species’ responses to the same environmental forcing factors vary according to their individual ecologies. As trees returned to central and northern Europe during the Allerød ca. 13.4–12.6 ka, the mammoth disappeared, while the giant deer flourished, albeit in restricted areas of North-West Europe. Then, as cold, open vegetation returned in the Younger Dryas (GS-1) ca. 12.6–11.5 ka, the giant deer was extirpated while the mammoth briefly re-expanded its range from Siberia into Northeastern Europe [23].
These changes illustrate the fluidity of species’ ranges on timescales of a few hundred years or less, but also the modulation of the species’ response by their differing habitat tolerances. In microcosm this reflects an ‘individualistic’ rather than ‘community’ response of species to environmental change, demonstrated for much larger sets of species in the Late Pleistocene of North America [9]. The result, in turn, is the constant shifting of community composition in a succession of non-analogue faunas. Lyons [17], in a new analysis of the Faunmap N. American dataset, shows that the result is somewhere in between the classic ‘Gleasonian’ (individualistic) and Clementsian (community integrity) models, as some groups of ecologically-related species do move approximately in concert, but with inevitable changes in community composition.
3 Loss of adaptive flexibility
Detailed morphological studies, and the advent of population-genetic approaches to Late Pleistocene faunas through the analysis of ancient DNA (aDNA), are demonstrating that the contraction of species ranges and abundances in the Late Pleistocene in some cases led to loss of genetic diversity and, probably, adaptive flexibility. An aDNA analysis of lions, for example [6], has shown that the extirpation of lions across all of Europe, northern Asia and North America at the end of the Pleistocene represented the loss of a mitochondrial clade distinct from all surviving Holocene lions (Panthera leo), corroborating the suggestion from morphology and Palaeolithic art [20] that the Holarctic population was a separate taxon (Panthera spelaea) probably adapted to the northern biome and now extinct.
Similarly, Dalén et al. [7] showed that for the arctic fox (Alopex lagopus), Late Pleistocene populations in low latitudes did not ‘migrate’ northward with the Holocene warming, but simply died out in situ, leaving existing higher-latitude populations to form the modern range, but with correspondingly reduced overall genetic diversity of the species.
For the mammoth (Mammuthus), the loss of more temperate-adapted populations (late M. trogontherii) after ca. 200 ka left the cold-adapted Late Pleistocene woolly mammoth (M. primigenius) more vulnerable to episodes of warming and afforestation [15]. A preliminary population-level aDNA study [3] indicates further loss of variation from two initial mitochondrial clades of M. primigenius in Siberia, one apparently disappearing before 40 ka, while only the second persisted to 12 ka and beyond.
The realisation that Late Quaternary forms, while closely-related to modern taxa, were distinctive and have now disappeared, has raised taxonomic issues, such as the status of Panthera spelaea in relation to P. leo [20]; or the Holarctic Late Pleistocene to Early Holocene Bison priscus in relation to living N. American B. bison [19]. Where there is evidence that the pairs were reciprocally monophyletic (i.e. their mitochondrial clades formed non-overlapping sister-groups), species status is defensible; or we may adopt the terminology of conservation biology and regard them, at the very least, as ‘Evolutionarily Significant Units’. The effect of recognising such populations as having had a unique and coherent evolutionary trajectory is to increase the perceived magnitude of the Late Quaternary extinction event.
4 From range contraction to extinction
Although bolide impacts [8] and ‘hyperdisease’ (see [18]) may have contributed to regional extinction events, the dominant causal factors for Late Quaternary extinctions, considered on a global scale, remain climatic and vegetational change on the one hand, and human hunting and disturbance on the other [4]. The persistent problem of the temporal coincidence of these factors, even when viewed on detailed time scales, is notable. For example, massive range contraction of the woolly mammoth was almost certainly climate-driven [23], yet the demise of the last mammoth population on Wrangel Island was roughly coincident with first record of palaeohunters on the island [1]. The last recorded occurrence of giant deer in its West Siberian refugium, ca. 8 ka BP, was at a time of both vegetational change and the arrival of the Neolithic [23]. Pending further evidence, it is plausible to regard such conjunctions not as ‘problems’ preventing us from identifying one of the two factors as causal, but as evidence in favour of a combined model whereby species only become extinct when subjected simultaneously to both climatic and human pressures [11,13,21].
