1 Introduction
When materials used in human restorative dentistry such as a glass-ionomer cement (GIC) or a modified composite were applied to dentin, exchanges occur between the substances in presence. Many studies have concentrated on characterizing the adhesive forces existing between the GIC or the compomer and the dentin. Several authors [1–8] have studied the mechanical properties of these materials, which vary according to whether a conditioner or a bonding system [9] is used or not. This variation has been analysed by interface-rupture tests, backed up by micromorphologic analyses.
Except for the widely studied case of fluoride release [10–17], the chemical characteristics of ion exchanges occurring at the interface have received relatively little attention. In particular, few researchers have tackled the question of the diffusion of the other elements present at the interface, such as aluminium, strontium, calcium, and phosphorus.
Forss [18] and Kudalkar et al. [19] showed that samples of polymerisable GIC put in solution for 1 to 120 days continued to release substances such as fluorine and strontium, and also aluminium, silicon, sodium and calcium.
Lin et al. [20] studied ion diffusion at the interface through two surface analysis techniques, XPS (X-ray Photoelectron Spectrometry) and SIMS (Secondary Ion Mass Spectrometry). Each one of the techniques demonstrated a particular property of the material, but only brought out one aspect of the sample. They were thus of specific interest only.
XPS enables a qualitative and semi-quantitative analysis to be made over a depth of the order of 40 Å. It detects all the elements of the periodic table, except hydrogen and helium, and the detection threshold is of the order of 1%. SIMS [21] has greater surface sensitivity than XPS. Its detection threshold can be as low as 0.01 ppm and volumes of 50 μm3 can be analysed. The process erodes the surface and provides profiles giving concentrations of the main and trace elements at the interface level.
In the present study, with the aim of analysing the time variations in the ionic interactions at the interface between a compomer or a polymerisable GIC and the dentine, we employed a device not previously used for this type of work: an electron microprobe. The microprobe [22] has a very low detection threshold, of the order of 0.01%, and can analyse volumes as small as 1 microtube.
It also has the advantage of being non-destructive, and the specimens can therefore be kept throughout the analyses.
The aim of the study was to evidence the diffusion of elements over time at the interface between (i) a glass-ionomer cement and dentin, and (ii) a compomer and dentin, from both the qualitative and quantitative points of view.
The results were obtained during the first year of a PhD [23] and were reported on several symposia.
2 Material and methods
The study concerned 52 human teeth. They were non-carious third molars extracted from 18- to 25-year-old patients (average age 21 years 3 months) for reasons of pathological development. The teeth were sectioned across the crown immediately after extraction. The cut was made with a diamond disk on a low speed saw (Isomet 2000) operating at 200 rpm. The section was a horizontal cut perpendicular to the occlusal plane, passing 1 mm below the deepest point of the central groove.
Two different materials were studied: a glass-ionomer and a compomer.
The glass-ionomer chosen was Fuji II LC (batch 071061 GC International Corp., Tokyo, Japan). This is a resin-modified ionomer. Unlike conventional GICs, it has the property of being photopolymerisable. It is composed of a powder and a liquid and requires the prior use of a dentin conditioner (Table 1). The conditioner makes the surface more suitable for the GIC to adhere to. It dissolves the smear layer, leaving the dentin tubuli open and the dentin surface clean. Cavity Conditioner (batch 030681 GC International Corp., Tokyo, Japan) was applied using a small ball of cotton wool. The solution was left for 10 s, and then rinsed to stop the action of the acids. The surface was then dried, but not dehydrated, with a soft blow of air. The GIC was prepared as recommended by the manufacturer: a 3-g spoonful was mixed with 2 drops of liquid using a plastic spatula on a block intended for this purpose. The cement was mixed for 20 s and the mixture was systematically weighed to check that the operating conditions were always the same. The GIC was placed between the two sectioned surfaces using a dental in a single application. Excess product was removed with a dental spatula before a 40-s polymerisation.
