1 Introduction
Bogert 1940 [1] found six snake genera having unusually thin (‘papilliform’, if not quasi filiform, unarmed with simple sulcus) and short (3–4 subcaudal scales long) hemipenes. Based on these features, he presented the six as his African colubrid group XVI, and associated them with a seventh genus: the Malgache (or Madagascan) Mimophis ([1], pp. 78 and 81).
The fact that these seven genera share features, such as opisthoglyphic teeth, with other genera, did not persuade Bogert ([1], p. 8) to group them in the subfamily Boiginae, a colubrid taxon essentially based on opisthoglyphic criteria. They have been alternately presented as the tribe Psammophini in the subfamily Colubrinae by Dowling in 1967 [2] or elevated to subfamily status – Psammophinae – by Bourgeois in 1968 [3]. One of the seven, Cerastes, has since been renamed Psammophylax (cf. Broadley [4]). Broadley [5] proposed and established an eighth psammophine genus, Dipsina (cf. Table 1), which until then had been inappropriately ‘sunk’ within Rhamphiophis.
Global distribution of the Psammophiini by genera and species numbers (Africa=continental Africa, Eur.=Europe, Madg.=Madagascar)
| Psammophiini | Number of species | References | ||||
| Eight genera | ||||||
| World | Africa | Asia | Eur. | Madg. | Branch [12] and: | |
| ∼43 | ⪢∼38 | 7 | 1 | 1 | ||
| Dipsina | 1 | 1 | Broadley [5]; Chirio and Ineich [21] | |||
| Dromophis | 2 | 2 | Loveridge [22]; Chippaux [23] | |||
| Hemirhagerrhis | 4 | 4 | Broadley and Hughes [13] | |||
| Malpolon ∗ | 2 | 2 | 2 | 1 | Bons and Geniez [24]; De Haan [25] | |
| Mimophis | 1 | – | – | – | 1 | Glaw and Vences [26] |
| Psammophis | ∼25 | ∼21 | 5 | Brandstätter [9]; Hughes [27]; Broadley [28] | ||
| Psammophylax | 3 | 3 | Broadley [4] | |||
| Rhamphiophis | 5 | 5 | Broadley [5]; Chirio and Ineich [21] |
∗ Malpolon monspessulanus is present on three continents, from eastern Iran to the Atlantic Ocean, in most regions having a Mediterranean-type climate. Malpolon moilensis (or Scutophis moilensis, cf. Brandstätter [29]), is present in more arid northern Africa and southwestern Asia.
Dowling and Duellman [6] consider the psammophine hemipenis as vestigial; however, their “less than 5 mm” has to be read as less long than five subcaudal scales. These authors slightly modified the former tribe name to Psammophiini, which they put – with more than the usual debate ensuing (see [7–10]) – into the subfamily Lycodontinae.
Zaher 1999 [10] recognized the subfamily Psammophiinae, but erroneously (pers. comm.) placed Dipsina and Dromophis among the ‘Boodontinae’. Böhme 1999 [11] preferred to regroup the three opisthoglyphic snakes of Europe, of which one is a psammophine, into the classic taxon Boiginae. Some authors, most notably Branch 1988 [12], Zaher [10] and Broadley and Hughes ([13], p. 14), followed Bourgeois [3] in considering psammophines – still informally – as Psammophi(i)nae. I concur with this latter approach since I documented in all eight genera (unpublished) – in addition to the extremely simple, perhaps vestigial, hemipenis – another unique morphological character for psammophines, namely the external narial valve.
This valve, described for the Montpellier snake Malpolon monspessulanus by Darevsky 1956 [14], has been overlooked in taxonomy, in spite of the fact that it bears the outlet of a special nasal gland that secretes pheromone-containing fluid [15]. The fluid is applied by the snake to its belly and tail during self-rubbing behaviour, which is briefly explained and discussed below. Regarding the hemipenis, Schleich et al. [16] for Malpolon and Brandstätter [9] for Mimophis relied on erroneously scaled drawings in Domergue [17,18], which led to an unjustified ‘inflation’ of the hemipenis as a taxonomic criterion. Indeed, Brandstätter ([9], p. 11) concluded that the Malgache endemic Mimophis “is a parallel genus, not connected with the continental Psammophiini by a common origin”.
