1. Introduction
Halogens can provide key insights into magmatic processes ranging from partial melting to volcanic eruptions [Webster et al., 2018] if their behaviour is fully understood from fluorine to iodine. However, iodine is the least investigated halogen in magmatic and volcanic processes, in relation with its lowest abundance both in magmas [Kendrick et al., 2012, 2014] and in volcanic plumes [Aiuppa et al., 2005]. It is nonetheless an important element as it impacts atmospheric chemistry through ozone depletion [Solomon et al., 1994], having the largest ozone-depleting efficiency [Cuevas et al., 2022].
As for most elements, iodine speciation controls its solubility, transport, and eventual elemental and isotopic fractionation between coexisting phases. Progresses have been made towards the understanding of its transfer between reservoirs, especially between magmas and aqueous fluids to assess the extent of its degassing [Bureau et al., 2000, Leroy et al., 2019], but not on its speciation in natural magmas. There are besides no available in situ data on iodine speciation in silicate melts, i.e. under high temperature (T) and high pressure (P) conditions. Available data on iodine speciation in silicate glasses have been obtained using Raman, X-ray photoelectron and/or X-ray absorption spectroscopies on borosilicate melts quenched from high P–T conditions [Cicconi et al., 2019, Morizet et al., 2021], in order to understand and better predict iodine behaviour and eventual mobility in nuclear waste glasses. Consequently, there is a lack of data on iodine speciation in natural magmas in general, and in particular at the high P–T conditions at which they form and ascend.
Here, we report in situ high P–T synchrotron X-ray diffraction data (XRD) on basaltic magmas, and Raman spectroscopy data on recovered quenched glasses. High P are necessary to dissolve sufficient amount of iodine so that its effect on magmas properties can be measured, but more importantly because arc magmas that are produced at greater depths hence greater pressures than oceanic ridge basalts are the most relevant to investigate iodine speciation, due to recycling of marine sedimentary components that bring iodine to the arc magma source [Muramatsu and Wedepohl, 1998].
The choice of Saint Vincent island (Lesser Antilles arc) basalt [Pichavant et al., 2002] and of Mount Etna basalt (from 2002/2003 South scoria [Gennaro et al., 2019]) was guided by the need to reflect a range of volatile-rich basalts, with high MgO Saint Vincent basalt being representative of one type of primary magma in subduction zones, and Mount Etna alkali basalt representative of later stage basalt differentiated through fractional crystallization and degassing.
2. Materials and methods
2.1. Glass synthesis
Starting natural basalt samples were ground, doped with NaI as iodine source, and with deionised milli-Q water added in the case of Saint Vincent basalt. When investigating the local environment of a trace element in a magma, one must reach a compromise between lowest amount possible and detection above noise level to avoid interaction between iodine ions that would occur for elevated concentrations. Two iodine levels were targeted, circa 3 wt% for the Saint Vincent basalt, and circa 1 wt% for the Etna basalt. Since iodine solubility increases with P, I-doping was done at 3.5 GPa and 1600 °C for the Saint Vincent basalt using platinum capsules welded at both ends, and at 1 GPa and 1350 °C for the Etna composition using gold–palladium capsules which have a lower T-stability but prevent Fe loss to the capsule unlike for Saint Vincent basalt that became almost FeO-free. Having two different FeO content turned out to be essential in assessing iodine local environment in the melt (cf. Section 3.2). For both compositions, I-free glasses were synthesized under the same conditions. High P–T conditions were generated by a Depth of the Earth piston cylinder press using a half inch talc-pyrex cell assembly with a graphite heater; T was monitored using a W/Re thermocouple, run duration at high T was one hour.
2.2. High P–T X-ray diffraction experiments
The recovered glass from piston cylinder press experiments was extracted from the platinum or gold–palladium capsule, crushed, and loaded in either graphite capsule (Saint Vincent basalt) or in single crystal diamond capsules with inner graphite caps and sealed under P by Pt–5%Rh caps (Etna basalt). High P–T conditions (Table 1) were achieved using a Paris-Edinburgh press with cell-assembly (Figure 1) as described in Yamada et al. [2011]. This cell-assembly is designed to optimize the sample signal by using low absorbing materials along the X-ray path (boron epoxy and hBN windows inside the MgO ring outside the graphite heater), while ZrO2 parts away from the X-ray path insure cell-assembly stability at high P–T conditions. Talc powder was added on top and bottom of graphite capsules, to act as fO2 buffer. Temperature was calculated from power-T curve calibrated against melting temperatures of salts [Kono et al., 2014], and P was calculated from the cell volume of MgO cylinder surrounding sample capsule [Kono et al., 2010]. Uncertainties on P and T are respectively 0.3 GPa and 80 °C. While most runs were carried at T above the liquidus, two experiments were run between solidus and liquidus T (TGH25 and TGH26).