Composition of materials
| Fuji II LC (GC) | |||
| Dentin conditioner | Glass Ionomer Cement | ||
| Cavity conditioner | Powder | Liquid | |
| – Polyacrylic acid | 15% | – Aluminium and strontium | – Dihydroxyethylmethacrylate 2HEMA 21 to 41% |
| – Citric acid | 5% | fluorosilicate glass | – Polyacrylic acid modified by methacrylate function 25 to 45% |
| – Metallic oxides | traces | – Glycol triethylene | |
| – Distilled water | 80% | – Dimethacrylate | |
| – Camphroroquinone | |||
| Dyract AP (De Trey Dentsply) | |||
| Bonding system | Dyract AP | ||
| Prime&Bond 2.1 | |||
| – Solvent acetone | – UDMA urethane dimethacrylate | ||
| – PENTA (penta dipentaerythrite acrylate monophosphate) | – TCB tetracarboxybutane (monomer containing two methacrylic | ||
| – TEGDMA (triethyleneglycoldimethacrylate) | radicals and carboxyl radicals) | ||
| – BISGMA (bis glycomethacrylate resin) | – Strontium fluorosilicate glass | ||
| – Photoinitiator, stabilizer | – Strontium fluoride | ||
| – Ethylamine hydrofluoride |
The compomer used was Dyract AP. This is a composite modified by adjunction of glass ionomer. It comprises a single-component adhesive, Prime & Bond 2.1, and comes in compules containing a single dose (Table 1). The dentin surface was rinsed and dried without dehydration. A first layer of adhesive was applied and, after 30 s, excess of solvent was eliminated with a puff of air. The UV lamp was used for 10-s polymerisation. The compomer was applied to cover both sectioned surfaces using a dental spatula and polymerised for 40 s.
The specimens were kept and aged in an incubator at 37 °C in a humid environment. They were divided into three groups. Group I received Cavity Conditioner + Fuji II LC, group II received Prime & Bond 2.1 + Dyract AP, and group III was a control group receiving no treatment (Table 2). After aging for time t (10, 20, 30 or 80 days), the specimens were coated in biological resin. For this, the teeth were first dehydrated with increasing grades of alcohol: at 70 °C for 24 h then at 90 °C for 24 h. They were then infiltrated and impregnated for 1 day in a solution of 50 ml basic resin and 0.5 g activator, and finally coated with the resin. The resin coating was applied by placing the specimens in polyethylene moulds and covering them with 15 ml of infiltration solution mixed with 1 ml hardener. The coated samples were cut perpendicular to the biomaterial/dentin interface with the saw. The cut surfaces were polished using progressively finer grained corundum disks (P800, P1200, P2400 and P4000) and finished using 6 microns then 3 microns diamond paste on Nap Pars cloths with blue lubricant followed by 1 micron with pink lubricant. In order to eliminate all impurity, the specimens were cleaned by sonication in a bath of 70-degree alcohol for 5 min. A 20-nanometer layer of carbon was deposited to facilitate the elimination of charges and the specimens were analysed by electron microprobe (Camebax SX 50) Samples were compared with standards (Sr/SrTiO3, Al/Al2O3, F/fluorapatite, Ca and Si/wollastonite, P/graftonite) in order to determine the chemical composition of the material analysed. All elements of higher atomic numbers than beryllium (4Be) could be detected. This device, which operates at a voltage of 15 kV, allows very rapid analyses of specimens (10 elements in 2–3 min) and without destruction. A video image of the specimen surface was provided by the video camera of the device. It was also possible to obtain secondary electron images (as in scanning electron microscopy), images of the distribution of an element for a given surface, and element distribution profiles. Analysis by X-ray powder diffraction (Co Kα radiation, curved position sensitive, CPS 120, INEL) showed that Fuji II LC was completely amorphous, whereas Dyract AP pointed out the beginning distinct peaks of crystallization with dhkl meas-I/I0: 3.34 (100), 2.90 (11), 2.05 (67), 1.75 (33).
Presentation of groups of specimens
| Aging time | Group I | Group II | Group III (controls) dentin |
| (days) | System cavity conditioner | system Prime&Bond 2.1 | |
| + Fuji II LC | + Dyract AP | ||
| 10 | 6 teeth | 6 teeth | 1 tooth |
| 20 | 6 teeth | 6 teeth | 1 tooth |
| 30 | 6 teeth | 6 teeth | 1 tooth |
| 80 | 6 teeth | 6 teeth | 1 tooth |
3 Results
All the results are expressed in weight of oxide for the cations and correspond to the average for the six teeth analysed per experimental subgroup. The dentin to which no material was applied presented a composition that did not vary with time.