Concerning Malpolon, the error of a hemipenis size four times enlarged has been pointed out and rectified by De Haan [19]. Concerning Mimophis, Joger et al. [20] found molecular clustering among Malpolon, Mimophis and Psammophis, and I present here additional arguments for a close relationship among these genera.
Table 1 presents the global distribution of the psammophine genera as currently understood, which includes the perpetual incertitude about the number and names of species in the genus Psammophis. In Table 2, names of species preceded by ‘cf’ reflect this problem. However, notes 1 to 4 in that table, referring to photographs and descriptions in the cited literature, should help in identifying the questionable taxa.
Psammophine species showing infralabial outlets (ILOs)
| Live (captive) specimens Collection CdH | Periodical ILO presence (in IL-shield serial nr left/right) | Alcohol-preserved specimens Collection MNHN-Paris | ILO presence (in IL-shield serial nr left/right) | |||
| Self-rub system | n | n | ||||
| Dromophis | ||||||
| Dromophis | lineatus | 2 | −/−, 4/− | |||
| P | lineatus Cameroon + RCA | 7 | 4/4 | praeornatus | 9 | −/− |
| P | praeornatus Cameroon | 1 | ? cf. > | > praeornatus 1020 | 1 | 5.6/5.6 |
| Psammophis | Psammophis | |||||
| P | condanarus Thailand | 1 | 4/4 | biseriatus | 1 | 5/5 |
| P | crucifer S-Africa | 1 | 4/4 | elegans | 20 | diverse |
| P | elegans Cameroon | 16∗ | 5/5 | punctulatus 1999-9281 | 1 | 5/5 |
| P | notostictus S-Africa | 1 | 4/4 | cf. sibilans Mauretania 1996 | 5 | −/−, 5/5 |
| P | phillipsi Cameroon + RCA | 74∗ | 4/4 | tanganicus 1895-427 | 1 | 5/5 |
| P | cf. phillipsi N-Cameroon | 3 | 4/4 or 5/5 | tanganicus 1895-428 | 1 | −/− |
| P | cf. phillipsi (1) ‘Nigeria’ | 3 | 4/4 or 5/5 | Details provided below the table | ||
| P | cf. rukwae (2) Cameroon | 8 | 4/4 or 5/5 | |||
| P | schokari Egypt + Morocco | 21∗ | 4/4 or 5/5 | |||
| P | cf. sibilans ‘Africa’ | 1 | 5/5 |
|
||
| P | subtaeniatus S-Africa | 1 | 4/4 | |||
| P | cf. subtaeniatus (3) ‘Africa’ | 5 | 5/5 | |||
| P | cf. subtaeniatus (4) E-Africa | 2 | 4/4 | |||
| P | tanganicus E-Africa | 2 | 5/5 | |||
| Mimophis | ||||||
| M(!) | mahfalensis Madagascar | 4 | 4/4 or 5/5 |
After finding territoriality in Malpolon monspessulanus ([15], cf. [19]), a behaviour pattern previously unknown in Serpentes and clearly related to both the self-rubbing behaviour and pronounced sexual dimorphism in this species (tail length excepted), I have since studied the behaviour in captive specimens of many other psammophine taxa. These are mostly smaller than M. monspessulanus and – compared to that or any other snake – seem to lack sexual dimorphism. In several of them, I observed heretofore non-described extrabuccal infralabial secretion outlets, which are periodically absent. This is the main subject of the present paper.
2 Material and methods
Captive representatives of all psammophine genera except Dipsina have been observed for 3–11 years in order to learn about their self-rubbing and other behaviour.
The first column of Table 2 lists those of particular interest for the present study. Numbers of individuals followed by an asterisk in the table include captive-born F1 or F1+F2 specimens. For several species, small groups of young and adult snakes, separated or together, were observed in indoor terraria of 0.12 m3, interconnected by transparent 8 cm ∅ tubes, with the entire structure available per species measuring 2–12 m long. The main features of the terraria included: dry, neutral-smelling wrapping-paper floors with many hiding places under bark, in cartons, vegetal litter and a few hot-beds, plus suspended branches and twigs, food and drinking water seasonally ad libitum, optional temperatures between 20 and 45 °C (March–September), 15 and 35 °C (October–February) and French Mediterranean sunlight through glass or netting panels. A video-camera (Sharp VL31) recorded snake activities during presumed and actual mating periods in daily sequences of two hours from sunrise, or – occasionally – when special behaviour was expected following uncommon visual or audible cues from the terraria. Such cues also led to photographing infralabial shields (IL-shields) of several snakes, in particular just after their skin shedding. Shed skins from most individuals have been dated and are preserved in the author's personal collection and will be in part deposited at the ‘Muséum national d'histoire naturelle’, Paris. MNHN-Paris psammophine specimens, preserved in alcohol, have also been examined and described for both external narial valve and infralabial shield structures.