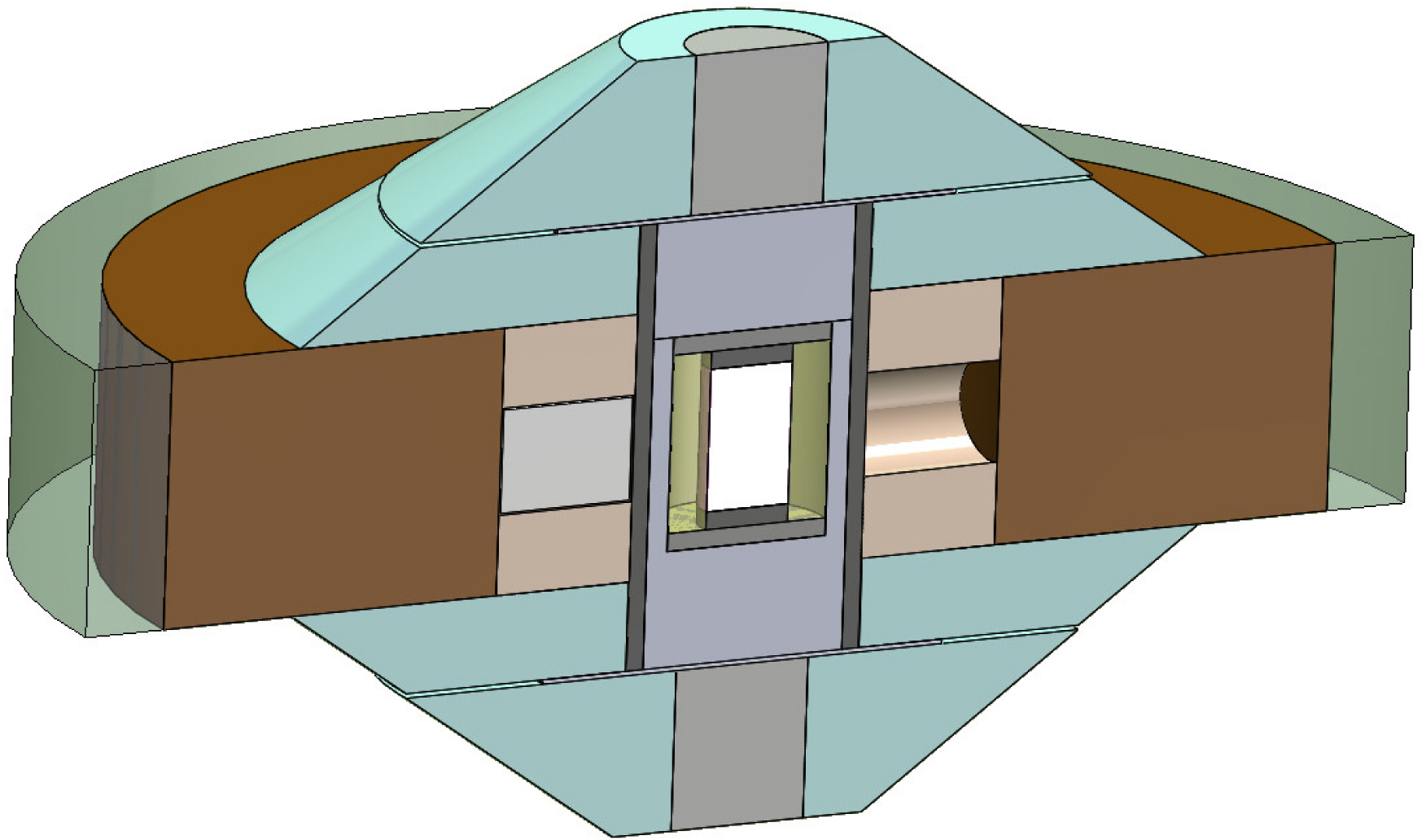
Sketch of the cell-assembly used for in situ high P–T X-ray diffraction experiments [Yamada et al., 2011]. Sample (inner white rectangle) is packed either in a graphite capsule or in a single crystal diamond cylinder (as drawn here, inner diameter: 1.0 mm), covered by inner graphite caps and sealed by Pt–Rh caps.
Chemical analyses (wt%). Starting and recovered samples
| Sample | SiO2 | TiO2 | Al2O3 | FeO | MgO | CaO | Na2O | K2O | I | Total∗ |
|---|---|---|---|---|---|---|---|---|---|---|
| P–T conditions | (Standard deviations) | |||||||||
| Saint Vincent basalt (glass), starting and recovered samples from XRD experiments | ||||||||||
| PC82∗∗ | 46.91 | 1.37 | 15.16 | 0.95 | 11.58 | 10.58 | 2.98 | 0.50 | 2.97 | 96.59 |
| 3.5 GPa-1600 °C | (0.51) | (0.27) | (0.09) | (0.15) | (0.18) | (0.08) | (0.58) | (0.04) | (0.22) | |
| PC83 | 47.95 | 1.36 | 15.57 | 1.24 | 12.33 | 10.94 | 2.84 | 0.46 | 2.90 | 95.59 |
| 3.5 GPa-1600 °C | (0.92) | (0.10) | (0.47) | (0.13) | (0.19) | (0.34) | (0.18) | (0.07) | (0.14) | (1.28) |
| APS run 13 | 47.16 | 1.30 | 15.42 | 0.70 | 12.42 | 10.78 | 2.55 | 0.38 | 2.26 | 93.2 |
| 4.7 GPa-1600 °C | (0.86) | (0.07) | (0.40) | (0.10) | (0.23) | (0.18) | (0.11) | (0.09) | (0.19) | (1.13) |
| APS run 26 | 51.14 | 1.14 | 16.52 | 0.58 | 12.97 | 11.57 | 2.41 | 0.48 | – | 97.06 |
| 4.9 GPa-1600 °C | (0.93) | (0.11) | (0.32) | (0.15) | (0.16) | (0.27) | (0.11) | (0.05) | – | (0.72) |
| Etna basalt (glass), recovered samples from XRD experiments | ||||||||||
| APS Etna2002 | 49.38 | 1.92 | 18.35 | 3.86 | 5.91 | 11.38 | 3.63 | 2.24 | – | 96.91 |
| 1.0 GPa-1250 °C | (0.46) | (0.15) | (0.40) | (0.37) | (0.14) | (0.25) | (0.16) | (0.24) | – | (0.79) |
| APS 20BaM19 | 46.79 | 1.94 | 17.31 | 3.88 | 6.88 | 11.49 | 3.82 | 2.06 | 0.80 | 95.20 |
| 1.3 GPa-1120 °C | (0.66) | (0.10) | (0.29) | (0.04) | (0.10) | (0.31) | (0.13) | (0.13) | (0.09) | (0.81) |
| Etna basalt (glass+clinopyroxenes), recovered samples from X-ray diffraction (XRD) experiments | ||||||||||
| APS TGH25 glass | 49.73 | 2.00 | 19.20 | 7.04 | 3.55 | 8.40 | 4.73 | 2.91 | – | 97.85 |
| 3.7 GPa-1450 °C | (0.97) | (0.05) | (0.28) | (0.34) | (0.09) | (0.32) | (0.17) | (0.21) | (1.19) | |
| APS TGH27 glass | 50.30 | 1.85 | 19.31 | 3.52 | 4.35 | 7.55 | 4.76 | 3.09 | 1.15 | 96.23 |
| 3 GPa-1550 °C | (0.58) | (0.11) | (0.54) | (0.23) | (0.16) | (0.17) | (0.21) | (0.19) | (0.10) | (0.17) |
∗ Note that ‘Total’ does not include water content, that varies from 1.6(0.3) to 3.8(0.5) wt% as measured only for PC83 and PC82 respectively Leroy et al. [2019].
