1 Introduction
De Haan [1] has reported on extrabuccal infralabial secretion outlets (ILOs) found in all observed live individuals of 19 species representing three psammophine snake genera. In all these individuals, the ILOs regularly change their external aspect and, most notably, are periodically absent.
The present paper deals with head pits, which have not been previously described and which most probably are not secretion outlets. They might be a kind of sense organs, are situated in the parietal region of psammophine snakes and have been found so far in seven species representing four genera. Remarkably, not all individuals of a concerned species exhibit these pits, a curiosity unrelated to sex. For this, and for other reasons noted hereafter, I consider these parietal pits (PPs) to be sporadically occurring. However, if present in an individual, PPs are permanently present, with a definite, consistent shape.
2 Material and methods
While looking with a pocket-lens for traces of infralabial secretion outlets (ILOs) in shed skins, I found small, epidermal structures in the upper head region, which happened to coincide with unreported parietal pits (PPs, Figs. 1–3). Representatives of all eight psammophine genera have been examined, the genus Dipsina only as an alcohol-preserved museum material (at MNHN-Paris), the other seven mainly as captive individuals and shed skins.

Adult Psammophis phillipsi (with PPs 1:1). PPs disposed symmetrically: one pit in each parietal shield (a black arrow leg represents 3 mm). Number, size and disposition of the pits in this example are the most frequently occurring in this and other psammophine species (cf. Section 3). [In the examined P. phillipsi (30–180 cm long), the most common external aperture ∅ is about 100 μm in hatchlings and 300–600 μm in adults.] Photo: A. Cluchier.

Hatchling P. phillipsi (with PPs 2:2). PPs disposed symmetrically, but showing two uncommon features combined, viz. two pits in each (unspotted) parietal shield and all pits having about twice the common size. The suture between the P-shields is 3 mm long. (Coll. CdH, Psammophis phillipsii No. F2-2000.5-1, alcohol-preserved.) Photo: A. Cluchier.
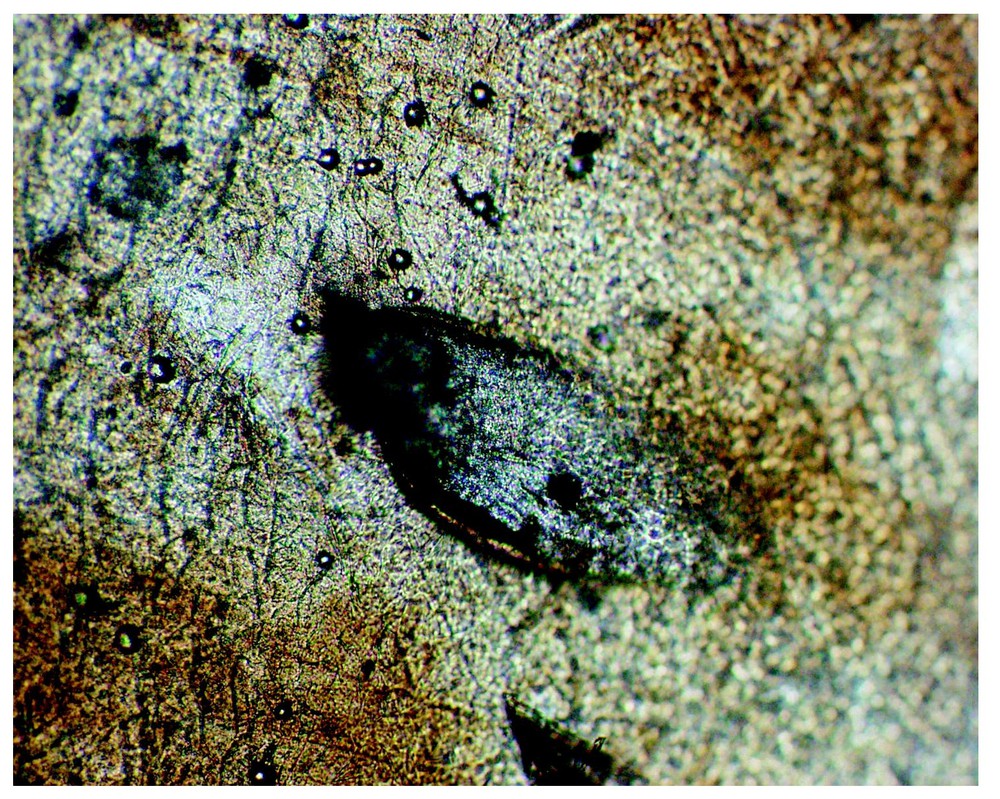
From an alcohol-preserved adult P. elegans (with PP 1:0). The left parietal shield shows a mussel-shell shaped aperture (450 μm long) of a PP in the outermost parietal shield layer, soaked off in the alcohol. The underlying pit wall and other structures, as shown in Figs. 5 and 6, have broken off and presumably are hidden in the epidermal layers remaining on the shield of this museum specimen. (Coll. MNHN-Paris, Psammophis elegans No. 1896-23.) Photo: A. Cluchier.