In addition, 2–6 individuals of 53 non-psammophine species, from all snake families except seasnakes and wormsnakes (s.l.), have been examined. None of the structures in question was found, but in the future it would be useful to search for them systematically, as well as for ‘parietal pits’ sensu De Haan 2003 [30].
3 Results
3.1 Different external secretion outlets on the psammophine snake's head
Representatives of all eight psammophine genera showed the presence of an external narial valve, similar to the one described by Darevsky [14] for Malpolon monspessulanus. This valve bears the outlet for the special nasal gland secretion that is applied by ‘self-rubbing’ to the belly and tail. Captive individuals of about 25 species representing seven of the eight genera (Dipsina was not available live) have been observed practising self-rubbing, using their narial valve outlets, explained below.
Table 2 lists about 20 species representative of three psammophine genera (Dromophis, Psammophis, and Mimophis) in which were found not previously reported extrabuccal infralabial secretion outlets (ILOs). These are situated in the 4th or 5th infralabial shield (IL), seldom between them or in the 6th IL, and most notably, are periodically present/absent. All captive individuals listed in Table 2 were not only observed practising self-rubbing (using their narial valve outlets), but those accompanied by conspecifics have also been observed practising what I would call ‘congener rubbing’, using their extrabuccal infralabial outlets (ILOs). Both these behaviours are discussed in Section 4.
Concerning the ‘Periodical ILO presence’ mentioned in Table 2, it should be noted that:
- – the Collection-CdH specimens were examined in vivo over at least three years, during which their ILOs were often absent; in the table, these temporary absences are not reported;
- – the MNHN-Paris alcohol-preserved specimens were examined for the presence or absence of ILOs and ILO traces. In Table 2, this is reported, e.g., as follows: 4/−, which means: “present in the 4th left IL-shield, absent in the right hand serial of IL-shields”.
Infralabial outlets (ILOs) were not found in representatives of the remaining five psammophine genera. Of these, Dipsina has been examined only from two alcohol-preserved specimens, while the others (Hemirhagerrhis, Malpolon, Psammophylax, Rhamphiophis) were represented by live specimens observed during at least four years.
3.2 Secretion outlets on infralabial shields: aspect and periodical presence/absence
In P. elegans and P. schokari, extrabuccal infralabial secretion outlets (ILOs) appear when the snakes are about 15 days old, i.e. some days after the first skin shedding. Their second slough does not show evidence of secreting activity from ILOs, but their third does. In P. phillipsi, ILOs appear some weeks after the first or just after the second slough, i.e. at age 1–4 months. The normal occurrence of the ILOs for each side is one or two in the 4th or 5th infralabial shield that touches both anterior and posterior sublingual shields (Figs. 1 and 4). In the genus Psammophis, this location coincides with the free mandibular space inside the mouth where the tips of the two elongated mid-maxillary teeth rest. This space is bounded by fleshy structures resembling salivary glands. In an infralabial (IL-) shield measuring 3×1.5 mm, two minuscule outlets spaced 1 mm apart may be conspicuous. If so, often one is open with a trace of dry secretion, while the other seems to be closing, or is already closed due to cicatrization. Some weeks before skin shedding, up to 1/4 of the IL-shield may be occupied by altered epidermis covering the outlets, of which only one seems functional at this stage. However, in about 20% of the examined skins showing the presence of an ILO, two outlets on a shield seem to have functioned simultaneously, shortly before shedding. A histological study is lacking so far.

P. phillipsi (a): Adult male. Underside head (max. head width here is 12 mm). ILO-traces in IL-shields serial numbers 4/4. Right 4th IL-shield showing advanced healing of recently heavily affected epidermis around the now closed ILOs. The depigmented area has started to be covered by whitish or dark pigment and may be completely covered within a few weeks. Same individual (a) as in Figs. 2 and 3. Photo: A. Cluchier.