∗∗ Data from Leroy et al. [2019]. Note that Etna basalt samples were recovered from XRD experiments still embedded in their diamond capsule, hence high quality polishing could not be achieved.
In situ high P–T experiments were conducted using energy-dispersive XRD on beamline 16-BM-B at the Advanced Photon Source (Argonne, USA). The incident beam was collimated by tungsten slits (0.3 mm vertical × 0.1 mm horizontal) and the diffracted signal was collected by an energy-dispersive germanium solid-state detector. In the molten state, X-ray diffraction data were collected at different 2𝜃 angles (2°, 2.7°, 3.5°, 5°, 7°, 10°, 15°, 20°, and 27°) thus covering up to 15 Å−1 in q-space (q = 4πE sin𝜃/12.398, where E is the energy of the X-rays in keV ranging up to 125 keV).
The multi-angle energy dispersive X-ray diffraction spectra were converted into the structure factor S(q) using analysis software package (aEDXD) program developed by Changyong Park [Kono et al., 2014]. The real-space radial distribution function, g(r), that described the short-order range structure (i.e. interatomic relations within 5–6 Å) was obtained by Fourier Transform of the spline smoothened S(q):
| (1) |
2.3. Starting and recovered samples analyses
Samples were polished for textural analyses using a Zeiss Ultra 55 field emission scanning electron microscope (SEM) at OSU Ecce Terra, Sorbonne Université, followed by chemical analyses (Table 1) carried at the Camparis center, Sorbonne Université, using a Cameca SX-FIVE electron probe microanalyser (EPMA) with accelerating voltage set at 15 keV, beam current at 4 nA, and a defocussed beam (7 μm radius).
Raman spectra were recorded on a Jobin Yvon Horiba HR460 spectrometer using a single-grating monochromator with 1500 gratings/mm and an argon laser (514.5 nm wavelength).
3. Results
3.1. Quenched texture and composition of starting and recovered glasses
Nanosize iodine droplets are observed on Saint Vincent basalt starting glasses (PC82 and PC83, Figure 2a), and could either be quench products or due to iodine oversaturation as those samples have the highest iodine content (Table 1). Such nanodroplets are not observed in other samples, including Saint Vincent glass recovered from XRD experiment which moreover was conducted at higher P (APS run 13) than during piston-cylinder press synthesis, i.e. at conditions of higher iodine solubility. Iodine was thus fully dissolved in molten basalts probed by XRD.
