1 Introduction
Nearly half of the antelope species (46%) are included by the International Union for the Conservation of Nature (IUCN) in the Red List of threatened species [1]. Among the species that occur in arid environments, this ratio increases to around 65%, and reaches 92% among the 13 species surviving in the harsh conditions of Sahara and Arabian deserts (Table 1).
IUCN conservation status of wild antelope species that occur in the Sahara and Arabian deserts [1,43]. nt=near threatened; cd=conservation dependent
| Species | IUCN Status 1996 | IUCN Status 2000 |
| Gazella dama, dama gazelle | Endangered | Endangered |
| Gazella leptoceros, slender-horned gazelle | Endangered | Endangered |
| Gazella cuvieri, Cuvier's gazelle | Endangered | Endangered |
| Gazella dorcas, dorcas gazelle | Lower Risk – nt | Vulnerable |
| Gazella subgutturosa marica, Arabian goitered gazelle | Lower Risk – nt | Vulnerable |
| Gazella gazella cora, Arabian mountain gazelle | Lower Risk – cd | Lower Risk – cd |
| Gazella rufifrons, red-fronted gazelle | Vulnerable | Vulnerable |
| Oryx leucoryx, Arabian oryx | Endangered | Endangered |
| Oryx dammah, scimitar-horned oryx | Critically Endangered | Extinct in the Wild |
| Addax nasomaculatus, addax | Endangered | Critically Endangered |
| Hemitragus jayakari, Arabian Tahr | Endangered | Endangered |
| Capra nubiana, Nubian Ibex | Endangered | Endangered |
| Ammotragus lervia, Barbary sheep | Vulnerable | Vulnerable |
Among the numerous tools used in conservation to re-establish endangered species (creation of protected areas, captive-breeding, ...), reintroduction is a commonly used, popular and suitable conservation procedure for vertebrates [2] which becomes a necessity once the species has been extirpated from the wild [3].
Many studies have emphasized the importance of maintaining genetic diversity in the reintroduced populations (e.g. [4–6]). After presenting some conservation genetic aspects of the Arabian oryx reintroduction, we focus on three factors recognized as determinant to increase the probability of success of vertebrate reintroductions [2,4,7,8], and evaluate how they apply to the case of the Arabian oryx reintroduction in Saudi Arabia:
- (1) The number of reintroduced animals;
- (2) The post-release monitoring of reintroduced populations;
- (3) The quality of recipient areas in term of coverage of vital requirements of the species.
2 The case of the Arabian oryx (Oryx leucoryx)
The Arabian oryx which formerly occurred throughout Arabian peninsula deserts was extirpated from the wild by hunting in the early 1970s [9]. The species was first reintroduced in Oman in 1982 [10]. In Saudi Arabia, the Arabian oryx was first released in 1990 into Mahazat as-Sayd [11], a fenced steppe desert protected area in west-central Saudi Arabia (2244 km2). Since 1995, Arabian oryx have also been reintroduced into ‘Uruq Bani Ma'arid, a sand dune protected area (12 500 km2) which lies in the western Rub-al Khali, one of the driest regions in the world [12].
The Arabian oryx is a social, large, white and desert antelope (80–100 kg), which survives indefinitely without access to drinking water in arid habitats [13,14]. Classified as a mixed-feeder but predominantly grazer [15,16], it can survive on poor quality forage [17], and decides of its range use according to the biomass and quality of forage available [16], and its seasonal food requirements [13]. Presumably because of the low food resources available in deserts, the Arabian oryx lives at low densities (around 0.016 oryx/km2 in Oman in 1996 [10] and in ‘Uruq Bani Ma'arid in 2001 [18]).
The climate of Saudi Arabia is characterized by hot summers and mild winters. Air temperatures in summer often exceed 45 °C [18,19]. The main characteristic of climate is that rainfall is unpredictable both spatially and temporally (i.e. precipitation at Mahazat as-Sayd ranges from 38 mm in 1999 to 253 mm in 1995).
3 Lessons from the reintroduction of Arabian oryx
Reintroduction is defined as an attempt to establish a species in an area which was once part of its historical range, but from which it has been extirpated or became extinct [3]. Three main factors increase the probability of success of vertebrate reintroductions [2,4,7,8], namely:
- – The number of reintroduced animals;
- – The post-release monitoring of reintroduced population;
- – The quality of recipient areas in term of coverage of vital requirements of the species.