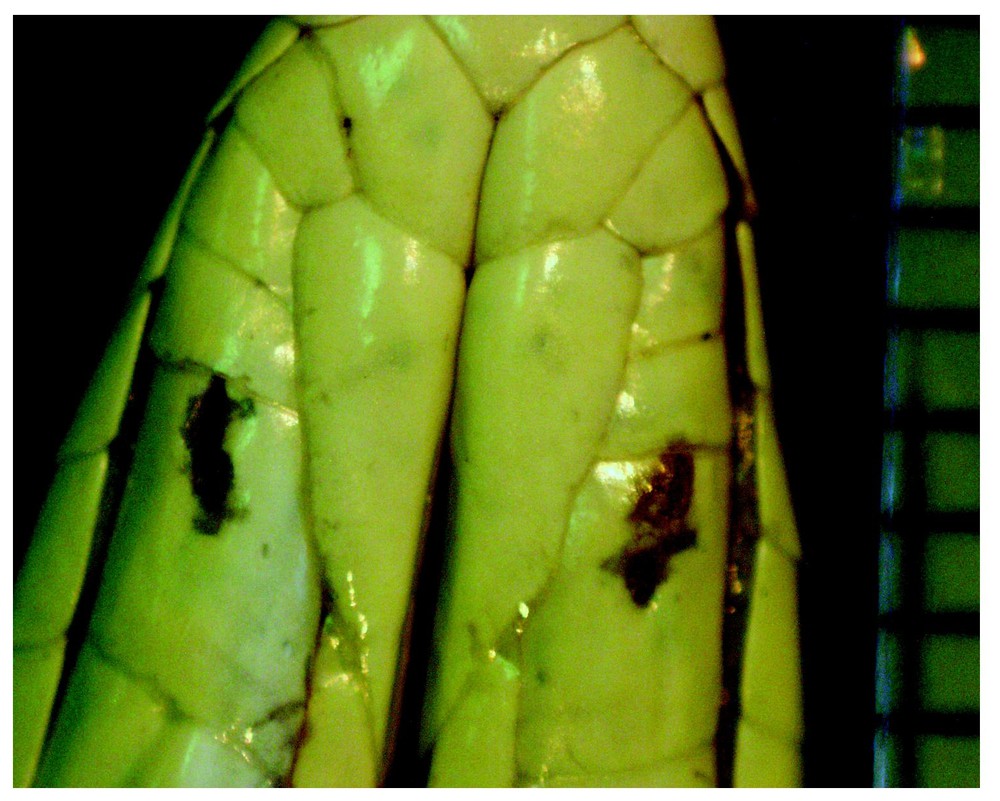
P. elegans (b): adult female: part of underside head. On the right side of the picture is a scale of 8×1 mm. On the left side of the picture, the right 5th IL-shield shows heavily affected, but cicatrized, epidermis. A covering crust was removed with the last shed skin. Ten days before, within this affected complex, two ILOs separated by about 1 mm seemed to be open. For details on the left IL-shield, see Fig. 5. Photo: A. Cluchier.
Figs. 1–5 show stages when ILOs or ILO traces are most conspicuous. After having temporarily disappeared, ILOs may come back just beside their former site in the shield, and one outlet may even re-emerge in an adjacent shield (cf. No. 1990-4635, MNHN-Paris: P. elegans, noted below Table 2).

P. phillipsi (a): enlarged details of Fig. 1. The right 4th IL-shield shows a smooth region without pigment (R), black and greyish pigment spots (S), and a rough ‘healing wound’ (W-W) around two disappearing ILOs. Same individual (a) as in Figs. 1 and 3. Photo: A. Cluchier.

P. phillipsi (a): enlarged details of Fig. 1. The left 4th IL-shield shows two recently healed ‘wounds’ on smooth skin without pigmentation, close to greyish pigment spots. The long plain black (pigment) spot is on the 4th Supralabial shield. At the right side is an unspotted part of the left anterior sublingual shield. (See also legend to Fig. 1.) Same individual (a) as in Figs. 1 and 2. Photo: A. Cluchier.