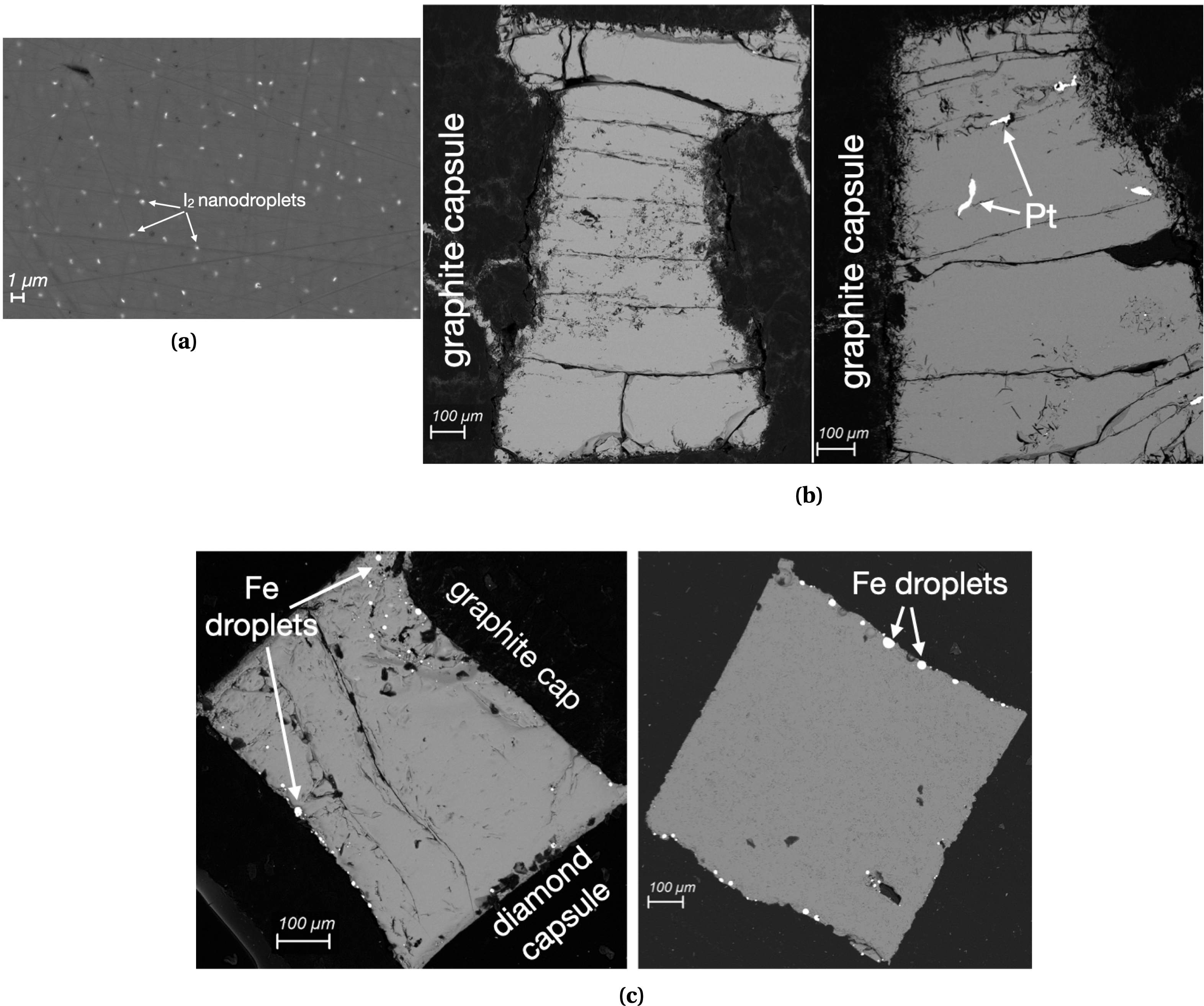
SEM images of quenched glasses recovered from piston-cylinder runs and in situ high P–T X-ray diffraction (XRD) experiments. (a) Starting sample PC82 (Saint Vincent basalt, I-doped). (b) Saint Vincent basalt in graphite capsule recovered from XRD experiments (left: run 13, I-doped, right: run 26, I-free). (c) Etna basalt recovered from XRD experiments (left: 20BaM19, I-doped, in diamond capsule, right: Etna2002, I-free, extracted from diamond capsule). Bright zones are either I2 droplets (a), Pt bits inherited from the previous piston-cylinder run using Pt capsules (b right), or metallic Fe droplets (c). Masquer
SEM images of quenched glasses recovered from piston-cylinder runs and in situ high P–T X-ray diffraction (XRD) experiments. (a) Starting sample PC82 (Saint Vincent basalt, I-doped). (b) Saint Vincent basalt in graphite capsule recovered from XRD experiments (left: ... Lire la suite
Recovered Etna basalt samples from XRD experiments, either I-doped or not, contain droplets of metallic iron (Figure 2c), indicating reduction from FeO. This is not observed in recovered Saint Vincent glasses, as those contained very little FeO in the starting glass (less than 1 wt%, Table 1), and remained homogeneous (Figure 2b).
3.2. Melt structure: X-ray diffraction
To investigate the effect of iodine on melt structure, both I-doped and I-free basaltic melts were probed. In case of co-existence of melt and crystals, the press was moved relative to X-ray beam position until area free of crystals could be probed. Amongst the three pairs of I-doped/I-free runs (Table 1), two provided sufficiently high quality data to extract radial distribution functions (APS runs 13/26 and APS TGH25/27, Figure 3). The structure factor, S(q), of the I-doped melts have a weaker first-sharp diffraction peak (highest intensity near 2 Å−1 on Figure 3 left panel) compared to the I-free melts, indicative of a lesser degree of medium-range order [Salmon, 1994], in other words, a lesser degree of polymerisation. APS runs 13 and 26 have different hydration levels in the starting samples (1.6 vs 3.8 wt% H2O), which also contributes to a lesser degree of depolymerisation in I-doped APS run 13. However Etna basalts were not hydrated prior to XRD experiments, but are similarly impacted by the presence of iodine.
