Version française abrégée
Les hyménoptères des figuiers ont fourni une série d'exemples, abondamment cités, d'évolution vers de faibles proportions de mâles et de variation des stratégies de reproduction. Chez les insectes pollinisateurs, une ou plusieurs femelles pénètrent dans la figue et pondent dans les fleurs. Quelques semaines plus tard, les mâles émergent les premiers dans la cavité de la figue et fécondent les femelles, souvent leurs sœurs. Puis les mâles percent un trou à travers la paroi de la figue, permettant la sortie des femelles. Il est généralement admis que les mâles meurent rapidement après le percement du trou. Ce système de reproduction mènerait à une forte compétition locale entre frères si les femelles ne produisaient pas, en réponse, de faibles proportions de mâles dans leurs pontes. Cette réduction de la compétition entre mâles par la réduction de leur nombre et le fait que les mâles en compétition soient souvent des frères ont été utilisés comme explication de l'absence de combats entre mâles, alors que les combats entre mâles sont rapportés chez beaucoup d'espèces d'hyménoptères parasites du système figuier–pollinisateur.
Cependant, les mâles d'une espèce de pollinisateurs sont connus pour se battre, ce qui suggérait que ce comportement pût exister chez d'autres espèces. À partir d'études de non-pollinisateurs, nous avons identifié une série de caractères morphologiques associés aux combats, comme des mandibules falciformes, un pronotum court et large, une fusion des mésonotum, métanotum et propodeum, des pattes plus déliées... Ceci nous permet, à partir de l'ensemble des descriptions de mâles de pollinisateurs et de notre collection de référence d'environ 200 espèces, d'établir une liste de 28 espèces sur environ 300 décrites, chez lesquelles nous prédisons que les mâles se battent.
Nous avons confirmé nos prédictions en observant le comportement des mâles chez seize espèces, neuf (appartenant à cinq genres) pour lesquelles les combats étaient prédits, sept (appartenant à quatre genres) pour lesquels un comportement pacifique était prédit. Les résultats étaient conformes aux prédictions. De plus, parmi les espèces combattantes, l'intensité des combats variait selon les espèces.
Les combats ne sont pas limités par l'apparentement entre mâles. Chez trois des espèces étudiées, nous montrons qu'en général, une seule femelle pénètre par figue pour pondre et que, dans de telles figues, les mâles se battent.
Nous avons constaté que des mâles de certaines espèces combattantes quittaient leur figue natale. Trois situations se présentent. Chez certaines espèces, les mâles ne se dispersent pas à partir de leur figue natale. Chez les autres espèces, certains mâles quittent leur figue natale par le trou percé par les mâles et parcourent les rameaux. Chez certaines de ces espèces, les mâles pénètrent dans d'autres figues par le trou de sortie percé par les mâles ; ils ne peuvent accéder qu'à des figues où il ne reste que quelques femelles à féconder. Enfin, chez Platyscapa awekei et Nigeriella excavata, certains mâles percent des trous d'entrée dans les figues et pénètrent dans celles-ci au stade où les accouplements vont débuter.
La distribution taxonomique des mâles combattants suggère que l'état ancestral soit non combattant et que le comportement agressif soit apparu au moins quatre fois de façon indépendante et peut-être six fois. De plus, les mâles de trois genres dispersent de leur figue natale, ce qui correspond à deux ou trois origines évolutives.
La morphologie des mâles associée aux combats permet potentiellement aussi la dispersion, car elle implique des pattes plus fines et plus allongées, un thorax plus court et raccourci et un gaster rétractile, moins encombrant. De plus, les mâles combattants ont des mouvements plus vifs et, à l'inverse des non combattants, se déplacent facilement sur des surfaces planes. L'association entre combats entre mâles et la capacité à la dispersion se retrouvent chez au moins deux espèces de parasites du système. La dispersion des mâles est probablement un caractère largement sous-documenté chez les hyménoptères des figuiers et devra être prise en compte dans les études sur les proportions de mâles dans les pontes et sur les combats.