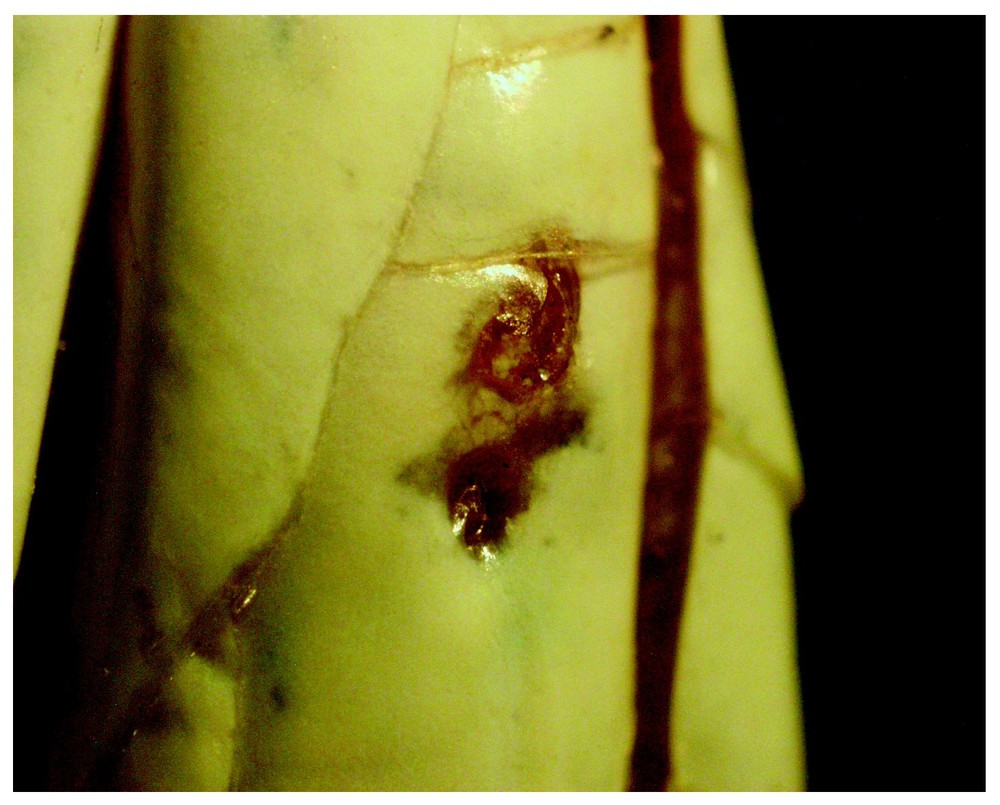
P. elegans (b): enlarged detail of Fig. 4. Left 5th IL-shield with two ILOs of which one is open (0.3 mm ∅) and recently reactivated at the margin of a cicatrized, but still depigmented region. The other is covered with the crusty results of epidermis affecting secretion. Altered epidermis extends to the suture between the 5th and 4th IL-shields. Photo: A. Cluchier.
In the alcohol-preserved MNHN-specimens listed below Table 2, no more than one outlet per corresponding IL-shield could be distinguished. In live specimens, even if outlets with altered surrounding keratine are present, they may easily be inconspicuous in dark pigment-spotted IL-shields (cf. Fig. 6). The outlet's aspect can vary from clean, minuscule and round (<0.2 mm ∅, not shown) to crusty and amorphous (shown in Figs. 2 and 5). This latter aspect is obviously due to an old epidermal layer having blocked and accumulated secretion between the old and new epidermis of an IL-shield. Subsequently, just after skin shedding, the IL-shield on the snake may either be crusty or – more frequently – clean but partly lacking in pigmentation, showing (at 10–20× magnification) underlying blood vessels and often a sort of cicatrized wound (Fig. 3).

P. phillipsi (c): adult female; part of underside head (max. width here is 14 mm), spotted with dark pigment. ILOs and/or ILO-traces in IL-shields numbers 4/4, which are less conspicuous than in the case of the unspotted IL-shields of the P. elegans in Fig. 4. Photo: A. Cluchier.
Renewed ILO activity occurs immediately after each shedding in most P. phillipsi, or – independently of several sheddings – remains absent during 9 or 10 months per year in Dromophis lineatus. These different periods seem to be related, at least partly, to reproduction cycles in the species concerned. Annual series of shed skins per individual snake bear witness to shape alterations or temporary absences of ILOs. They may give an indication (cf. Fig. 7) of the periodicity of the secreting activity.