At t=10 days for Fuji II LC (Table 3), only fluorine and strontium had diffused. The difference revealed by analysis was of the order of 1% between dentin far from and close to the material. For Dyract AP, no migration was observed (Table 4).
Analysis of distribution of elements (expressed in % of weight of oxide for cations) near interface for Fuji II LC (Al = aluminium; P = phosphorus; Sr = strontium; Ca = calcium; F = fluorine; Si = silicon)
| Element | Aging time | Dentin | Dentin | Material | Material | |
| (days) | (50 μm from | (10 μm from | (10 μm from | (50 μm from | ||
| interface) | interface) | interface) | interface) | |||
| Al | 10 | 0 | 0 | 19.7 | 19.5 | |
| 20 | 0 | 0 | 20.5 | 20.4 | ||
| 30 | 0 | 1.9 | 19.5 | 23.1 | ||
| 80 | 0.9 | 3.1 | 10 | 15.2 | ||
| P | 10 | 29.8 | 29.7 | 1.4 | 1.5 | |
| 20 | 34.8 | 35.1 | 3.2 | 1.3 | ||
| 30 | 33.2 | 30.8 | 1.3 | 1.4 | ||
| 80 | 28.7 | 28.5 | 8.8 | 2.1 | ||
| Sr | 10 | 0.2 | 0.3 | 25.1 | 24.5 | |
| 20 | 0.1 | 0.2 | 24 | 24.3 | ||
| 30 | 2 | 4.9 | 19.4 | 21.5 | ||
| 80 | 0.2 | 5.4 | 10.5 | 15.7 | ||
| Ca | 10 | 36.3 | 35.9 | 1.1 | 1 | |
| 20 | 41 | 45.3 | 1.3 | 0.15 | ||
| 30 | 40 | 34.8 | 1.2 | 1 | ||
| 80 | 38.7 | 34.8 | 6.7 | 1 | ||
| F | 10 | 0.6 | 0.7 | 3.3 | 3.6 | |
| 20 | 0.8 | 0.9 | 3.5 | 4.8 | ||
| 30 | 0 | 1.2 | 4.7 | 7.3 | ||
| 80 | 0.6 | 1.5 | 2.8 | 7 | ||
| Si | 10 | 0 | 0 | 32.3 | 30 | |
| 20 | 0 | 0.5 | 30.2 | 29.2 | ||
| 30 | 0.05 | 0.04 | 32.8 | 28.6 | ||
| 80 | 0.03 | 0.7 | 21.7 | 23.7 |
Analysis of distribution of elements (expressed in % of weight of oxide for cations) near interface for Dyract AP (Al = aluminium; P = phosphorus; Sr = strontium; Ca = calcium; F = fluorine; Si = silicon)
| Element | Aging time | Dentin (50 μm | Dentin (10 μm | Material (10 μm | Material (50 μm | |
| (days) | from interface) | from interface) | from interface) | from interface) | ||
| Al | 10 | 0 | 0 | 13.4 | 13.4 | |
| 20 | 0.8 | 0.6 | 12.7 | 12.7 | ||
| 30 | 0 | 0 | 13.82 | 13.82 | ||
| 80 | 0 | 0 | 11.35 | 11.35 | ||
| P | 10 | 33.69 | 27.64 | 1.99 | 1.99 | |
| 20 | 29.6 | 27.3 | 2.2 | 2.2 | ||
| 30 | 33.85 | 34.4 | 2.97 | 2.97 | ||
| 80 | 24.55 | 33.36 | 2.63 | 2.63 | ||
| Sr | 10 | 0 | 0.03 | 23.36 | 23.36 | |
| 20 | 0 | 0.1 | 25.2 | 25.2 | ||
| 30 | 0.2 | 0.25 | 25.1 | 25.1 | ||
| 80 | 0.16 | 0.04 | 17.02 | 17.02 | ||
| Ca | 10 | 42.58 | 41.14 | 0.19 | 0.19 | |
| 20 | 43.7 | 42.3 | 0.1 | 0.1 | ||
| 30 | 41.86 | 42.72 | 0.36 | 0.36 | ||
| 80 | 40.78 | 38.45 | 0.52 | 0.52 | ||
| F | 10 | 0.78 | 0.57 | 4.87 | 4.87 | |
| 20 | 0.72 | 0.68 | 4.94 | 4.94 | ||
| 30 | 0.73 | 0.67 | 5.04 | 5.04 | ||
| 80 | 0.06 | 0.02 | 5.79 | 5.79 | ||
| Si | 10 | 0 | 0.08 | 18.28 | 18.28 | |
| 20 | 0 | 0 | 19.1 | 19.1 | ||
| 30 | 0.04 | 0 | 19.58 | 19.58 | ||
| 80 | 0.03 | 0.04 | 18.06 | 18.06 |
At t=20 days for Fuji II LC, the analysis revealed, as at 10 days, a slight migration of fluorine and strontium (Table 3). For Dyract AP, no migration was observed (Table 4). The percentages of calcium and phosphorus tended to zero as the analysis area moved towards the compomer. The percentages of fluorine, strontium, aluminium and silicon tended to zero when the analysis area moved towards the dentin (Table 4).