Comment expliquer l'existence de combats entre mâles très apparentés ? Nos résultats confirment que l'apparentement entre mâles n'est pas par lui-même un obstacle, car les combats ont souvent lieu entre plein-frères, ce qui est prédit par la théorie de la sélection de parentèle lorsque les interactions se produisent exclusivement entre apparentés. Un autre obstacle serait que les femelles devraient limiter la compétition entre mâles en pondant peu de mâles. Un certain nombre de facteurs augmente cependant la proportion de mâles qu'une femelle doit pondre, de sorte qu'il y a compétition entre frères. Ce sont par exemple les cas où il y a plusieurs fondatrices, la nécessité de prévoir qu'il pourrait y avoir plusieurs fondatrices, la ponte d'un excès de mâles comme garantie contre une éventuelle mortalité... Il n'y a donc pas d'obstacle de ce point de vue-là pour l'évolution des comportements agressifs.
Pourquoi le comportement agressif a-t-il évolué chez certaines espèces et pas chez d'autres ? Nous avons constaté que chez un certain nombre d'espèces combattantes, les mâles expulsent les femelles de leur galle juste après les avoir fécondées, ce qui interdit les fécondations multiples. Ce comportement n'a jamais été observé chez des espèces non combattantes. Ceci ferait que le sex ratio opérationnel deviendrait progressivement biaisé vers les mâles, une situation qui sélectionne pour les combats. De plus, il semble que toutes les espèces où les mâles se battent sont associées à des figues à structure interne très simple, permettant aux mâles d'accéder à toutes les femelles sans avoir à se glisser entre les ovules. Ceci autoriserait l'évolution de la morphologie combattante.
1 Introduction
Fig wasp mating ecology is fascinating and has delivered textbook examples of skewed sex ratios resulting from local mate competition, and of alternative mating strategies. One or a few females of the pollinating species crawl into a fig to lay their eggs in the flowers on the inside of the fruit. The males hatch first and inseminate the females, mostly their sisters, inside the fig. Then the males chew a tunnel through the fig wall in order to release the females. The males are believed to be helpless on the outside of the fig and useless after the tunnel has been chewed [1,2]. They either die inside their natal fig or slip to the ground and their imminent deaths. This mating history leads to extreme local mate competition between brothers and as a result, mothers produce very female biased sex ratios [1]. In this way, mothers can reduce futile competition between her sons. This reduction of the potential conflict between brothers as well as the fact that interacting males are related is believed to result in the absence of fighting in pollinating species [1].
The absence of fighting amongst pollinator males is in stark contrast to many of the non-pollinating species inhabiting the same figs [1,3–5]. These non-pollinators often fight lethally over females. A number of theories have been proposed to explain this difference. (i) Non-pollinating males are not related to each other. (ii) Their sex ratios are closer to equality leading to more potential for conflict between males over mating opportunities [1]. (iii) Working on non-pollinating fig wasps, Vincent [5] argued that the mating site correlates with fighting morphologies and behaviours and may determine if males can develop these traits. Males that mate in the confined spaces between the fig's internal seeds and galled flowers cannot evolve bulky fighting morphologies, whereas those that mate in the cavity of the fig can do so. Indeed Bean and Cook [6] showed that in a non-pollinating wasp species, small males had reduced adaptations to fighting, and that this supposedly allowed them to manoeuvre and mate in areas of the fig where flowers are tightly packed, while large males which were better adapted to fighting could only mate in the cavity. (iv) Furthermore, the operational sex ratio of species that mate in the cavity of the fig is extremely male-biased, since females tend to reach the cavity one by one, favouring fighting [7]. Because pollinators mate between the galls, presumably engaging in a scramble-type competition [1,8], the apparent lack of fighting in pollinator wasps was in line with these theoretical expectations.