3.1 Genetic management of Arabian oryx herd at the NWRC
The goal of our management has been to maintain the initial genetic diversity through careful breeding and to limit inbreeding level of animals to be reintroduced.
The degree of relatedness within the original oryx herd of the NWRC is unknown, and the Arabian oryx microsatellite loci discovered up to now are not sufficiently polymorphic to carry out a large-scale parentage inference analysis [20]. Consequently, we considered original animals with unknown parents as “founders” [21].
The policy implemented was to balance the 48 putative founder representations within each captive generation (and reintroduced populations). Ultimately, we built-up a captive oryx population recognized as the most polymorphic of all captive herds (heterozygosity varying between 0.425 and 0.785 [20]), with the class of rare alleles (frequencies 0–0.1) being the modal class (L-shaped allele frequency distribution), suggesting that no recent management-related bottleneck has occurred [20]. The NWRC herd is currently composed of more than 240 individuals (Fig. 1).
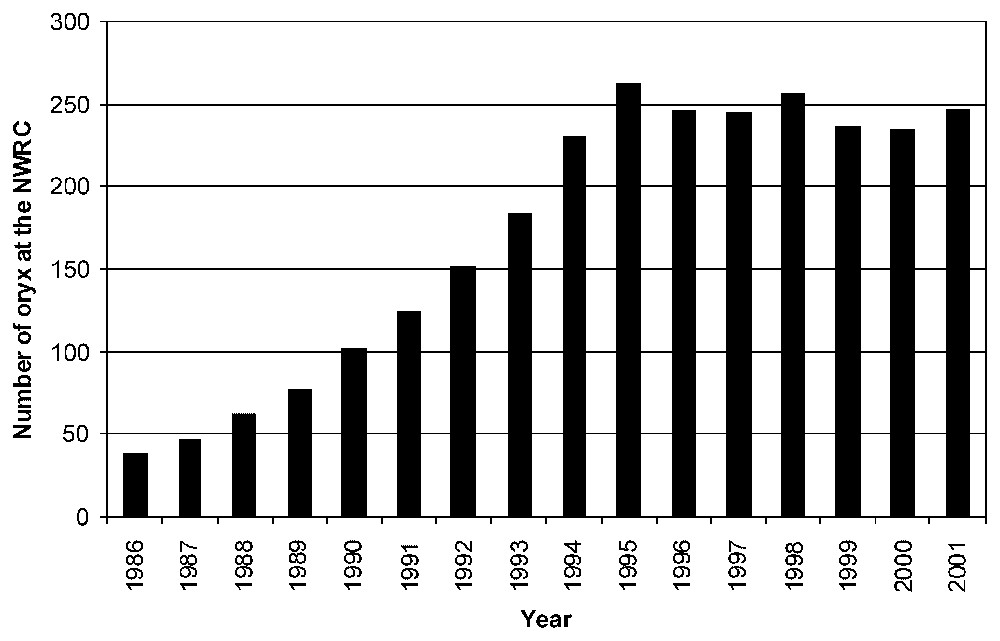
Development of captive-bred Arabian oryx population at the NWRC. Herd growth reduction is mainly due to reintroduction effort increase since 1996.
However, recent genetic analysis have suggested that perhaps as much as 50% of the neutral genetic variation present in the pre-extinction population of Arabian oryx is absent from contemporary populations [20]. Yet, the high rate of intrinsic population growth in reintroduced populations has suggested that this “lack” of polymorphism did not constraint population establishment a least in the short term (e.g. mean intrinsic growth rate of 40 and 43% before drought period respectively in Mahazat as-Sayd and ‘Uruq Bani Ma'arid; see also [22]). Indeed, a majority of the captive oryx populations in the Arabian peninsula are essentially confronted with housing saturation difficulties (Ostrowski, pers. com.) and infectious diseases [23–25], often transmitted by neighbouring livestock.
3.2 Number of reintroduced animals
According to Fischer and Lindenmayer [2], Griffith et al. [4], and Wolf et al. [7,8], vertebrate reintroductions success is increased when more than 100 individuals are released.
Between 1990 and 1993, we reintroduced 72 Arabian oryx into the fenced Mahazat as-Sayd protected area; animals derived from the captive breeding stock held at the NWRC, and from foreign private or national collections [11]. In 2001, we estimated the re-established population size at about 450 animals [26] (Fig. 2).

Mahazat as-Sayd oryx population estimates between 1990 and 2001 using three different estimators. MPE: Minimum Population Estimation (from field workers census); Transect: Transect count using Distance; MR: Mark-re-sighting index.