At t=30 days for Fuji II LC, the migration of fluorine and strontium had increased and aluminium had started to migrate (Table 3). No diffusion was observed in Dyract AP.
At t=80 days for Fuji II LC, the migration of fluorine, strontium and aluminium had intensified. Calcium and phosphorus exchanges appeared from the dentin towards the GIC. The elements emitted from the pasting interface are clearly identified on distribution images (Figs. 1–6). The amounts of calcium and phosphorus were between 5 and 10% in the material near the dentin (Table 3). The diffusion of fluorine, strontium and aluminium varied in parallel. In Dyract AP, there was no migration of the elements (Table 4).
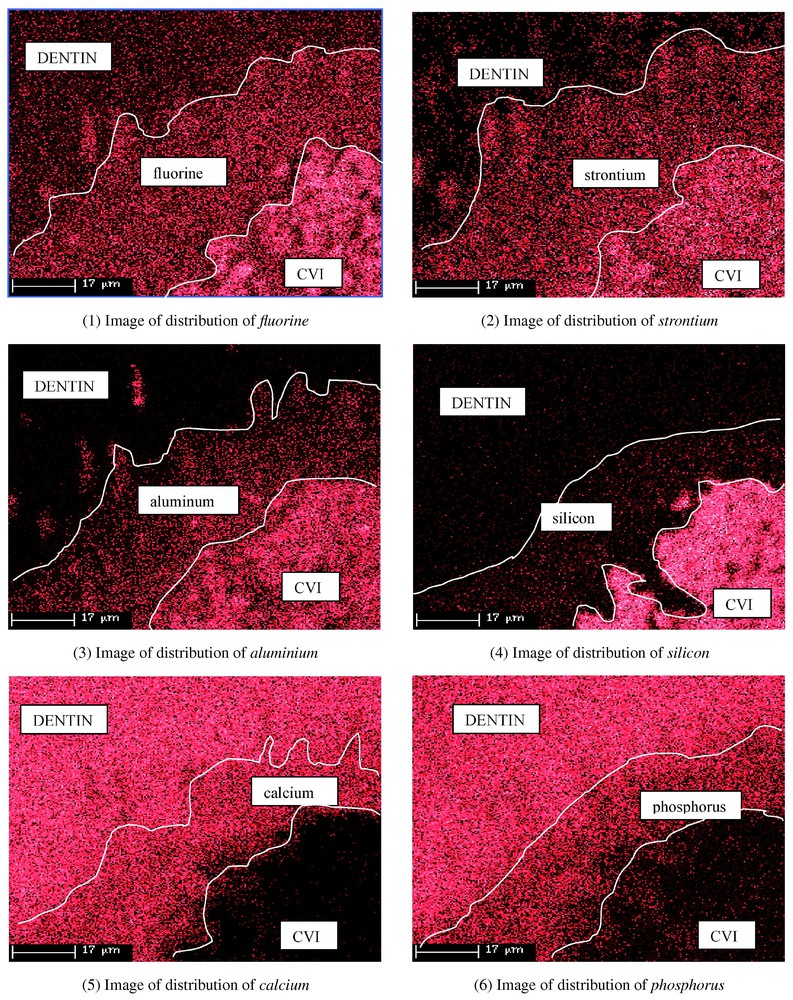
The density of points corresponds to the percents of present elements.