However, the males of one fig-pollinating wasp had been documented to fight [9] and West et al. [10] recently argued that the hypothesis that high relatedness should reduce fighting is fallacious, since brothers compete locally, cancelling out any conflict-limiting effects higher relatedness may have had [11,12]. This suggests that fighting may have been overlooked in pollinating species and that alternative factors, such as interspecific variation in the accessibility of females to be mated or variation in mating behaviour, may shape the evolution of fighting.
We report here that fighting, and the associated and characteristic morphology, evolved at least four and possibly six times in pollinating fig wasps. We also show that the males of three fighting genera disperse actively, leading to non-local mating. We suggest that the physical environment within the fig and its consequences on mating behaviour, the operational sex ratio and male dispersal could be important factors that can prevent or promote the evolution of fighting.
2 Materials and methods
2.1 Male morphology
From studies on fighting non-pollinators we identified several traits associated with fighting such as falcate (sickle-shaped) mandibles; large head; long antennal scape (first segment); antennae not projecting forward; pronotum broader than long; mesonotum, metanotum and propodeum strongly fused. Morphological studies of the pollinating wasps were reviewed to identify the occurrence of these traits in pollinators. Species with fighting traits were designated as ‘Potential fighters’. All published drawings and descriptions of pollinating fig-wasp males, cited in Wiebes' general revisions of Agaonidae [13–15], were perused to identify such males. Pollinating fig wasp males of the INRA reference collection were also examined (roughly 200 species). All species names in the following are according to Wiebes's revisions.
2.2 Fighting behaviour
We observed directly whether a number of species that were predicted to fight from their morphology, really fought and whether species that were predicted not to fight really did not (list of species in Table 1). Figs were observed at the development stage when male wasps had just hatched from their galls and had started to look for and mate with females. Figs were split in half and viewed under a dissecting microscope at an appropriate magnification. As the aim was not to quantify fighting behaviour, but to establish whether or not fights occurred, observations were terminated when several fights had been observed. In the case of non-fighting species, we observed the wasps for at least five hours, during which time we artificially increased the numbers of males in the half we watched to increase the number of interactions between males.
Species in which behaviour was observed. Dispersers A: enter other figs through the existing exit hole. Dispersers B: chew own entrance hole in other figs. Dispersers C: observed to disperse on the figs and branches, but no monitoring of whether they subsequently entered into figs
| Species | Fighting (predicted) | Fighting (observed) | Dispersal | Locality∗ |
| Alfonsiella binghami | yes | yes | A | N |
| Alfonsiella longiscapa | yes | yes | C | T |
| Alfonsiella sp. indet. A (ex F. craterostoma) | yes | yes | A | Pta |
| Alfonsiella sp. indet. B (ex F. ‘petersii’) | yes | yes | A | N |
| Alfonsiella brongersmai | yes | yes | C | T |
| Elisabethiella glumosae | no | no | no | IGR |
| Elisabethiella comptoni | no | no | no | N |
| Elisabethiella stuckenbergi | no | no | no | T/Pta |
| Elisabethiella socotrensis | no | no | no | T |
| Nigeriella excavata | yes | yes | B | LT |
| Allotriozoon heterandromorphum | yes | yes | no | N/IGR |
| Platyscapa awekei | yes | yes | B | IGR/Pta |
| Platyscapa soraria | no | no | no | Pta |
| Courtella michaloudi | yes | yes | no | T |
| Courtella armata | no | no | no | N |
| Pegoscapus mexicanus | no | no | no | M |
∗ N = Nelspruit (South Africa), Pta = Pretoria (South Africa), IGR = Itala Game Reserve (South Africa), LT = Louis Trichardt (South Africa), T = Tanzania, M = Miami (Florida).
