1 Introduction: the ins and outs of calcium
Calcium is of fundamental importance for many biological processes. For example, one of the earliest events in the development of multicellular organisms, egg activation at fertilization, is triggered by an influx of calcium [1]. The death of neuronal cells induced by the neurotransmitter glutamate is also mediated by a raise in the intracellular calcium concentration ([Ca2+]i) [2,3]. The pro-death or pro-survival outcomes for cells of changes in [Ca2+]i depend on its amount, kinetics and source of entry into the cell [2,3]. Thus, since it is essential that [Ca2+]i is maintained within strict limits there are multiple cellular mechanisms that buffer, sequester and accumulate Ca2+ and changes in concentration are usually highly localized within the cell.
Calcium is an abundant ion and multicellular organisms constantly need to monitor and adjust Ca2+ levels outside cells ([Ca2+]out) in body fluids to maintain a stable environment. This process is termed calcium systemic homeostasis and it requires an extracellular calcium detector. Before the molecular identification of this sensor, it had been known for many years that a raise or a decrease in systemic ionized calcium (which is normally ) lowers or increases respectively levels of parathyroid hormone (PTH) in the blood [4]. PTH is a hormone secreted from the parathyroid gland whose three principal actions are to favour calcium conservation from kidney, uptake from intestine and, at longer term, release from bone (Fig. 1). Hence, PTH secretion acts to balance changes in [Ca2+]o through a negative feedback mechanism (Fig. 1).
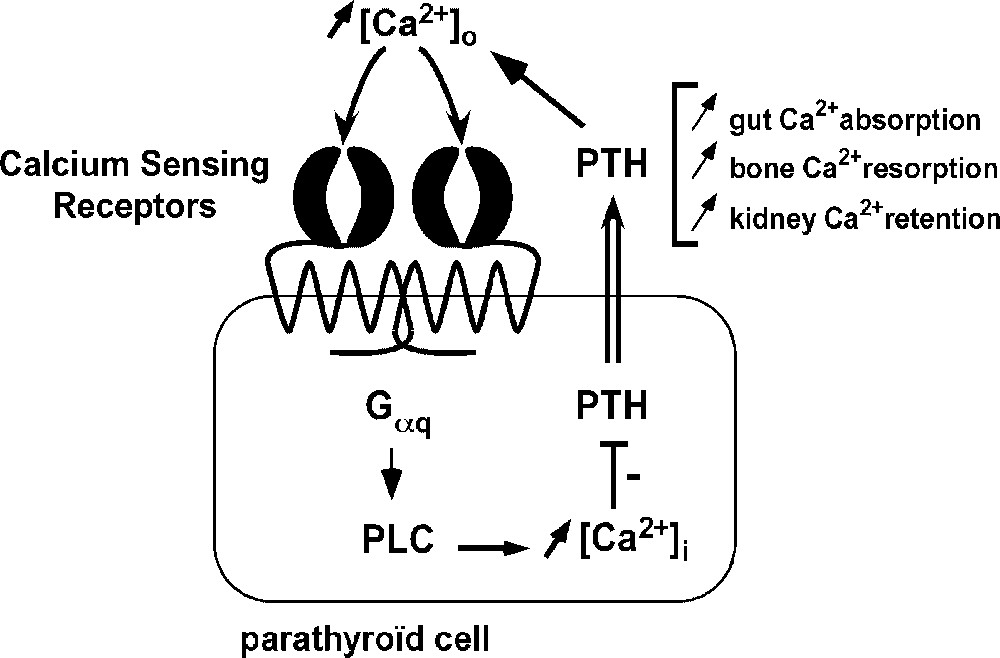
Role of the Calcium Sensing Receptor (CaSR) in calcium homeostasis and parathyroid hormone (PTH) secretion. Homodimers of the CaSR situated at the parathyroid cell membrane sense changes in extracellular calcium concentration ([Ca2+]out) by their external flytrap lobes. A raise in [Ca2+]out is transduced by the CaSR into intracellular signal cascades leading to a decrease in PTH secretion. Since PTH acts to increase [Ca2+]out, CaSR-mediated PTH release inhibition ensures return to normocalcemia through a negative feedback mechanism.