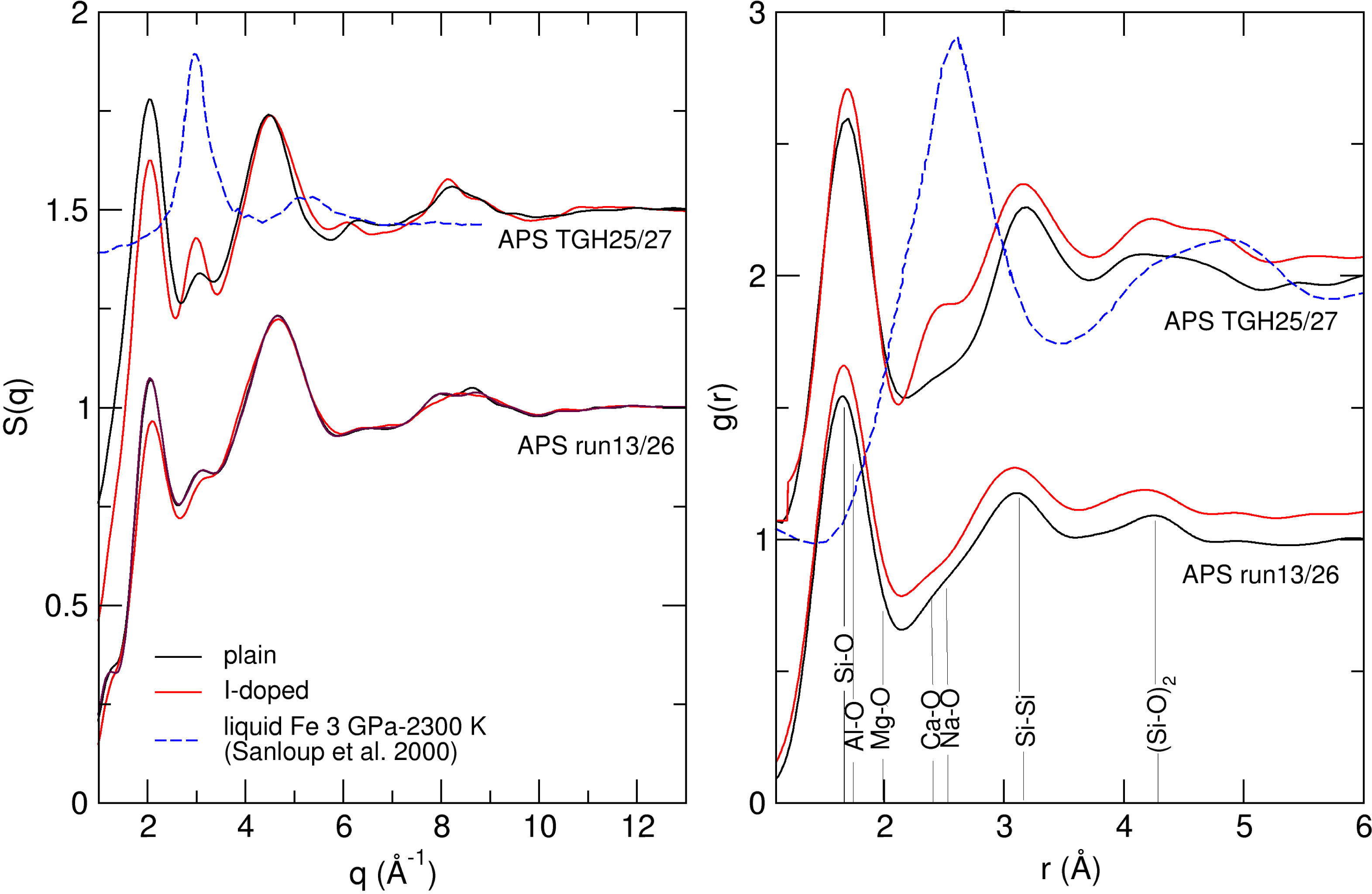
Right panel: structure factors, S(q); left panel: radial distribution functions, g(r).
The effect of iodine on the radial distribution function g(r) (Figure 3 right panel) is less pronounced. The small contribution near 3 Å−1 on S(q) for run APS TGH27 stems from dispersed Fe droplets in the magma, and translates into a contribution peaking at 2.5 Å−1 on g(r), consistently with reported XRD data on molten Fe [Sanloup et al., 2000] and with the observation of Fe droplets on recovered samples (Figure 2c). Interestingly, we do not observe this contribution of molten Fe for the I-free run APS TGH25, attesting that reduction of Fe was less extensive in the I-free basalt (Table 1). Diffusion of hydrogen through the Pt–Rd caps, and/or diffusion of C from the inner graphite caps, can not be excluded as other causes of Fe reduction, but the difference between I-free and I-doped samples is significant.