2.3 Male dispersal
We observed that males of some fighting species left their natal fig and entered another fig on the same tree, sometimes 50 cm or more distant. In the species investigated for male behaviour, the terminal branches containing figs and leaves were scanned for any males and on detecting one, it was observed continuously until it disappeared into a fig. In cases where males appeared to excavate a new entrance hole into the fig, the twig bearing the fig was taken to the laboratory and observed under a microscope for confirmation. The progress of a number of such males was followed simultaneously under the microscope. For both Nigeriella excavata and Platyscapa awekei, we split open one fig, several hours later to determine if the immigrant male was mating inside the fig or not. To confirm that males from Allotriozoon heterandromorphum do not disperse, we placed ten males on the outside of the fig and observed their behaviour.
2.4 Relatedness of fighting males
To establish whether fighting occurred in the evolutionary context of brothers fighting amongst each other, foundress numbers were established for four species. Figs were collected after females oviposited but before the offspring emerged. These figs were split open and the central cavity and bracts were searched for the remains of the foundresses. In the species examined females did not leave figs after ovipositing, but males did disperse and this can lower the relatedness between interacting males. For the species Platyscapa awekei, Alfonsiella binghami and Alfonsiella sp. indet. A, we also observed whether fighting occurred in figs that had a single foundress and contained no exit/entrance hole.
3 Results
3.1 Male morphology
A series of traits were found to co-occur and constituted what was assumed to be a fighting syndrome. They included falcate mandibles; large head; long antennal scape; antennae not projecting forward; often located in separate toruli instead of in a central depression; elongate legs; narrower tibia and femur; reduced dents on fore tibia; pronotum broader than long; mesonotum, metanotum, and propodeum strongly fused (Fig. 1). For many species only a few of these traits could be assessed from the original descriptions and illustrations. From the morphological analysis, we predicted that most pollinating fig-wasp males would be peaceful, but that probably all males of the genera Alfonsiella, Nigeriella and Allotriozoon and some males of genera Pegoscapus, Platyscapa and Courtella should engage in fighting (Table 2). In fact, Hamilton [1] suggested that Alfonsiella species might not be pollinators, because males have such large mandibles. As a whole, we noted one or several characters suggesting the occurrence of fighting males in the descriptions of 28 of the roughly 300 known fig-pollinating wasp species.