Parietal shield structures in several captive individuals and their shed skins have been described and photographed using light microscopy (Olympus DP11 digital camera, combined with an Olympus Om-system bellows macrosystem and Zeiss optiphot objectives f=16 mm and f=25 mm). Some examples are given in the Section 3. Many annotated shed skins from free-living Malpolon monspessulanus and other (captive) psammophine snakes are preserved in my personal collection. The parietal structures of several alcohol-preserved snake specimens from MNHN-Paris and my own collection have also been described with the help of a ‘Wild Heerbrugg’ binocular light microscope, No. 250609.
3 Results
Table 1 shows in which species parietal pits (PPs) have been found so far, and the frequency of occurrence among the examined individuals. The PPs occur in numbers of one to four per individual snake. All PPs have a definitive shape, and only increase in size in correlation with the general head growth of an individual snake.
Psammophine individuals with or without sense-organ-like parietal pits. Also indicated: Self-rub system, M or P, adopted by the observed species (cf. De Haan, 2003 [1])
| Examined live individuals or shed skins | Presence of parietal pits in n individuals | Self-rub system | Examined alcohol-preserved individuals | Presence of parietal pits in n individuals | |||||
| Collection CdH | n | Collection MNHN-Paris | n | ||||||
| Dromophis | Dromophis | ||||||||
| lineatus Cameroon + RCA | 7 | 6 no, | 1 yes | P | lineatus | 2 | 2? | ||
| praeornatus Cameroon | 1 | 1 no | P | praeornatus (2) | 10 | 7 no, | 3? | ||
| Psammophis | Psammophis | ||||||||
| elegans Cameroon | 16∗ | 3 no, | 13 yes | P | elegans (3) | 20 | 17 no, | 2?, | 1 yes |
| phillipsi Cameroon + RCA | 74∗ | 29 no, | 45 yes | P | cf. sibilans Mauretania 1996 | 5 | 5 no | ||
| cf. phillipsi N-Cameroon | 3 | 2 no, | 1 yes | P | tanganicus 1895-427, 428 | 2 | 2? | ||
| cf. subtaeniatus (1a) ‘Africa’ | 5 | 4 no, | 1 yes | P | |||||
| cf. subtaeniatus (1b) E-Africa | 2 | 1 no, | 1 yes | P | |||||
| Malpolon | Dipsina329, 1973-429 | ||||||||
| monspessulanus | 71 | 62 no, | 9 yes | M | multimaculata | 2 | 2 no | ||
| Rhamphiophis | Rhamphiophis | ||||||||
| rubropunctatus | 2 | 2 yes | M | acutus | 6 | 6 no | |||
| maradiensis (3) | 3 | 1 no, | 2? | ||||||
| 1989-2740, 2741A, 4300 | |||||||||
| n followed by an concerns mainly individuals born in captivity. | oxyrh. oxyrhynchus | 10 | 10 no | ||||||
| (1a) = P. sudanensis in Spawls et al. 2002 [3]. | (2) For collection n°s see De Haan 2003 [1] | ||||||||
| (1b) = P. orientalis in Spawls et al. 2002 [3]. | (3) Details are given below |
In the examined Psammophis phillipsi (30–180 cm long), their most common external aperture ∅ is about 100 μm in hatchlings, and 300–600 μm in adults.