Despite these physiological observations, the identity of the extracellular calcium sensor remained elusive. Experiments performed in vitro using parathyroid cells in culture suggested that the extracellular calcium detector is situated at the cell surface. Indeed, increasing the concentration in divalent or trivalent cations less membrane permeable than calcium (such as Mg2+ or Gd3+, which are now established calcium sensing receptor activators) mimicked calcium effect in that these ions induced intracellular calcium mobilization and decrease in PTH secretion [5,6]. It is interesting to note that in contrast to most stimulus-regulated secretion systems where increasing [Ca2+]i favours secretion, the raise in [Ca2+]i in the parathyroid following activation of the extracellular calcium receptor is linked to a reduction in PTH secretion [7].
2 Cloning of the Calcium Sensing Receptor (CaSR)
In 1993, Brown and colleagues screened poly(A)+RNAs from a bovine parathyroid gland library for their capacity to induce Ca2+-activated Cl− current in Xenopus laevis oocytes following Gd3+ addition [8]. This expression cloning strategy yielded a single 5.3-kb clone that when expressed conferred the ability to recruit transduction cascades following elevation of [Ca2+]o, and thus reproduced one of the major features of native calcium sensing receptor expressed in the parathyroid [8]. Since the identification of this clone (named BoPCaR for Bovine Parathyroid Calcium-Sensing Receptor), the receptor for extracellular calcium has been identified in numerous species including salamander [9], rat [10], human [11], and in a wide range of tissues including bone, intestine, pancreas and brain (for an extensive inventory see [12]). Interestingly, a closely related receptor has been identified in dogfish shark and salmon [13], whose possible function would be to detect alterations in water salinity and orientate these fishes during their migration.
3 The CaSR is a G Protein-Coupled Receptor
Sequence analysis of the CaSR has revealed that it comprises a fusion of two distinct domains that have proved successful during evolution [8]. One domain is the seven-transmembrane-spanning topology that is characteristic of members of a G-protein-coupled (GPCR) proteins, a family of proteins that is widely expressed in eukaryotic cells and play fundamental roles in transducing signals as diverse as light and hormones [14] (Fig. 1). The other domain is extremely large (605 amino acids in BoPCaR [8]) extracellular Venus flytrap domain that is a hallmark of bacterial detectors for nutrients and ions (Fig. 1). The CaSR shows significant homology with the metabotropic glutamate (mGluR) and GABAB receptors. These neurotransmitter receptors also contain 7TM and Venus flytrap domains and all three receptor families belongs to the class III of GPCR superfamily [8]. The ability to detect extracellular cations seems to be conserved among this family of receptors since metabotropic glutamate and GABAB receptors are sensitive to changes in [Ca2+]out [15,16].
The events that occur downstream of CaSR activation are complex and can be mediated via several different signalling pathways. The functional CaSR assembles as a homodimer [17] that recruits the heterotrimeric Gq protein, resulting in stimulation of phospholipase C activity and subsequent calcium mobilization through the generation of inositol-1,4,5-triphosphate and protein kinase C activation [18] (Fig. 2). The CaSR can also bind to and activate Gi/o and G12/13 resulting in an inhibition of adenylate cyclase activity and stimulation of Rho-GEF (a Guanine nucleotide Exchange Factor for the small G protein Rho), respectively [18] (Fig. 2). In addition the CaSR can bind directly to the Filamin-A protein and this interaction is necessary for stimulation of the Extracellular signal Regulated Kinases ERK1 and ERK2 [19,20] (Fig. 2). Recent data suggest that CaSR-induced ERK1 and ERK2 activation is also, in part, dependent on the dynamic release of heparin bound-EGF (HB-EGF) through metalloproteinase activation and subsequent transactivation of the Epidermal Growth Factor Receptor (EGFR) [21]. Interestingly, antibodies that neutralise HB-EGF and drugs that block metalloproteinases or EGFR activities (CRM 197 and AG 1478, respectively) attenuate CaSR-mediated secretion of PTHrP (PTH related peptide), indicating a physiological role for this membrane spanning signalling mechanism [21].