P. elegans (d): juvenile, aged 3.5 months: Part of its third shed skin showing, by transparency, the respective crusts (∼0.2 mm ∅×0.2 mm thick) removed, by skin shedding, from two ILOs of which one was situated in the suture between 4th and 5th left IL-shields, the other on the 5th left IL-shield. At the right side: suture with the left anterior sublingual shield. Photo: A. Cluchier.
3.3 ‘Rubbing’ behaviours
Self-rubbing (i.e. performed by a snake with the outlet of the closed left or right narial valve pressed onto the belly and then onto the tail), was filmed for the first time in representatives of Dromophis, Hemirhagerrhis and Mimophis. Two systems of self-rubbing have been described by De Haan 1982 [35]. The P-system (for Psammophis) is adopted by Psammophis and Dromophis; the M-system (for Malpolon) by five of the other six genera. The missing genus, Dipsina, which probably uses the M-system, has not yet been reported to practise self-rubbing. The systems P and M are indicated in Table 2 and briefly explained in the discussion below. No other basic self-rub system has been found, but within both system groups minor differences per species, related to the number of ventral shields, have been observed.
Congener rubbing (i.e. performed by a snake with the chin and infralabial outlets pressed onto the back and neck of a conspecific individual) has been observed most easily in Psammophis species. It seems to be frequent and ‘rather automatic’ when an individual crawls along the length of a congener's back, but rare and ‘purposeful’ if done with zigzag movements only on the congener's neck. The zigzagging has been observed in P. phillipsi and is performed exclusively by adult females on an adult male's neck, whereas the back-length stroke is practised by both sexes, but especially by males onto females. (Additionally, in P. phillipsi often two or more individuals lie together on the look-out, with their heads alternately resting one upon the other.)
4 Discussion
In the Crocodilia and several Testudines, there exist mandibular epidermoid holocrine glands, probably related to social behaviour [36]. Their extrabuccal outlets or protuberances are permanently visible but some of them may change shape according to activity levels of the gland. In the Serpentes, two other types of externally secreting cephalic glands are known. Their respective outlets are permanently visible. One is found in the tribe Psammophiini (Colubridae), situated externally in the narial valve, connected to a special nasal gland and related to self-rubbing behaviour (this paper; cf. [14,19,35,37]).
The other is found in the genus Echis (Viperidae), situated in the last supralabial shield [38]. A histological analysis revealed its connection with a supralabial gland having both merocrine and holocrine properties, but the role of its secretion has yet to be investigated [39]. In the case of the periodical infralabial outlets reported here, no study has yet analysed the secretion or revealed the type and the location of its source. However, the function of the secretion involved seems to be explained by the results of the behavioural observations of which the following are discussed more in detail.
Many authors consider the psammophine self-rubbing (or ‘self-polishing’ or ‘grooming’) as a means of preventing evaporative body water loss through the skin, based on histochemical findings, physiological experiments and a non-tested hypothesis of Dunson et al. [37]. I argued that this hypothesis incorrectly assumes that in Malpolon monspessulanus the whole body is covered with the nasal secretion [15]. Like Dunson et al. (loc. cit.), Mantel and I (unpublished) have ascertained that the secretion, when leaving the narial valve outlet, is watery and contains a mixture of mainly proteins and fatty acids. However, as Darevsky 1956 [14] had already indicated, it is applied by self-rubbing via the narial valve outlets, in two long, rather compact zigzag patterns onto the belly and the tail, where it then dries quickly, and I have shown that it subsequently serves a function of chemical marking [15,19].
Self-rubbing acts are carried out by the snake only under safe conditions. The subsequent marking works mostly ‘automatically’ by crawling, resulting in slightly sticky secretion particles left behind [14,15]. These are afterwards detected and interpreted by the marker and conspecifics, in a lingui-vomeral way. In Malpolon monspessulanus, a species showing pronounced sexual dimorphism (uniformly coloured, stout males, up to 2.30 m long, and spotted, slender females, up to 1.40 m long), I observed chemical marking with ventrally scraped off nasal secretion particles [15,19]:
- • of hunting routes traced on substrate by all individuals, irrespective of sex and age;
- • of conspecifics in holes and other dark places, where the marking afterwards serves for mutual recognition, prevents panic and favours clan formation, but might also discourage aggregation since given individuals risk becoming the prey of opportunist conspecifics;
- • of individual territory limits traced upon and between optical landmarks by certain adult males, limits which they guard and defend as well as their respective females against most male conspecifics and diverse allospecific intruders during 6–8 weeks in May/June;
- • by a territorial male, marking his female and one or two vassal-males, which also – if not mainly – mark themselves dorsally by frequently creeping under the territorial dominant.