Sixty-seven captive-born F1 and F2 P. phillipsi, with a 60% frequency of PP occurrence, showed PPs in the left:right parietal shield in the following numbers, dispositions and proportions:
- • 1:1 41% PPs paired symmetrically: one pit in each parietal shield; (Fig. 1).
- • 2:2 6% PPs paired symmetrically: two pits in each parietal shield; (Fig. 2).
- • 1:0 or 0:1 6% asymmetrically: one pit in one parietal shield, no pit in the other shield.
- • 2:0 or 0:2 3% asymmetrically: two pits in one parietal shield, no pits in the other shield.
- • 2:1 3% asymmetrically: two pits in the left and 1 pit in the right parietal shield.
- • 3:0 1% asymmetrically: three pits in the left parietal shield, no pits in the other shield.
- • 0:0 40% Absence of PPs (sensu: parietal pits of the present study, disregarding the ‘classic’ pit-like structures briefly reviewed in the Section 4).
In these 67 individuals, the PP frequency among the sexes could not yet be definitively established, due to the extremely small psammophine hemipenis and the lack of sexual dimorphism (cf. [1]). However, the seven parents, among which three (underlined), showed, respectively: 1:1, 1:0, , in females and 1:1, , 0:0 in males.
Among the 40 snakes born in captivity featuring PPs (=60% of 67 individuals), five showed them located eccentrically compared to those in Figs. 1 and 2, e.g., touching the suture between the two parietal shields. I did not find PPs inside the suture.
Among the same 40 snakes, three showed PPs about two times larger (cf. Fig. 2) than the PPs in Fig. 1, and one had PPs being (or being part of) a long slit in the P-shields. I also found in other psammophines such parietal slits, accompanied or not by a ‘normal’ (i.e. minuscule) parietal pit. In the case of one P. elegans individual, both the slit and the pit in each P-shield were situated in perfect symmetry and exactly as those depicted in a drawing of Rhamphiophis maradiensis by Chirio and Ineich 1991: Fig. 2B [2]. I think these details on the drawing represent PPs as understood in the present study.
In 67 P. phillipsi, on the day of hatching, two had PPs in the course of development: towards PP opening or PP closure? Indeed, on P-shields epidermal cells were disposed in a few concentric circles around and covering a point where, in 41% of the hatchlings, a PP was located, i.e. approximately as in Fig. 1. These ‘covered PPs’ (= c) were disposed as c:1 (= one c on the left shield and one open PP on the right) and as c:c. Postnatally, no further development took place.
In 58 randomly-chosen French Malpolon monspessulanus, I found a similar case among the only four individuals having at least one PP.
In M. monspessulanus, the seemingly low occurrence of PPs appeared to be remarkably more frequent among seven affiliated NE-Moroccan Coast individuals (a female and four of her six male/female offspring have PPs, two do not) than among the randomly chosen 58 SE-French ones (4 have PPs, 54 do not) and six Egyptian ones from unspecified location(s) (all six do not). Both the five Moroccan and the four French PP-featuring snakes bored their PPs in left : right dispositions of 1:1, 1:0 and 0:1, which means in proportions remarkably different from those found in P. phillipsi.
In 13 of 16 P. elegans individuals, PPs occurred in numbers, sizes and in left:right proportions comparable to those found in P. phillipsi.
In specimens preserved in alcohol, it is not easy to find sporadically occurring PPs. The presence of PPs in an epidermis that has absorbed alcohol and that is often pitted with various injuries has mostly been ascertained using bifocal light microscopy, but this concerned the external aspect only. The epidermal wall of the internal structure could not be detached from the parietal shields. Only normal skin shedding seems to enable this.