To better evidence the contribution of iodine atoms to the radial distribution function, we used the APS run13/26 datasets, that are not impacted by the contribution of molten Fe to the XRD signal. The difference between reduced radial distribution functions, G(r) = 4πr𝜌(g(r) − 1), for I-doped and I-free basalts was calculated after normalisation of g(r) to the Si–O contribution (Figure 4). Note that while this procedure enables to evidence interatomic distances, it is not sufficient to calculate accurate coordination numbers. To do so, the full g(r) should be simulated against the sum of all partial pair distribution functions, but this is challenging for such small differences. Two interatomic distances are visible at 2.2 Å and at 3.5 Å (Figure 4), and due to the normalisation procedure to the Si–O contribution, we cannot exclude that distances shorter than 2 Å also exist. These contributions are either I-related or enhanced contributions due to the presence of iodine. XRD is sensitive to the electrons, the intensity of the signal evolves with Z2 (Z, atomic number). Iodine being a very heavy element, its scattering is 4 times stronger than that of Fe, and 14 times stronger than that of Si. Hence the likeliest possibility is that differences on radial distribution function between I-doped and I-free basalts are due to iodine, and not to other elements even if their abundances may vary slightly. The potential contribution of Fe–O nevertheless needs to be discussed, as its contribution to g(r) in a basalt is at 2.07 Å and 3.4 Å (Fe–O and Fe–Fe interatomic distances respectively [Guillot and Sator, 2007]), all other main interatomic distances being different (see position of main cation-oxygen interatomic distances on Figure 3). However, it cannot be the case since FeO content is similar between I-free and I-doped Saint Vincent basalt, and this content is lower than 1 wt% hence the expected contribution is very low. These additional contributions in I-doped G(r) can neither be attributed to an eventual P difference between I-doped and I-free basalts. The maximum P (4.9 GPa) reached in these experiments can induce changes of coordination number for some cation-oxygen bonds, but it is way too modest to induce a contraction of interatomic distances. Na–O for instance contracts by 0.02% between 0 GPa and 5 GPa [Karki et al., 2018], all other main cation-oxygen distances change even less.
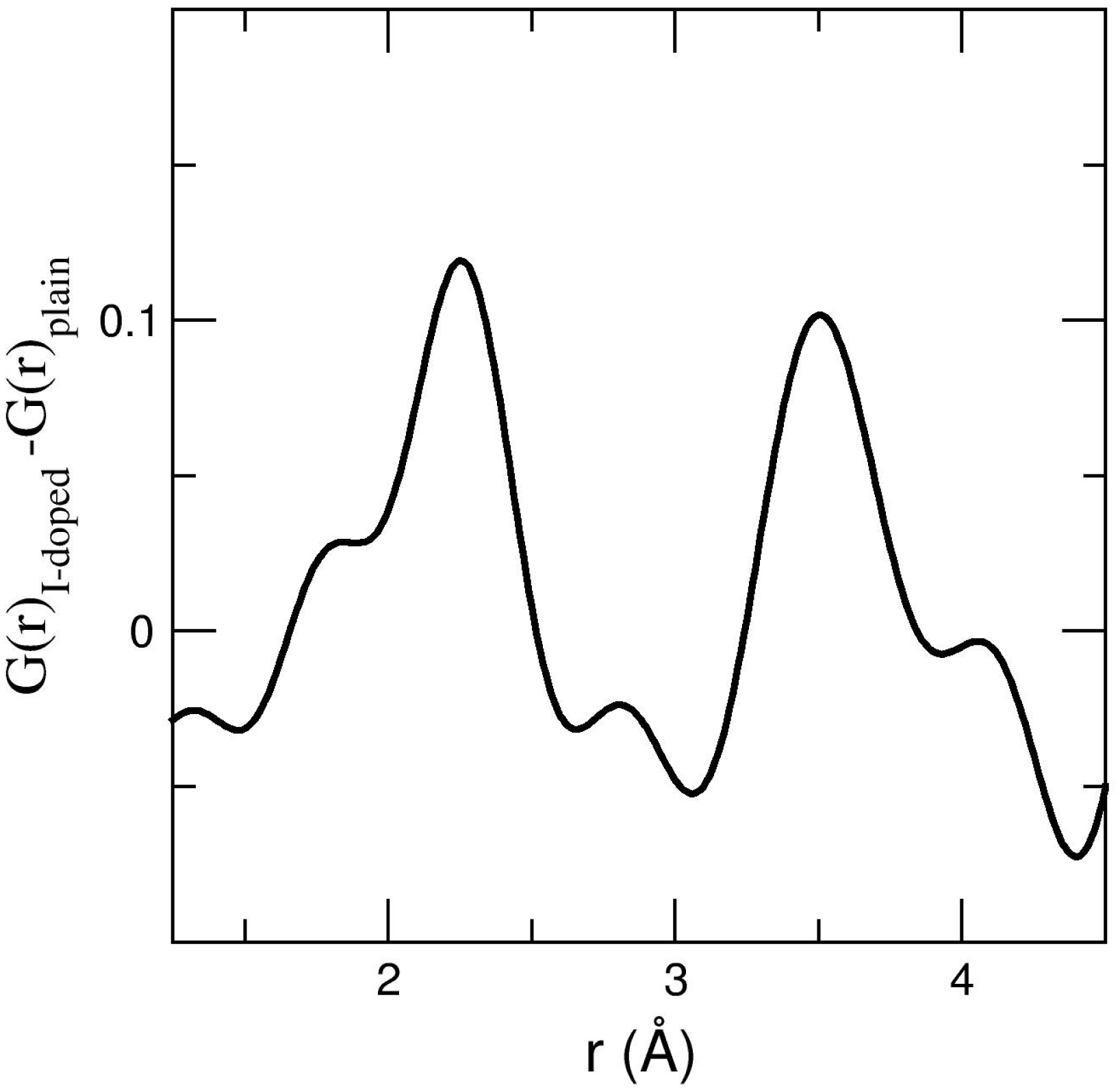
The iodine contribution to the reduced G(r) radial distribution function, as obtained from the difference between APS run 13 and APS run 26 XRD datasets.