Since 1995, 139 Arabian oryx issued from the NWRC have been reintroduced into the ‘Uruq Bani Ma'arid protected area [27]. By 2001, the population had increased to 200–220 animals (Fig. 3). Further releases are planned to avoid bottleneck phenomena in case of prolonged demographic stagnation. It appears at present premature to deem the success of this reintroduction project.
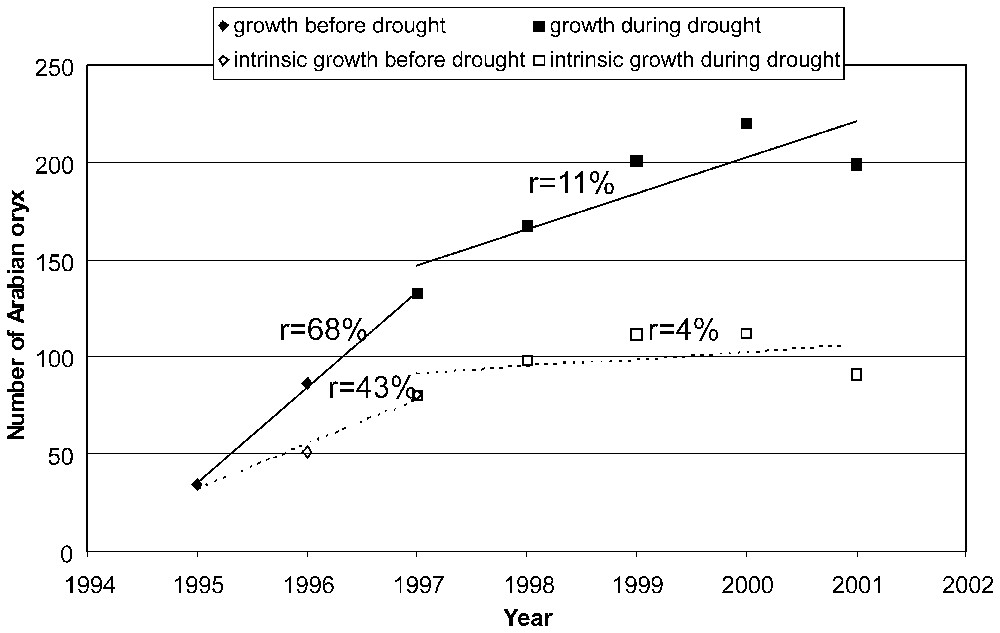
Estimated growth rates of the Arabian oryx population in ‘Uruq Bani Ma'arid protected area before and during drought period (r: mean relative growth rate).
In the case of a fenced, predator-free area like Mahazat as-Sayd, or an unfenced protected area as Yalooni in Oman (40 animals released [10]), the number of animals released, lower than recommended [2,4,7,8] did not seem to limit the oryx population re-establishment.
3.3 Post-release monitoring of reintroduced population
Monitoring of reintroduced populations is a key factor to evaluate the success of reintroductions and to implement management policies.
Because in arid environments, wild antelopes usually survive at low densities, estimators of population size have a low accuracy, owing to the small number of individuals encountered during surveys. We observed such inaccuracy even in the closed and highly surveyed oryx population of Mahazat as-Sayd, where coefficient of variation of transect estimators (Distance software; [28]) often exceeded 50% [29] (Fig. 2).
We have tried two different strategies to offset this weakness:
- – We use three different methods of population size estimation in Mahazat as-Sayd (Fig. 2): cumulated births and deaths recorded by field workers, transect counts and mark-re-sighting index [30]. This monitoring effort allows us to cross-check convergent indications, and to carry out surveys only twice a year.
- – In ‘Uruq Bani Ma'arid, we rely on an intensive post-released population monitoring (e.g. 184 cumulative days spent in the protected area by the field researcher in 2001). We also use aerial surveys (18 aerial survey sessions were carried out in 2001) to improve the monitoring efficacy for these large desert antelopes. Transect counts are not used because of the low number of oryx encountered during surveys (density of around 0.016 oryx/km2 in 2001 [18], against 0.20 oryx/km2 in Mahazat as-Sayd [26]), and hilly ground. However, because during summer, oryx aggregate (mean herd size is 4.3 during summer and 2.5 during winter) in a small part of the reserve (around 1200 km2 [18]) where trees provide them with shade, we use during this period total count method coupled to a mark-re-sighting index to estimate their number.