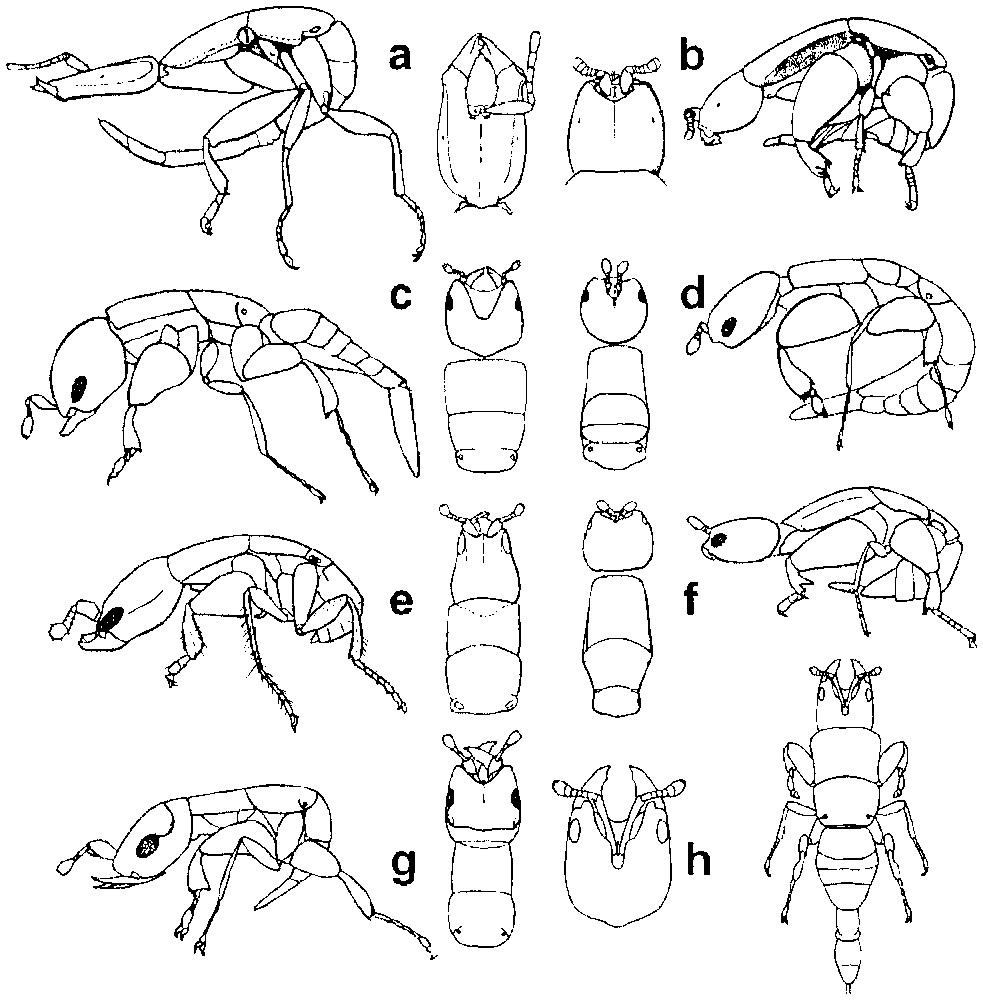
Fighting (F) and non-fighting (NF) fig pollinating wasp males. (a) Courtella michaloudi (F); (b) Courtella armata (NF); (c) Platyscapa awekei (F); (d) Platyscapa soraria (NF); (e) Alfonsiella longiscapa (F); (f) Elisabethiella stuckenbergi (NF); (g) Nigeriella excavata (F); (h) Pegoscapus astomus (F). Note falcate mandibula, strong head, elongate scape (first antennal segment), and shortened strong thorax, in fighting males. Fighting males can retract the gaster: (a) extended position, (e, g) retracted position. Note the elongate segments of the fore tarsus in (a) compensating for their reduced number, and suggesting non-fighting ancestry, (h) redrawn after Grandi [28].
The 28 species whose morphology suggests male fighting and species in the same genera that are not expected to fight
| Genus | Males suspected to fight | No suspicion of fighting |
| Courtella | sylviae, penicula, wardi, hladikae, michaloudi, | hamifera, bekiliensis, malawi, armata, gabonensis, |
| medleri | camerunensis | |
| Pegoscapus | astomus, flagellatus | aemulus, aerumnosus, aguilari, amabilis, ambiguus, assuetus, attentus, augusta, baschierii, bifossulatus, brasiliensis, |
| carlosi, cumanensis, danorum, elisae, estherae, franki, | ||
| gemellus, grandii, groegeri, herrei, hoffmeyeri, insularis, | ||
| jimenezi, kraussii, longiceps, lopesi, mariae, mexicanus, | ||
| obscurus, oroczoi, philippi, piceipes, silvestrii, standleyi, | ||
| tomentellae, tonduzi, torresi, tristani, urbanae, williamsi | ||
| Platyscapa | awekei, binghami, etiennei, desertorum, arnottiana | quadraticeps, soraria, corneri, ishiiana, coronata, fischeri, tjahela |
| Nigeriella | all 4 described species | |
| Alfonsiella | all 7 described species, plus 2 additional investigated in this study | |
| Allotriozoon | 2 species |
3.2 Fighting behaviour
With the exception of A. heterandromorphum, males fell into two clear categories; they were either (1) oblivious of each other, often chewing holes side by side into the same female-containing gall without any interaction between them, or (2) they fought at almost every conceivable opportunity where two males were vying to mate with the same female (for each species, over 10 fights were observed before observations were interrupted). For instance, during the mating of one female N. excavata that took 17 min in total, eight males were involved in 10 fights over the female; three of these lasted longer than a minute, five were shorter interactions and two attacks were from behind. As a result of these interactions, there were three sequential displacements of the mating male by the victor.