Amongst reported I–X bonds for iodine compounds, the 2.2 Å distance is shorter than iodine–iodine (2.7 Å) or iodine–metal bonds (3.2 Å–4.0 Å), and closer to iodine–oxygen bonds reported for crystalline iodates (1.8–2.2 Å range), the longest I–O bonds corresponding to medium intramolecular bonds with a single covalent bond character [Gautier-Luneau et al., 2010, Abudouwufu et al., 2020], indicative that we are not looking at
3.3. Melt structure: Raman spectroscopy
Some starting and recovered samples were analysed by Raman spectroscopy (Figure 5). We note that it is difficult to obtain Raman spectra on these natural compositions due to an inherent high level of fluorescence, and in particular in the intramolecular water range (e.g. [2800–3600] cm−1). Iodine–iodine signature is visible on starting sample PC82, with bands at 114 cm−1 and 154 cm−1, consistently with the observation of nano-size iodine (I2) bubbles by SEM (Figure 2a), bands that are also observed in the I-richest borosilicate glass in Cicconi et al. [2019] and were attributed to NaI bonds but seem more consistent with I2 signal. There is no visible iodine–iodine nor metal–iodine contribution on any spectra measured on samples recovered from XRD experiments. Instead there is the systematic presence of four small but clear vibrational bands in I-doped samples at 615 cm−1, 647 cm−1 (this one on top of a broader band by comparison with I-free samples), 1098 cm−1, and 1177 cm−1. Interestingly, there is also a broad band circa 640–660 cm−1 in I-doped borosilicate glasses (Figure 5), albeit not discussed by the authors. While we are most likely observing oxidized iodine in these glasses, it is not in the form of
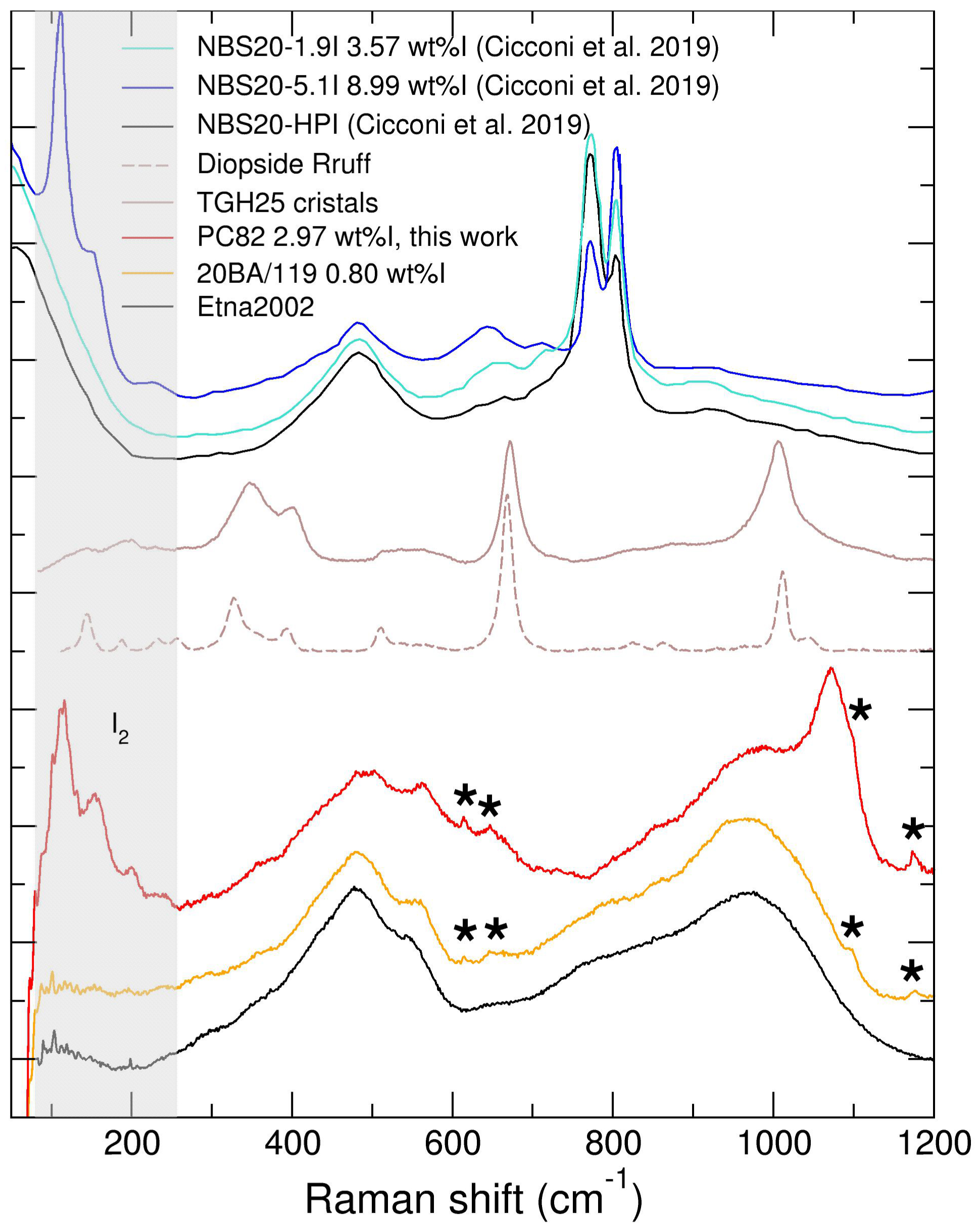
Raman spectra collected on an iodine over-saturated sample (PC82), and on two recovered samples from in situ X-ray diffraction experiments (I-doped 20BA/M19 and I-free Etna2002), Raman spectra collected on I-doped and plain borosilicate glasses (NBS20) [Cicconi et al., 2019] are shown for comparison. For I-doped basaltic glasses, I2 related modes are observed below 200 cm−1 for PC82 (grey shaded area), and iodate-related modes are marked by asterisks. Note that PC82-Saint Vincent basaltic composition (3.8 wt% H2O) is less polymerised, as seen by the increased band near 1070 cm−1. For borosilicate glasses, the strong bands in the [740–840] cm−1 are related to borate rings and boroxol. Masquer
Raman spectra collected on an iodine over-saturated sample (PC82), and on two recovered samples from in situ X-ray diffraction experiments (I-doped 20BA/M19 and I-free Etna2002), Raman spectra collected on I-doped and plain borosilicate glasses (NBS20) [Cicconi et al., 2019] are ... Lire la suite
4. Discussion
Iodine induces changes in the silicate melt network structure, observed here by a lesser degree of polymerisation as probed by X-ray diffraction, and reported on the basis of Raman spectroscopy on Fe-free alkali-rich felsic magmas [Faranda, 2023], and Fe-free borosilicate glasses [Cicconi et al., 2019, Morizet et al., 2021]. While this behaviour is noticeable for experimental levels of I-doping in the order of 1 or more wt%, and is a relevant property in the context of nuclear waste glasses, it is not expected for natural levels of I content in magmas. The network modifying role of I however does indicate that I is not retained passively in the voids or in the ring structure of magmas, nor that it removes Na or K ions from the melt oxides otherwise the opposite effect would be observed, i.e. enhanced polymerisation.