The existence of major, climatically-driven range changes, from the LGM onwards, can therefore be seen as part and parcel of the extinction process, whether or not humans were also involved. For both Mammuthus and Megaloceros, a series of major range changes are evidenced through a period of up to 20,000 years before their final extinction (Fig.2). From this point of view, extinction is seen not as an instantaneous event - the loss of the last population or even individual - but as an extended process of net range reduction over thousands or tens of thousands of years. On the other hand, such range shifts and contractions have undoubtedly happened during previous Quaternary cold-warm cycles, and did not lead to extinctions on this scale, presumably because species survived in refugia and re-expanded on the return of favourable conditions. The proximal causes of the demise of the final refugial populations – human or environmental – therefore remain fundamental to explaining Late Quaternary extinctions. Thus, both the longer-term process of range contraction and the extirpation of the terminal populations are necessary to explain extinction – neither is sufficient explanation by itself.
5 Terminal refugia and extinction debt
As suggested by Koenigswald [14], the initial contraction of a species from an originally broad range often represented a retreat to its ‘core’ area, where it had survived during previous unfavourable episodes. The contraction of European warmth-loving species such as straight-tusked elephant (Palaeoloxodon) or interglacial rhinoceros (Stephanorhinus) to the Mediterranean region after the Last Interglacial; or of Mammuthus to northern Siberia after 14 ka, are examples. Further contraction, into much smaller refugia, would have been more unpredictable and influenced by complex regional and local factors. However, the identification of these terminal refugia, and of events within them, is clearly critical for determining the final causes of extinction.
The pinpointing of such refugia and defining their geographical extent are difficult within the limitations of palaeontological data. The revelation that mammoths had survived into the Middle Holocene on Wrangel Island [25] was sensational. However, a detailed survey of the mammoth's originally vast range is virtually impossible, so it should not have been surprising when a second mid-Holocene refugium, on the Pribilof islands [10] came to light. Similarly, the discovery of a Holocene refugium for the giant deer (Megaloceros) in the eastern Ural region [23] was made essentially by chance during a programme of radiocarbon dating, but this relatively rare species remains poorly-sampled except in North-West Europe, so the existence of further Holocene refugia is entirely plausible.
However, even if further very late refugia are discovered, the overall pattern, at least for Mammuthus primigenius and Megaloceros giganteus, is clear: a formerly extensive range contracted over time into one or more very small refugial areas, and when these disappeared the species was extinct (Fig.2). It is notable that, in the case of these species at least, the refugial populations survived for a considerable length of time after the collapse of the species’ main range. In the case of the woolly mammoth, the large majority of the range collapsed around 14 ka; some mainland populations remained until ca. 11 ka on current evidence; but the Wrangel population persisted for a further ca. 6,000 years before dying out. Similarly, giant deer never regained its former range after the LGM, and appears to have been severely restricted after 12 ka, but survived for at least a further 3,000 years in its western Siberian refugium.
This interval between major range collapse, and extirpation of the terminal populations, is similar to the ‘extinction lag’ posited for modern populations surviving in relict patches of habitat following major disturbance [5] (Fig.2). In turn this has led to the concept of ‘extinction debt’ – species consigned to small, vulnerable populations being regarded as essentially doomed to extinction in the near future [16]. As was probably the case in the Late Quaternary, and even more so in today's anthropogenically altered world, such populations are extremely sensitive to changes in critical biotic, abiotic and human factors. Thus, Barnes [2] showed that the likelihood of extirpation of isolated populations of elephants in West Africa through the 1980s, when poaching and habitat destruction were at an unprecedented level, was strongly inversely correlated with the size of the habitat patch containing the population.
Whether a species can survive changing environments depends on many factors, but the ability to expand or shift its range into newly emerging areas of suitable habitat may often prove critical [12]. Mammal species in the Late Quaternary were able to shift their ranges over large distances relatively rapidly [9,17,23]. However, as illustrated by the Late Pleistocene history of the giant deer (Megaloceros), they did not always succeed in occupying all available areas of apparently suitable habitat, nor of re-occupying formerly inhabited areas on the return of apparently favourable conditions following an interval of range restriction. Whether a species was able to do so would have depended on a complex interplay of factors including their population density in the source area, dispersal rate in relation to speed of habitat change, and the environment of the target area, especially the presence of competitors or predators, including humans. In cases where the source and target ranges are separated by intermediate areas of unsuitable habitat, the dispersal of a species from one to the other may prove difficult or impossible. Although hard to demonstrate palaeontologically, this may well have been the case for terminal populations of Late Quaternary species, and it will almost certainly be a major problem for species faced with anthropogenically-induced ecosystem shifts in the future.
Acknowledgement
This work was supported by the Natural Environment Research Council, grant NE/D003105/2.