Fights were always in the context of a receptive female. We observed no injuries resulting from fighting, but the winner monopolised mating with the contested female. The males of fighting species moved faster than the non-fighting males that appeared to require a substrate behind their dorsal surface to remain stable.
Males fought in all the observed species whose morphology predicted that they were fighters and vice versa (Table 1). In each species, fighting followed a stereotypical pattern. In A. heterandromorphum, when two males were vying for the same female, they either pushed each other away or, less frequently, they fought with clear exchanges of bites and displacement of the resident male (only three fights observed). In P. awekei, males engaged in slow but powerful biting of each other's heads. In Courtella michaloudi, males used their long mandibles to grab other males by the thorax, lift them up, and throw them to one side within the fig. Lifting other males was made possible by their very long legs (Fig. 1). In Alfonsiella species and N. excavata, males moved fast and used their upwardly curved mandibles to attack the undersides of their opponent's thorax. When males were standing on opposite planes, they grabbed hold of each other's antenna and pulled and pushed their opponent around. Although the general pattern was very clear, in one instance three males of the usually peaceful P. soraria vying for the same female did appear to bite at each other, despite the fact that their small mandibles hardly allow it and despite their slow movements. This limited observation does not contradict our one-to-one fit between morphology and behaviour, because the intensity of the interaction was weak and only occurred rarely. Nevertheless, it suggests that limited sparring may occur even in generally non-fighting species. This is a necessary initial condition for an arms race to result when the correct ecological situation prevails.
3.3 Male dispersal
Although dispersal by pollinating fig-wasp males had not previously been documented, we observed that the males of some fighting species left their natal fig and entered another fig on the same tree (Table 1). Males were regularly observed to disperse at over 50-cm distance from their natal fig. We found two dispersal patterns in the fighting wasps: first, males from Alfonsiella simply entered figs that already had an exit hole. These males generally froze momentarily at the exit hole with their heads inserted into the fig and either entered the fig or walked to the next. Second, P. awekei and N. excavata males chewed an entrance hole from the outside of the fig and took up to six hours to do so (observation of 16 figs into which a total of 23 male P. awekei were cutting an entrance hole and of three figs into which three male N. excavata were cutting an entrance hole). In P. awekei, sometimes a male gave up chewing an entrance hole, and often died shortly afterward and the entrance hole was a bit later continued by another male. In other instances, one male would be chewing the entrance hole through the ostiole, while another male would be staying nearby either waiting until the hole was finished or until the other male gave up, or the two males would take turn at chewing. In both N. excavata and P. awekei, males seemed to almost always choose to chew at figs in which male activity was to begin within a few hours. It was then easy to observe them mating inside the fig they had entered mixing up with the very few resident males that had already emerged from their gall and started mating. Population genetic data suggest that the males of Alfonsiella sp. Indet. A manage to mate after dispersal [16]. Dispersal is thus a goal-directed and fitness-enhancing trait and not a mere side effect of chewing the exit hole.
Despite careful monitoring, C. michaloudi and A. heterandromorphum males were never observed to disperse. When deposited on the fig surface, the males of A. heterandromorphum continued their ‘internal’ behaviour on the outside of the fig; they appeared to be searching for females on the hairy exterior of the fig fruits. This is in stark contrast to a species like Alfonsiella sp. indet. A where, in 15 min, one male visited 24 figs, walking directly to the area where the exit tunnel is normally eaten, inspecting the hole if it was present, and walking to the next fig, finally disappearing into the last one.
Interestingly, the non-dispersing C. michaloudi and A. heterandromorphum have very reduced eyes, while dispersing males have large to very large eyes for agaonid wasps.
3.4 Relatedness of fighting males
Three of the species investigated for foundress number usually had a single foundress only, while in the fourth species half the figs were singly founded (Table 3). Since males of these species disperse, relatedness between interacting males may be lowered. Note that males of only three of the six fighting genera have been observed to disperse. In the three species investigated for behaviour in single foundress figs (P. awekei, A. binghami and Alfonsiella sp. indet. A) males, i.e. brothers, always fought when the opportunity arose.