The coexistence of reduced iodide (I−) and oxidized iodate (
The potential role of FeO is highlighted here by the amount of reduced iron that is higher for I-doped samples than I-free samples ran for similar duration at high T (TGH25 and TGH26, Table 1), indicating that FeO was the likely source of oxygen to form periodates, leading to the formation of metallic Fe. This effect is unfortunately not clear for I-free Etna2002 compared to I-doped 20BA/M19, with similar FeO content in the quenched glass which could result from Etna2002 shorter run duration. In the case of Saint Vincent basalt experiments, the starting glass was already almost FeO free (Table 1) after piston-cylinder press iodine and water doping using platinum capsules. Hence the most obvious source of oxygen was water for this composition.
Iodine speciation in basalts as an oxide is quite unique amongst halogens elements. X-ray absorption spectroscopy measurements on glasses recovered from high P–T conditions have mostly targeted chlorine and bromine speciation so far. Chlorine bonds to network modifying cations [Evans et al., 2008, Thomas et al., 2023], in particular to Ca, Fe and Mg, to a lesser extent to Na, and in the case of the most SiO2 rich melts also to Si [Thomas et al., 2023]. The higher affinity of chlorine for alkaline-earth cations than for alkaline cations was confirmed for borosilicate glasses [Jolivet et al., 2023]. Bromine speciation in silica-rich magmas changes between low P, with Br–Na bonds and a hydration shell, to a closer oxygen environment above 2 GPa [Cochain et al., 2015, Louvel et al., 2020] although whether oxygen belongs to water molecules or to the silicate network could not be deciphered. Bromine speciation besides remains to be investigated in basaltic compositions. The bulk silicate Earth (BSE) contains approximately ten times more Br than I [Kendrick et al., 2017, Guo and Korenaga, 2021], the Br/I ratio raises in MORB and arc basalts (circa 50, [Kendrick et al., 2014]), and even slightly more in atmospheric volcanic plumes (58–87, [Aiuppa et al., 2005]). Differences in Br and I speciation in magmas could, at least partially, underpin this behaviour.
5. Conclusion
While iodine is generally thought as present in nature either as iodide (I−), iodate (
Interstingly, formation of iodates has also been observed during circulation of water–iodide solutions through volcanic rock cores at ambient conditions [Neil et al., 2020], on the basis of UV spectroscopy measurements. This was interpreted as resulting from the retention of oxidized I by minerals in volcanic rocks, concomitantly with reduction of ferric ions. In conjunction with the present results, this points out that future studies should be dedicated to elucidating simultaneously the speciation of I and Fe in magmas to fully understand I behaviour in petrologic and volcanic processes.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Acknowledgments
Natural basaltic samples from Saint Vincent and Mount Etna were provided by G. Prouteau from Institut des Sciences de la Terre d’Orléans. We acknowledge K. Curtis-Benson for providing parts for cell assemblies and arranging shipments before and after experiments at the Advanced Photon Source, K. Béneut for use of the Raman spectroscopy platform at IMPMC, O. Boudouma for SEM measurement and N. Rivodini for EPMA analyses at OSU Ecce Terra, Sorbonne Université. This work was supported by the European Research council under the European Community’s Seventh Framework Programme (FP7/20072013 Grant Agreement No. 259649 to CS), TG was supported by the ANR Projet de Recherche Collaborative VOLC-HAL-CLIM (Volcanic Halogens: from Deep Earth to Atmospheric Impacts, ANR-18-CE01-0018) and is grateful for an EU Marie Skłodowska-Curie Fellowship “ExCliso” (Project ID 101017762), QC was supported by CSC scholarship (#201806340094). HPCAT operations are supported by DOE NNSA’s Office of Experimental Sciences. The Advanced Photon Source is a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.





 CC-BY 4.0
CC-BY 4.0