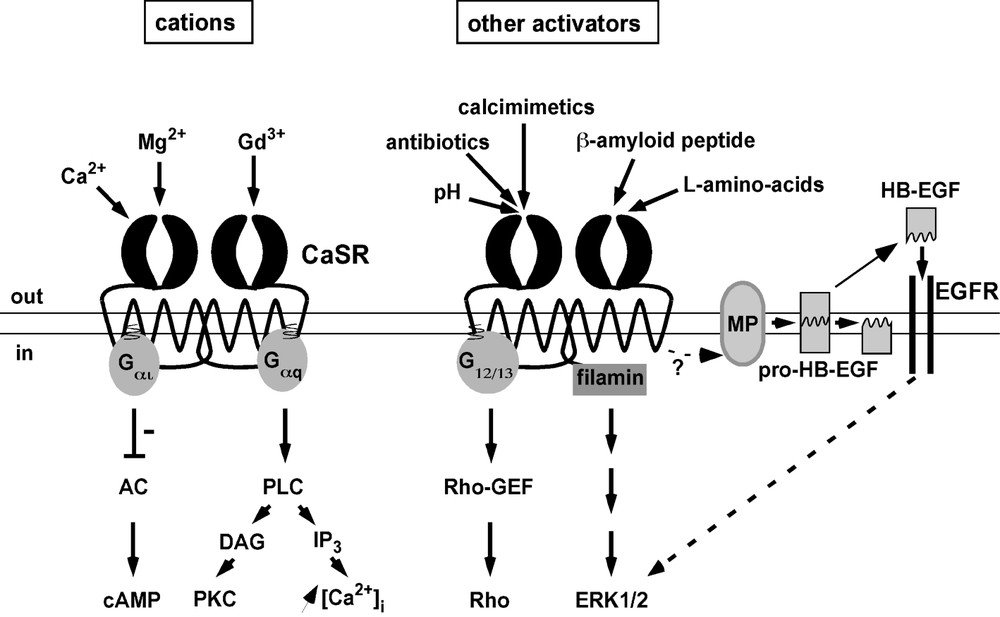
Signalling and multi-sensing by the Calcium Sensing Receptor. A plethora of substances stimulates CaSR activity leading to the recruitment of multiple intracellular pathways. These transduction cascades are detailed in the text. Abbreviations are: AC (Adenylate Cyclase), cAMP (cyclic AMP), PLC (Phospholipase C), DAG (diacylcerol), PKC (Protein Kinase C), IP3 (inositol-1,4,5-triphosphate), Rho-GEF (Rho-Guanine nucleotide Exchange Factor), ERK1/2 (Extracellular signal Regulated Kinases 1 and 2), MP (metalloproteinases), HB-EGF (Heparin Bound-Epidermal Growth Factor), EGFR (EGF receptor).
4 The CaSR is associated with human disease
As expected for a sensor playing a pivotal role in detecting and adjusting [Ca2+]o, the CaSR is crucial for the maintenance of systemic calcium homeostasis. In human, inactivating or activating mutations in the gene coding CaSR cause hypercalcemia or hypocalcemia, respectively [22–24]. More precisely, loss of function CaSR mutations are associated with familial benign hypercalcemia (FBH) and neonatal severe primary hyperparathyroidism (NSHPT), a lethal disease resulting in skeletal demineralization and multiple fractures that can be corrected by the surgical removal of the parathyroid gland of the newborn [24,25]. Individuals having FBH or NHSPT bear respectively one or two defective copies of the CaSR gene. This gene dosage feature is reproduced in transgenic animals where heterozygous mice (CaSR+/−) mimic FBH whereas homozygous mice (CaSR−/−) exhibit the typical hallmarks of NHSPT including elevated levels of calcium and parathyroid hormone in the serum, parathyroid hyperplasia, bone abnormalities, retarded growth and premature death [26]. Conversely, gain of function CaSR mutations are associated with hypocalcemia [23]. As for hypercalcemia, this human disorder could have its murine replica since a recent study describes that the Nuf mouse model, to date known to display cataract, is also subjected to hypocalcemia, reduced levels of plasma parathyroid hormone and carries an activating CaSR mutation [27]. In addition, autoantibodies directed against the CaSR have been identified in plasma of patients presenting hypocalcemia [28] or hypercalcemia [29,30], suggesting that the disease has an auto-immune component.