Additionally, I observed that the guarded female M. monspessulanus uses her cloacal scent gland secretion for direct marking – with tail held upwards – the flanks of the territorially dominant male. This does not provoke any reaction in the male, but does lead to sudden retiring on the part of an outsider female trying to establish body contact with the male [19].
In the present study, captive Psammophis elegans (slender male and female adults: 110–170 cm in length) and P. phillipsi (moderately stout male and female adults: 120–180 cm in length) show a strong tendency to stay by day on tree stems of their body diameter or less. In nature, these and other Psammophis species, though often considered ‘essentially terrestrial’, are reported, copulating or looking for prey, in trees or bushes a few meters above ground (cf. [9,12,33,40–44]). Even P. schokari, a species well known to be desert dwelling, and as such purely terrestrial, also stays in trees, as may be seen in oases (J.-P. Baron, pers. comm.; P. Geniez, pers. comm.).
Contrary to the swollen and armed everted hemipenes of many other snakes, the everted short, smooth and quasi-filiform or ‘papilliform’ hemipenis, unique to the Psammophiini tribe, enables immediate uncoupling. So, frequent mating on exposed ground, as done by most ‘Malpolon’ system self-rubbers, and – more particularly – in trees and shrubs, as done by several species showing the ‘Psammophis’ system, implies no risk that coupled bodies and vital parts might hook onto twigs if it is urgent to flee and hide (partly unpublished; cf. [15]).
The ‘Malpolon’ system of self-rubbing and most of the subsequent markings by belly-to-back contact, as well as direct markings with cloacal scent gland secretion, are feasible only when the snakes can move on a firm and broad substrate, and have access to objects providing solid counterbalance. In contrast, the ‘Psammophis’ system of self-rubbing permits Psammophis and Dromophis to carry out the performance on the ground as well as in trees, without risk of falling out (unpublished, cf. illustrations of the two systems in [35]). However, the nasal secretion applied onto the belly and tail in a non-compact streak pattern, to be subsequently scratched off, obviously does not enable efficient marking of conspecifics in trees. There, it is carried out by ‘congener rubbing’, i.e. by direct infralabial outlet (ILO) contact with a conspecific individual.
The most obvious, if not spectacular, ILO contact is made with the head zigzagging, shown by females holding the forepart body floating (in a ‘cantilever’ position, cf. [45]), while the hind-part and tail ensure balance. In Psammophis phillipsi couples, I observed the female zigzagging her chin onto the neck and head of the male immediately after an outsider female had joined him. And, as did the cloacal scent in M. monspessulanus, the infralabial ‘scent’ of P. phillipsi peacefully repelled the outsider.
In M. monspessulanus, the dorsal scales are grooved, which facilitates scratching off and retaining the slightly sticky particles of nasal gland secretion film from a conspecific's belly [19,46]. The other M-system self-rubbing species have smooth, ungrooved dorsal scales, which might be in relation to the observed non-cannibalistic characters of these snakes.
Among the P-system self-rubbers, so far, I have found more or less grooved dorsal scales only in the rather small and robust Psammophis crucifer. Nevertheless, marking conspecifics on tree stems by ILO contact appears to be easy since the ILO secretion seems to adhere extremely well on smooth dorsal and other scales, perhaps due to a keratine-etching secretion component. The presence of such a component is presumed because of the periodically damaged infralabial epidermis observed in and around the ILOs shortly before certain skin sheddings. However, the entire secretion, once applied to a mate's body, is invisible to human eyes.
In the American whip snake Masticophis taeniatus (Colubridae), the male has been reported as passing waves of S-shaped loops of his foreparts under the chin of a female during mating preliminaries [47]. In my study, a quite active adult female Masticophis mentovarius, very similar in appearance to P. phillipsi, has been under observation for three years for possibly having a kind of ILO. So far, she has not shown an ILO, but she also lacks the presence of a conspecific male, or any other snake. Remarkably, however, and perhaps important in the present context, she sheds her skin 10 times every year as compared to 4–6 times annually in P. phillipsi and all other captive psammophines mentioned herein.