The proportion of figs that contain a single foundress for four species of fighting pollinating fig wasps
| Species | Number of crops | Proportion of single |
| (number of figs) | foundress figs | |
| Platyscapa awekei | 4 (358) | 0.79 |
| Alfonsiella binghami | 3 (116) | 0.87 |
| Alfonsiella sp. Indet. A | 12 (665) | 0.93 |
| Nigeriella excavata | 3 (223) | 0.54 |
4 Discussion
Despite the notion that fighting does not occur between male pollinating fig wasps, we observed fighting in a number of genera and it may occur in almost 10% of all species. What is even more remarkable is that it evolved a number of times independently. The distribution of the genera with fighting males (Nigeriella not included in phylogenies) within the published phylogenies of fig-pollinating wasps [17,18] shows that it is a derived trait. Indeed, males of the basal genus Tetrapus and males of most genera are very clearly non-fighting. Preliminary results from a molecular phylogeny based on longer sequences and encompassing all pollinating genera indicate that Elisabethiella and Alfonsiella are sister genera, while the position of Nigeriella is not yet fully ascertained (Carlos Lopez Vaamonde, pers. comm.; Jousselin, Erasmus and Greeff, unpublished data). Since at least two genera (Courtella and Platyscapa), probably three (plus Pegoscapus, for which no behavioural observation is available for P. astomus and flabellatus) and a pair of sister genera (Alfonsiella and Elisabethiella) are polymorphic among species for fighting, fighting evolved at least three times independently, and possibly as many as six times, in pollinating fig wasps.
Another remarkable finding of this study is that pollinator males of three genera disperse from their natal figs and secure mating elsewhere. This partial breakdown of local mate competition between brothers should result in less female biased sex ratios. Indeed, this has been found for Alfonsiella sp. indet. A [16]. A less biased sex ratio could result in an increase in the degree of local mate competition.
Male morphology associated with fighting also allows dispersal as it involves thinner, more elongate legs, a broader, shortened thorax, and the capacity to retract the gaster, so that it becomes less cumbersome (Fig. 1); fighting males move faster than non-fighting males and they are capable of walking on a flat surface whereas non-fighting males fall over helplessly. Wingless male dispersal also occurs in a number of non-pollinating male fighting fig wasp species [6,7], confirming the connection between adaptations to fighting and potential dispersal capacity. Male dispersal may also necessitate physiological adaptations. The internal cavity of the fig is often rich in CO2 [19]. Males are active in this atmosphere but at least in some species become indolent and clumsy when exposed to the normal ambient atmosphere. This is the case for Platyscapa quadraticeps [19]. Hence the evolution of male dispersal in Platyscapa awekei may have involved the evolution of a male physiology that is not inhibited by the ambient atmosphere. Male dispersal is probably largely under-documented in non-pollinating fig wasps and needs to be taken into account in studies investigating sex ratios [20,21] and fighting [10]. Due to the similarity between adaptations for fighting and for dispersal, and due to incomplete descriptions, we cannot exclude that within our list of species for which male fighting is suspected, some could be dispersing non-fighters.
Given that fighting possibly evolved six times and dispersal three times along with it, a number of questions are raised. Is there any link between these two traits and what initiated their evolution? Both fighting and dispersal can result from high local mate competition between brothers; the former, because the ameliorating effects of relatedness are cancelled out when competition is this local [11], and the latter, to reduce competition between relatives [22,23]. That local mate competition should exist in pollinating species is surprising, since mothers produce their sex ratios exactly to eliminate this competition [1,8]. A number of factors may increase the sex ratios, so that the potential for competition exists. These are: multiple foundresses [8], the need for insurance males against mortality [24,25] and against subsequently arriving foundresses [26], and higher female mortality due to parasitic wasps (J. Pienaar and J.M. Greeff, unpublished data).