There is, therefore, considerable interest in the development of CaSR agonists and antagonists for the treatment of disease. On one hand, CaSR agonists (calcimimetics) are being developed to inhibit PTH secretion and treat primary and secondary hyperparathyroidisms [31]. On the other hand, CaSR antagonists and other drugs that indirectly stimulate PTH secretion through a decrease in CaSR activity (CaSR antagonists = calcilytic compounds) could in parallel provide potential anabolic therapies for conditions such as osteoporosis [31].
5 The CaSR is a multi-sensor
It seems that the primary physiological function of the CaSR is to detect extracellular calcium in order to restore normocalcemia. However, as mentioned, the receptor also responds to other substances such as Mg2+ or Gd3+. The list of substances modifying CaSR activity is, in fact, extremely long and diverse suggesting that the CaSR could have several roles. For example, CaSR activity is stimulated by an increase in extracellular pH [32], by antibiotics such as neomycin [8] or by the amyloid β-peptide fragment (which is produced in excess in Alzheimer's disease) [33] (Fig. 2). Extensive listing of ions and compounds that act on the CaSR are reported elsewhere [12,25]. Interestingly, CaSR activity is also stimulated by a mixture of l-amino acids [34] (Fig. 2) and l-amino acids such as l-phenylalanine regulate PTH release [35]. Thus it has been suggested that the CaSR could be involved in the molecular basis of the known effect of dietary protein on calcium metabolism [34]. Given the plethora of substances acting on the CaSR, one emerging question is if these extracellular clues are encoded similarly or differently by the receptor. Interestingly, it has been reported that calcium and amino acids trigger different patterns of intracellular calcium oscillations [36] suggesting that differential coding can occur depending on the extracellular signal acting on the CaSR.
6 The CaSR mediates intercellular communication
When a substance, whatever its nature, induces a raise in [Ca2+]i, a large proportion of the ion is exported to the extracellular space through activity of the plasma-membrane calcium pump ATPase (PMCA; Fig. 3) [25]. This results in an increase in local [Ca2+]o and thus, possibly in activation of CaSR situated at the plasma membrane of neighbouring cells. Hofer and colleagues have tested this hypothesis and have shown that calcium extruded from one cell following calcium mobilizing agonist stimulation can increase levels of calcium in the surrounding medium sufficiently to activate CaSR on the surface of adjacent cells in a paracrine manner [37] (Fig. 3). This calcium efflux can also activate CaSR on the same cell, in an autocrine manner [38] (Fig. 3). Further support for the concept of extracellular calcium-mediated communication, a recent study in gastric cells demonstrated that the extracellular calcium rise generated by treatment with carbachol (which mimics the cholinergic signal received during digestion processes) is both necessary and sufficient for the induction of pepsinogen secretion [39].