Fig. 3 shows the mussel shell shaped orifice of a PP in the outermost parietal shield layer, accidentally removed when I took a preserved Psammophis elegans out of its alcohol. Earlier examination of shed skins had revealed structures that underlie the PP apertures (Figs. 4 and 5). Indeed, at each regular shedding, a pocket-shaped cuticle is removed together with the old epidermal layers oberhäutchen and stratum corneum. The cuticle appears to have covered the wall and floor of an obviously pocket-shaped head pit along with nerve-like fibres. A non-epidermal looking vesicula fixed to and under the pocket floor is also removed with the shed skin, and is hard to detect; it appears as a flattened, rather transparent balloon of various shapes. A sketch has been made (Fig. 6) to highlight some of the details in Fig. 5.
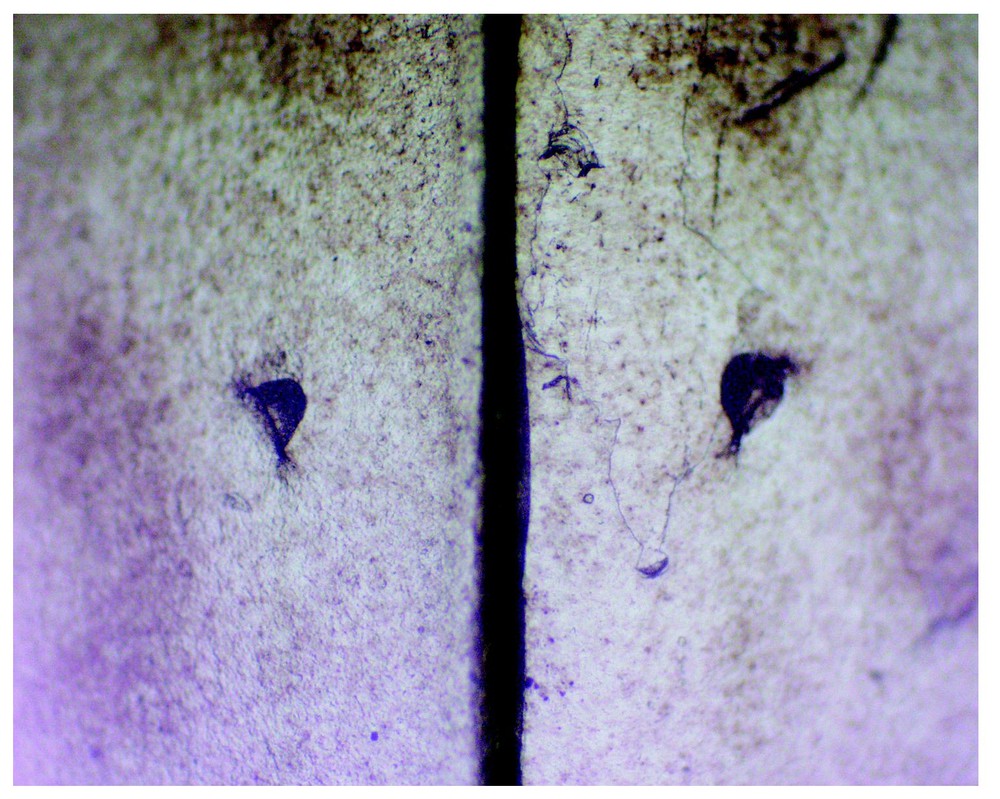
From a 10-day-old P. elegans (with PPs 1:1). Part of its first shed skin bears witness to PP presence in the parietal shields (roughly 2×3 mm each), of which a part of the common suture is shown here as a large vertical separation. The PP orifices are about 100 μm in ∅. (Coll. CdH, Psammophis elegans No. F1-2000.5-1, first slough 00.5.18, mounted on slide.) Photo: A. Cluchier.