An alternative scenario is that dispersal evolved first, and since relatedness of dispersers to resident males is zero, fighting may easily evolve subsequently. If true, this scenario would be able to explain fighting in the three genera where males disperse. However, a genetic study on one of the dispersing species, Alfonsiella sp. indet. A, showed that 90% of mating is between sibs [16]. For a pollinating fig wasp this is a very high level of sibmating and it must stem from high levels of local relatedness. A general survey of 22 non-fighting pollinator species by Herre et al. [8] recorded only six species where the expected relatedness between competing males was higher. Yet, none of the 16 species with less relatedness between males fight. If dispersal leads to so little non-local mating, and since so many figs are entered by only a single foundress, fighting may well have evolved in the context of brothers competing against each other. Present information suggests that due to competition being restricted between relatives, the ameliorating effects of relatedness have been negated [10–12]. Nevertheless, none of the observed fighting led to injuries, although the way Alfonsiella and Nigeriella fight suggests it can be lethal. Hence further studies will be necessary to establish whether relatedness among interacting males limits the severity of fights.
A number of other factors may also set the fighting species apart from other pollinators and facilitate the evolution of fighting. First, during our observations of male behaviour, we noted that after mating with a female, the males of Alfonsiella, Nigeriella, and Allotriozoon (but not P. awekei) enlarge the mating hole, grab the female by her antennae and pull her out of the gall. This behaviour precludes multiple mating, as males will only mate with females still inside their galls. This behaviour was previously only known from Alfonsiella fimbriata [27]. As a result of this atypical behaviour among fig pollinating wasps, the supply of mateable females should decrease rapidly leading to a male biased operational sex ratio that favours fighting. In all other agaonid wasp species for which mating behaviour has been described, including the non-fighting Courtella armata (this study), females stay in their gall after mating and seem to be mated several times, so that the operational sex ratio remains female biased. Expulsion of females into the fig cavity is only feasible because of a large fig internal cavity, a trait we observed in figs pollinated by fighting Alfonsiella, Nigeriella, Allotriozoon, and Courtella species. Hence, operational sex ratio may be an important factor affecting the evolution of fighting.
Second, the figs that host the fighting species of Allotriozoon, Platyscapa, and Courtella all appear to have an exceptionally simple internal structure with very few layers of loosely packed galls and seeds. Indeed figs pollinated by A. heterandromorphum and by C. michaloudi only presented two layers of flowers at fig maturity despite their large numbers of females flowers (over 500), while figs pollinated by P. awekei, N. excavata, but also some of those pollinated by Alfonsiella are very small also, leading to few layers of flowers. In these species, fighting morphology probably does not limit access to females.
In summary, we recorded two novel behavioural patterns, fighting and dispersal, in pollinating fig wasps. This is surprising, because female biased sex ratios should act to limit competition between males. Even so, fighting possibly evolved six times and dispersal three times under conditions presumably marked by high local mate competition. Additional ecological conditions, such as a male-biased operational sex ratio and simple fig morphology, may also play an important role in shaping the evolution of these traits. Comparative quantitative data on fighting intensity, wasp mating and dispersal behaviour, and on fig internal structure will be required to test these hypotheses.
Acknowledgments
We thank the NRF and CNRS for a combined S&T grant, Tony Ware for field assistance and Kwazulu-Natal Nature Conservation Service for accommodation at Itala Game Reserve. Work in Itala Game Reserve was done under permit number 2985/1999. Drawings of Fig. 1a, b, e and f are by Emmanuel Barrau. Georges Michaloud provided figs of F. natalensis leprieuri and F. bubu. Carlos Lopez Vaamonde provided access to unpublished molecular phylogenies. We are grateful to J. Seger, A. Raman, S.A. West, E.A. Herre, J. Bronstein, R. Harrison and D. Yu for comments on the manuscript. Sadly, the late W.D. Hamilton was already ill when we sent him our first fighting observations and we will never know his views on these.