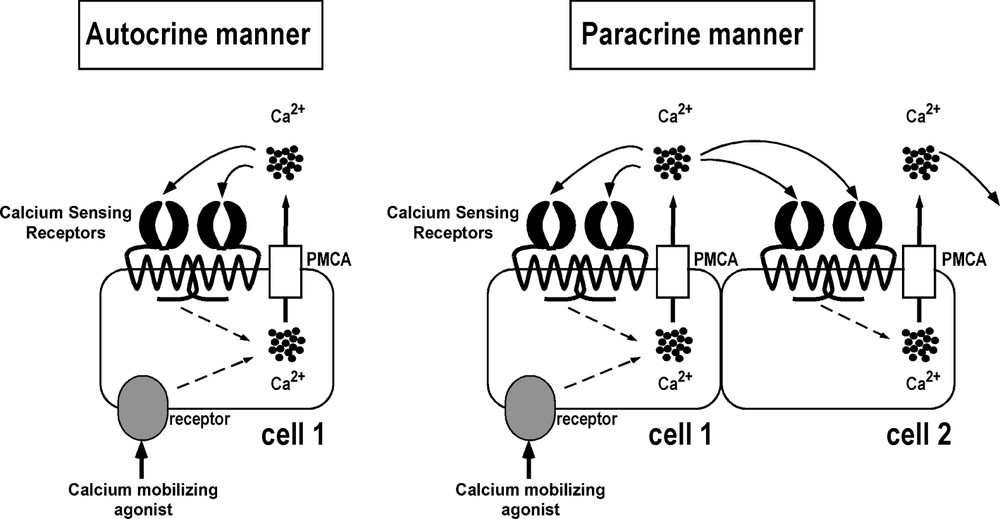
Cell-to-cell communication mediated by the Calcium Sensing Receptor. Following an increase in intracellular calcium concentration ([Ca2+]i) induced by a calcium mobilizing agonist, some calcium (represented by black circles) is extruded from the cell by the Plasma-Membrane Calcium pump ATPase (PMCA). This results in an increase in local [Ca2+]o and activation of CaSR situated at the surface of the same cell (cell 1) in an autocrine manner and of the neighbouring cell (cell 2) in a paracrine manner. CaSR increases [Ca2+]i, leading to a rise in calcium in the surrounding media and propagation of the signal to other adjacent cells.
It is important to note that in intact tissue the extracellular space volume to cell volume is very small. Since internal stores maintain a total calcium concentration of several mM, substantial changes in the [Ca2+]o can occur in vivo. For example, the extracellular calcium concentration is subjected to notable variation during synaptic activity between nerve cells [40,41] and can reach levels of in the resorption lacunae during bone erosion [42]. Such changes would be detected by the CaSR, activation of which could then lead to the propagation of the extracellular signal to neighbouring cells through the use of the single messenger calcium. Given that the most economic mechanisms are often selected during evolution, it is conceivable that this ‘low-cost’ communication process may be present in tissues subjected to substantial variations in calcium and expressing the CaSR. Obvious candidate tissues include bone [12], the CNS [10,12] and the pancreas [43].
7 Perspectives on the CaSR
The CaSR is expressed in non-parathyroid tissues and responds to a variety of agonists suggesting that this is a multifunctional receptor that may fulfil different roles in different cell types. As for most membrane proteins including GPCRs [44], the mechanisms the determine CaSR trafficking, surface expression and localisation are of crucial importance but remain almost completely unknown. Here, we describe the current state of knowledge and propose some possibilities on future directions to investigate these important and unsolved issues.
7.1 The CaSR and the brain
The neuronal functions of the calcium sensing receptor are still largely elusive [12,45]. Nonetheless, several publications have shown that the CaSR [45,46] is expressed in the mammalian brain [10,12,45]. Significantly, immunocytochemical studies have suggested that the GPCR occur in discrete punctate localizations throughout the brain that appear to be associated with nerve terminals [10]. CaSR mRNA expression rises in hippocampus at a time when Long Term Potentiation (LTP) [47] can first be induced and persists at high levels during the period when brain development is proceeding most rapidly [48]. In LTP, there is a long-lasting increase in synaptic efficiency following appropriate stimulation of the synapse. This phenomenon is believed to be an underlying cellular mechanism for memory formation and consolidation. It is well established that in LTP, as in many forms of synaptic transmission, intracellular calcium signalling is of fundamental importance [47]. Of particular interest in the context of this review, it is now becoming clear that dynamic changes in the levels of extracellular calcium at and around synapses also occur [40,41]. For example, using a novel optical method to measure [Ca2+]out, it has been recently demonstrated that brief trains of synaptic transmission in the hippocampus CA1 region induce transient depletion of extracellular calcium [41]. It is thus tempting to imagine that these changes in [Ca2+]out would be detected by the CaSR that is present at these synapses. Deactivation of the CaSR will lead to inactivation of corresponding intracellular signalling cascades that, in turn, would modulate synaptic responsiveness and plasticity.