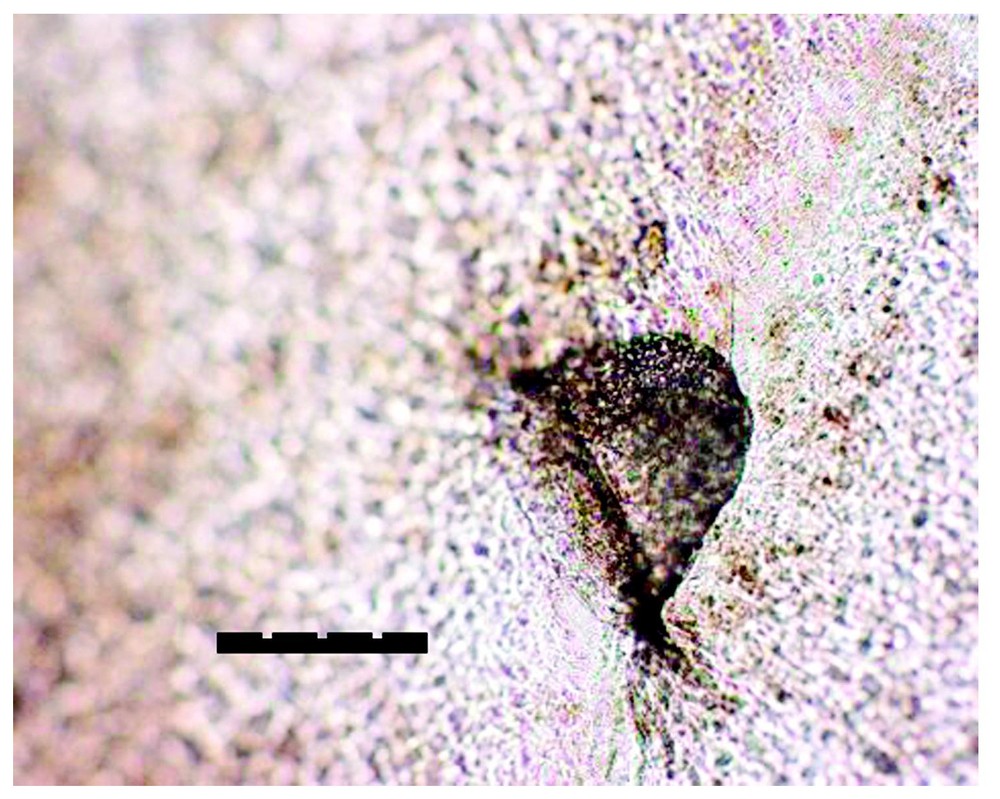
From a 10-day-old P. elegans (with PPs 1:1). Part of its first shed skin shows the left PP print in Fig. 4, magnified. The bar =100 μm (0.1 mm). (Coll. CdH, Psammophis elegans No. F1-2000.5-1, first slough 00.5.18, mounted on slide.) Photo: A. Cluchier.

Sketch, made from light microscopy image magnified 400×, to highlight some details of Fig. 5. (Original drawing by Myriam Comet.) A: Elliptical shaped PP orifice, about 0.1 mm in maximal ∅, with a thickened rim of unidentified fibres at the external surface of the parietal shield and lying, rostrally directed, in a mussel-shell shaped depth (cf. Fig. 3). B, C: PP wall and floor of stratum intermedium type epidermal cells. D: Mainly transparent, non-epidermal looking vesicula, shaped – in this case – like a fairly well inflated balloon. In other cases, D may have all sorts of flattened-balloon shapes (except in the case of ‘parietal slits’, A, B and C are more or less similarly shaped in the parietal pit prints found in the various psammophine species).
In addition, Table 1 indicates which one of the two self-rub systems (M- or P-system) has been adopted by the psammophine species [1]. Occurrence of PPs seems to be unrelated to a particular self-rub system.
Thus far, PPs have been found for certain in individuals of seven species belonging to four genera of the Psammophiini-tribe. Other species and genera of this tribe could also easily be concerned, as may be gathered from the data provided here.
No PPs were found in the individuals of several Psammophis species that have not been listed here, but are in Table 2 in [1], among which 21 P. schokari.
Nor were PPs found in the following captive individuals showing the M-self-rub system: four Hemirhagerrhis kelleri, six Malpolon moilensis, six Mimophis mahfalensis, seven Psammophylax rhombeatus.