According to Fischer and Lindenmayer [2], the probability of success of mammal reintroductions increases if supportive measures are taken. However, the supply of food and water during summer does not appear as a viable option for the closed oryx population of Mahazat as-Sayd protected area, as it would allow the population to grow when density dependence would normally be controlling numbers [31].
3.4 Quality of the reintroduction areas
It is recommended that reintroduction sites cover the vital requirements of the reintroduced species and that causes of its original decline are removed from these sites [3].
3.4.1 Coverage of vital requirements
Arabian oryx require shade to survive during summer and sufficient forage supply [32,33].
It is therefore important to select reintroduction sites that can fulfill the summer shading requirements of this species [32].
Contrary to the preconceived idea that surface water is a limiting factor of antelope populations dwelling in deserts, it seems that these species are more dependent on the preformed water in the forage than on drinking water [13,14,33]. Indeed, Arabian oryx may survive indefinitely without drinking water [13,14]. Because biomass and quality of forage available are strongly related to the occurrence of unpredictable rainfall [34–37], the long term prediction of forage availability and as a consequence of oryx release schedule are difficult (Fig. 4).

Illustration of the “feed-back” management process on Arabian oryx reintroduction according to biomass and quality of forage available.
The effect of rainfall on herbivore population dynamic has been well documented (e.g. [31,34,37,38]). Prolonged drought in arid habitat may lead to herbivore population collapse (e.g. [39]). In ‘Uruq Bani Ma'arid protected area, a severe drought period between 1997 and 2001 [18] has decreased the intrinsic growth rate of the oryx population (Fig. 3).
3.4.2 Alleviation of causes of decline
3.4.2.1 Habitat degradation and interspecific competition.
Although habitat degradation and interspecific competition did not appear to be responsible for the oryx extirpation from the wild [40], they represent a potential threat to the successful re-establishment of wild populations.
Although lowly populated, it is likely that the habitat used by the pre-extinction populations of Arabian oryx has changed. In ‘Uruq Bani Ma'arid, further habitat degradation has been minimized by controlling the number of human settlements within the reserve [11]. Tree cutting is a source of concern because oryx need the shade provided by trees to retreat from direct solar radiations in summer [32]. A ban on tree cutting inside the protected area must be enforced, despite local bedus still use wood as a source of energy. The recent development of eco-touristic activities constitutes an additional concern related to habitat degradation. The negative impact of such activities on the protected area will have to be evaluated and ultimately controlled.
Oryx compete on forage with camels. Levels of tolerance still need to be addressed scientifically. Although a certain level of competition is tolerable, we suggest that it may become threatening under the combined effect of drought conditions and oryx population increase. Level of grazing control, by limiting the number of domestic livestock permitted inside the protected area, must rely on a quantitative and scientifically-based approach.
3.4.2.2 The poaching threat.
Over hunting is the main cause of decline or extinction of desert antelopes [40]. Although living at low densities in a vast habitat, oryx are highly vulnerable to hunting. Their destruction is eased by the fact that:
- – They are conspicuous from a long distance (up to 3 km) because of their white and highly reflective coat [33,41];
- – They leave conspicuous tracks in the sandy areas where they dwell;
- – They display fairly little stamina when running (presumably because of its high water and energy cost);
- – They can be easily chased by car when in open flat areas;
- – They have to shade under trees during summer [32], and tree locations are known from local inhabitants;
- – They use to aggregate in areas where rainfall occurred [36], also known from local inhabitants.
4 Perspectives
We may generalize the lessons learnt from the Arabian oryx reintroduction in Saudi Arabia to desert antelope reintroductions:
- (1) Monitoring and management of desert antelope reintroduced populations appear more important than the number of released animals;
- (2) Because of the low accuracy of desert antelope population size estimators, we recommend to implement an intensive monitoring and use a set of estimators to assess the reintroduced population size and growth rate;
- (3) Timing of release must match the biomass and quality of forage available. Because this parameter is difficult to predict in an habitat where rainfall is unpredictable, reintroduction plans must be flexible;
- (4) Long term reintroduction success of desert antelopes is highly dependent on the enforcement of regulations to avoid the hunting of these rare species [40]. With the predicted climatic aridity increase [42], this effort is of particular importance, as animals will probably leave the relative safety of their drying habitats and approach human settlements.
Acknowledgements
We are very grateful to Dr. Joe Williams for useful comments and criticisms on the manuscript.