This view of a possible role for CaSR in synaptic plasticity is supported by two sets of recently published data. First, a nonselective cation channel can be activated by decreases in extracellular calcium at single neocortical nerve terminals [46]. However, the channel activity is insensitive to the calcimimetic NPS R-467, suggesting that the receptor expressed at these nerve terminals is not the classic parathyroid-type CaSR. Secondly, the CaSR heterodimerizes with another class of GPCR, known to be important for LTP [49], the metabotropic glutamate receptors (mGluR1 and mGluR5) in neurones [50]. CaSR and mGluR1 co-immunoprecipitated from bovine brain and co-localized in the juvenile rat brain (in the cerebellar Purkinje and molecular layers and in the hippocampus in areas CA1, CA3 and dentate gyrus) [50]. Moreover, the heterodimers appeared functional since both receptors internalized upon glutamate stimulation when co-expressed in HEK 293 cells. However, the attractive possibility that CaSRs are [Ca2+]out sensors at synapses and act to modify transmission and/or plasticity remains to be definitively established.
Another intriguing area of brain research in which the CaSR is implicated is phenyloketonurea, a disease associated with toxic amounts in l-Phe. As mentioned, an interesting property of CaSR is that it binds l-amino-acids in addition to calcium [34]. It is well established that in phenylketonuria, phenylalanine induced central nervous system toxicity arises from l-Phe concentrations greater than 0.2 mM in the cerebrospinal fluid. The CaSR is activated at concentrations exceeding 0.1 mM and therefore could play a role in this neurotoxicity. Furthermore, since the CaSR is coupled to the PLC/IP3/Ca2+ pathway, one interesting question is whether or not over-activation of CaSRs by l-Phe may cause damaging increases in [Ca2+]in leading to neuronal death, as observed with toxic doses of, for example, NMDA [2].
The CaSR is expressed in non-neuronal cells in the brain (i.e. oligodendrocytes [51], astrocytes [52], microglia [53]) which suggests that this GPCR could take part to processes associated to these cell types. Glial cells serve many diverse functions including generally sensing and regulating the extracellular ionic and nutrient environment to support neuronal viability and specifically regulating rapid changes at and around synapses. While the precise role(s) and function(s) of glial CaSR remain to be determined, they will certainly be involved in mediating appropriate glial cell responses to changes the local environment. For example, one interesting but speculative possibility is that CaSR agonists are released during brain trauma following cell damage. Consequent CaSR activation could signal astrocytes to form the glial scar.
7.2 Intracellular trafficking of the CaSR
Internalization and trafficking mechanisms are now clearly established as essential regulators of GPCR functions [44]. The CaSR, like many GPCRs, undergoes ligand-induced endocytosis [50]. Despite this early observation, the molecular rules governing its trafficking remain unclear. To begin to shed light on these mechanisms, we have generated a CaSR tagged with the pH-sensitive GFP molecule named Super-Ecliptic pHluorin (SEP). SEP is a GFP variant which is essentially non-fluorescent at pH values of <6.0, and whose brightness increases with pH values of up to 8.5 [54]. As shown in Fig. 4, SEP was inserted in the extracellular N-terminal domain of the CaSR. Since plasma membrane-targeted proteins are delivered to the surface via the secretory pathway in vesicular compartments that have an acidic lumen, SEP-CaSR should exhibit low fluorescence inside the cell. In contrast, surface-expressed SEP-CaSR should be brightly fluorescent as the SEP is exposed to extracellular media at pH 7.4. Using transfected HEK 293 cells, we have verified that the SEP-CaSR is mainly visualized at the cell surface (Fig. 4). In agreement with previous reports using SEP as a tag [55], lowering extracellular pH triggered a decrease in the surface fluorescence whereas incubation with NH4Cl (that increases intracellular pH inside the cell) illuminated the entire receptor population (Fig. 4).