4 Discussion
The analysis of the distribution of the elements at and near the interface showed an absence of diffusion when the material applied was Dyract AP, whatever the age of the samples (Table 4). But it demonstrated continuous, progressive exchanges between Fuji II LC and the dentin (Figs. 1–6). After 10 days, diffusion had started for two elements, fluorine and strontium, over a thickness of about 10 μm in the dentin (Table 3). After 80 days, the fluorine has penetrated to about 50 μm. GICs' potential for releasing fluorine ions has been under study for more than 20 years. Kato et al. [24] showed that fluorine could penetrate up to 360 μm in a dogtooth and that it diffused easily along the tubuli in the dentin. The release of fluorine is associated with a reduction in the incidence of caries [11–17]. The diffusion of strontium into the dentin also started after 10 days and continued progressively up to 80 days (Table 3). Few workers have taken an interest in the migration of strontium from GICs into the dentin, although some studies describe the selective release of elementary components in contact with pure water or water acidified with lactic acid [18,19]. Our study provides evidence of the diffusion of strontium in dentin in vitro and then that of aluminium, which only starts to migrate after 20–30 days (Table 3). Aluminium diffusion thus occurs later, but in the same direction. The apatite structure of dentin is able to accept some cations, in particular strontium and aluminium [25,26].
The migration or diffusion in the case of Fuji II LC may be helped by the fact that the material is amorphous, as shown by the X-ray diffractogram. In the absence of a crystal lattice, elements are likely to be more mobile. Furthermore, fluorine exchanges by substitution of hydroxyl radicals are well known in natural apatites [27].
According to Wilson et al. [28], the glass-ionomer cements adhesion to the dental structures is due to the absorption of the polyacrylate constituent of the cement on the hydroxyapatite crystals of the dentine. This phenomenon is accompanied by phosphoapatite migration.
This spontaneous adhesion by ion exchanges between the cement and the dental structures is one of the most significant advantages of glass ionomers.
This adhesion is made possible by the attack of the cement's polyalkenoid acid on the dentine surface releasing the calcium and phosphate ions. These ions are then attracted to the adjacent layer of the cement to neutralize the acidity during the setting thus creating a layer enriched in ions at the tooth's interface. With this type of adhesion, the tooth and restoration are entirely combined.
The total absence of migration with Dyract AP, in sharp contrast with the Fuji LC II observations, can be explained by the use of the dentin conditioner before application of the glass-ionomer. This can develop an ion exchange surface, as Chigira et al. [29] pointed out.
The solution can rearrange dentin debris without opening the tubuli or demineralising the surface of the tooth too much. In addition, it reduces the surface energy of the dentin. On the other hand, in the Prime & Bond/Dyract AP system used without a dentin conditioner, the presence of the system adhesive seems to prevent any diffusion from the compomer towards the dentin, including diffusion of fluorine. According to Eliades [30], the major role of the adhesive is to stabilize the structure of the dentin region ‘impregnated’ with the adhesive. On the distribution images concerning Dyract AP, the interface shows a scalloped contact area that seems to correspond to a slight attack of the Prime & Bond, which appears to behave as a barrier inhibiting any diffusion. It should be noted that the manufacturer has recently started to recommend the use of an etching gel (De Trey Conditioner 36 Dentsply, Konstanz, Germany) containing 36% phosphoric acid before the bonding system is applied.
The absence of diffusion from the compomer can be explained by its crystallization, a process that does not take place in the amorphous Fuji II LC. Dyract AP thus accepts foreign ions less easily and, for the same reasons, the ions composing it are less mobile.
5 Conclusion
Because of its great sensitivity, the electron microprobe proved to be an excellent instrument for studying the diffusion of elements in various materials. This technique enabled us to highlight reciprocal diffusion of various elements of Fuji II LC and dentin at their interface. In addition to fluorine, strontium and aluminium were also clearly seen to migrate. In parallel, a distinct, although less marked, tendency was noted for calcium and phosphorus to migrate towards the glass ionomer. In the future, we intend to determine the characteristics of the diffusion zones obtained by identifying the structure of the products formed, notably by microdiffraction of X-rays, and to analyse specimens aged for more than 120 days, trying to plot the migrations and distributions. At the same time it may be possible to confirm or invalidate the absence of diffusion in Dyract AP when there is prior use of a dentin conditioner.
After these first results, further investigations are planned on other materials.