The tiny Eastern Barksnake Hemirhagerrhis kelleri may move quickly on the ground ([41], p. 184) or vertically on trees [48]. This snake, with its adult length of 30–40 cm, is one of the smallest psammophines. My captive specimens perform the M-system of self-rubbing on the ground but also on reliably supporting clustered twigs and loose tree bark. In this species, though rather arboreal, I have not found ILOs so far.
Mimophis, the only psammophine genus living in Madagascar, where it is endemic, is – like the former taxon – observed as being terrestrial and arboreal (cf. [49]). It is represented by Mimophis mahfalensis, perhaps a monotypic species, which reaches 85 cm in length, and may be slender or rather stout. Its M-system of self-rubbing has been observed in my captive specimens, which were moving on the ground, in leaf litter. It is most notable that this M-system self-rubber periodically shows ILOs (cf. Table 2).
At least eleven Psammophis species have not yet been studied concerning their rubbing behaviour. However, it seems plausible that they will show ILOs and congener rubbing in addition to the P-system of self-rubbing, as do their presently studied congenerics.
If so, the Malgache Mimophis mahfalensis should be the only snake performing congener rubbing by ILO-contact (like arboreal Psammophis) and self-rubbing as per the M-system (like terrestrial Malpolon).
More study on psammophine snakes, in the field as well as in the laboratory, should elucidate to what extent environmental conditions have played, or still play, a role in the developmental process of behaviour occurring in connection with different secretions used as chemical markers and a tiny, ‘papilliform’, perhaps vestigial, hemipenis.
It remains to be seen if the results of a histochemical study, which is now in preparation, will support the following hypotheses based on my previous findings and on various published snake phylogenies:
- (1) the cloacal scent gland outlet has – in evolutionary terms – preceded the narial valve outlet, and this in turn preceded the infralabial outlet;
- (2) the three secretions involved help compensate for the presumable drawback for a snake of having a short and thin hemipenis and might even reinforce the presumed advantages of such an organ;
- (3) the ILO-secretion also compensates for the presumably inhibited functions of the two other secretions, when certain psammophines that are bigger than barksnakes, ‘choose’ to stay on twigs or tree stems for social activities.
If borne out by careful studies, these hypotheses might finally provide a clear answer to Bogert's 1940: 83 [1] incitement to discover how snake species with the ‘papilliform hemipenis’ manage to maintain this male mating organ inserted in the female's cloaca.
Whatever the link may be, Mimophis demonstrates a pre-eminent glandular and rubbing-behavioural relation to the variety of mainland psammophines, thus supporting, as far as needed, both Bogert's morphological ‘hemipenial arrangement’ [1] and the results of the molecular analyses provided by Joger et al. 2001 [20].
Acknowledgments
For their help to my research, I am grateful to Jean-Pierre Baron, Aaron M. Bauer, Sophie Berland-Timmel, Charles-P. Blanc, Wolfgang Böhme, Xavier Bonnet, Marc Cheylan, Laurent Chirio, Alexandre Cluchier, Myriam Comet, Yves Dachy, Marguerite de Haan, Aaron de Haas, Jeroen Determan, Benjamin Drucker, Bernard Dupont, Gabriel du Rivau, Goran Dušej, Jean Garzoni, Philippe Gaucher, Philippe Geniez, Francis Girard, Claude-P. Guillaume, Michel Guillod, Olivier Filippi, H.-W. and Patty A. Herrmann, Frans Hooykaas, Barry Hughes, Diederik Hülsmann, Ivan Ineich, Sandrine Isnard, Ricardo Jesu, Ulrich Joger, Piet Mantel, J.-C. Miranda, Jean Nicolas, Saı̈d Nouira, Annie Orth, Olivier S.G. Pauwels, Bas J. Peeters, Jean-Luc Quinquarlet, Giovanni Schimmenti, Klaas Stapert, A. and T. Steehouder, Max Sparreboom, Bernard Thorens, Rafi Toumayan, Garth Underwood, Denis Vallan, Roland Vernet, Hussam Zaher, and Peer Zwart. I am also grateful to James Aronson for his linguistic corrections and excellent suggestions to improve the manuscript, and to Ms Gregg Colin, Jan Lamberts, Angela Lubbers, Jean-François Timmel and an anonymous reviewer for their comments and additional improvements.