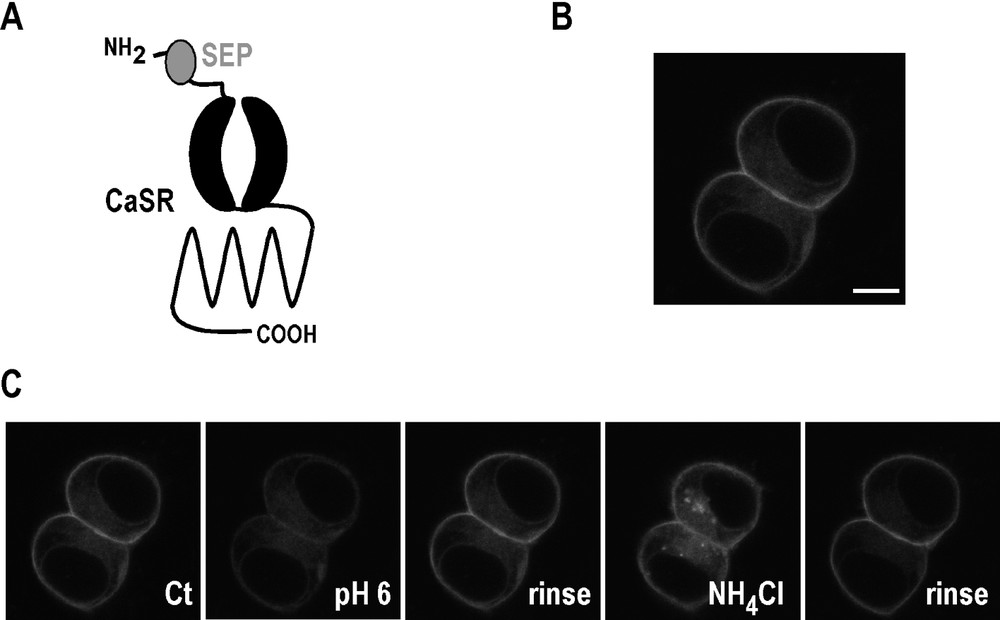
Properties of Super-Ecliptic pHluorin (SEP) tagged CaSR. (A) Schematic representation of Super-Ecliptic pHluorin–CaSR. (B) Confocal image of typical SEP-CaSR fluorescence distribution in two live, cultured HEK cells transfected with SEP-CaSR. Note that fluorescence is mainly present at the cell surface. Scale bar: 10 μm. (C) SEP-CaSR surface expression assessed by monitoring fluorescence intensity in response to pH changes. Cells expressing SEP-CaSR were imaged by live confocal microscopy. Reducing extracellular pH from pH 7.4 (Control, Ct) to pH 6 causes a decrease in fluorescence as surface SEP-CaSR is eclipsed. Surface fluorescence is restored following a rinse with the pH 7.4 control solution. Application of a solution at pH of 7.4 containing NH4Cl (50 mM), which rapidly equilibrates cellular pH levels, causes a sharp increase as all the SEP-CaSR fluorescence in the cell is revealed. The intracellular fluorescence disappears following a rinse with the control solution.
Given that internalized receptors are rapidly targeted from a pH 7.4 at the cell surface to low-pH endosomes, it is possible to monitor endocytosis of a receptor tagged with this pH-sensitive molecule by following decreases in fluorescence in defined membrane regions after agonist addition [54]. Therefore SEP-CaSR will be a useful tool to provide new insights into the molecular mechanisms involved in CaSR trafficking.
8 Concluding remarks
Arising from the discovery of the CaSR, the concept has emerged that calcium is an external ligand in addition to its well documented role as intracellular second messenger [56,57]. So far, the best-characterized role of CaSR is in calcium homeostasis via fine-tuning of PTH release from the parathyroid gland. Mutations or autoantibodies leading to abnormal levels of this receptor activity are linked to human hypo- or hyper-calcemia. However, the finding that the CaSR can propagate signals from cell to cell by calcium waves, the fact that it is expressed in a variety of tissues and that its activity is modified by a wide range of substances strongly suggest that the CaSR has many additional functions and that greater understanding will have far-reaching implications.
Acknowledgements
The authors expressed their gratitude to Drs Stéphane Martin and François Maingret for critical reading of this manuscript. We also thank Drs E. Nemeth (NPS Pharmaceuticals), S. Snyder (John Hopkins, Baltimore), A. Jensen (Copenhagen, Denmark), and A. Spiegel (NIH, Bethesda) for the generous gift of reagents that has allowed us to enter in the Calcium Sensing Receptor research field. We are grateful to the Wellcome Trust and the MRC for financial support. T.B. is supported by a Wellcome Trust fellowship (ITRF).