4 Discussion
Small (∅ 30–300 μm) cephalic structures, generally called pits, exist in many snakes, including Psammophiini. They are situated on diverse upper head shields, and may look like pits in shed skins by microscopic light transparency; however, they do not have substantial depth, nor are they more permeable to different dyes than other parts of the shields [4]. They are often accompanied by much smaller (∅ 15–25 μm) and more numerous, convexly elevated tubercles, which are also present on the underside of the head. The following roles have been suggested: a tactile role for the tubercles, which is presumable on the basis of the work of Aota 1940 [5], and a radiation sensing role for the so-called ‘pits’ [4]. For these pits, however, including the more documented ones found on the heads in several species of Alsophis, Dasypeltis and Mehelya (cf. [4]), I suggest a function comparable to that of the pit-like structures on the apex of dorsal body and tail scales in most Colubridae and Viperidae. These so-called ‘apical pits’ are not genuine pits and appear to serve as final joints between old and new skin, enabling correct shedding at an opportune moment: Chiasson [6] and Chiasson and Lowe [7] propose the term apical stigmata for these structures. I examined ‘head pits’ of alcohol-preserved Alsophis and Dasypeltis specimens at MNHN-Paris and did not find their structure comparable in any way to the psammophine parietal pits (PPs), as described in the present paper.
The sporadic occurrence of the newly found parietal pits (PPs) among conspecific individuals may suggest the influence of some ecological phenomenon. Should this be the case, it existed before the worldwide impact on the environment resulting from human activities over the last century. Indeed, specimens of Psammophis elegans at MNHN-Paris show that in the period 1895–1896, parietal pits occurred sporadically as well (see details below Table 1).
So far reported in this paper, the occurrence frequencies of the PPs found in NE-Moroccan, Egyptian and French Malpolon monspessulanus might suggest a relation between these frequencies and definite populations. However, ongoing investigations seem to weaken this possibility.
The location of the pits on the top of the head might indicate the existence of a close connection with a cerebral structure. This is the case for the parietally-situated head pits connected to balancing-organs in sharks or for the vestigial parietal eye connected to the pineal complex in the Tuatara and in many lizards [8]. In snakes, a balancing-organ with neuromasts, such as occur in fish, has never been found. Sadia et al. [9] studied the pineal complex in three colubrid snakes, among which one psammophine: Psammophis schokari. First, these authors did not find evidence of even rudimentary photoreceptor cells in the pineal parenchyma of the examined snakes. Further, they observed what is known for snakes in general, i.e. that the pineal of the three species showed remarkable similarity to the mammalian pineal, which implies absence of a connection with the parietal region. It would be surprising if the absence of such a connection in P. schokari were due to a general absence of PPs in this species. In my study so far, no PPs were found in P. schokari. However, as shown in Table 1, Psammophis phillipsi should offer excellent material to test for the possible presence of a connection between the pineal and PPs.
5 Conclusion
Superficially, and according to the ‘prints’ of their internal structure, which are visible in shed skins, the here reported parietal pits (PPs) do not resemble any earlier documented parietally situated ‘head pits’ in snakes. A possible relation between the presence of PPs and specific behaviour patterns has not yet been noted. Since the sporadicity of the PPs represents an additional enigma and a histological study is lacking so far, further discussion here of the possible functions would be premature.
Acknowledgements
I would like to thank for most valuable help to my research or for improvement of the manuscript James Aronson (CNRS-Montpellier, France), Sophie Berland-Timmel (MNHN-Paris, France), Wolfgang Böhme (ZMFK-Bonn, Germany), Laurent Chirio (MNHN-Paris), Alexandre Cluchier (EPHE-Montpellier, F.), Mrs Gregg Colin (Paris), Myriam Comet and Klaas B. Stapert (Balaruc-les-Bains, F.), Marguerite de Haan (Loiras, F.), Jean Garzoni (Lausanne, CH), Michel Guillod (Servion, CH), Ivan Ineich (MNHN-Paris), Ulrich Joger (HLM-Darmstadt, Germany), Angela Lubbers (Goirle, NL), Saı̈d Nouira (Ecology Lab., Fac. of Sc., Tunis), Olivier S.G. Pauwels (IRSNB-Brussels), Jean-François Timmel (Paris) and an anonymous reviewer.



