1 Introduction
The yeast Candida albicans is responsible for superficial or systemic opportunistic infections named candidosis: the pathogenic form of the fungi appears and provokes disease among weak patients (one of the most common responsible for nosocomial infections) and immuno-compromised subjects. It exhibits a variety of morphological forms ranging from unicellular budding yeast to hyphae [1]. The dimorphism ability to switch between yeast and filamentous forms is often considered to be necessary for virulence to cause medical infections. Nevertheless, formal proof of this relationship between morphological form and virulence remains lacking [2]. It is important to incorporate a more sophisticated understanding of the respective surface properties of different fungal forms to appreciate molecular mechanisms responsible of fungal pathology. As suggested recently by Gow et al. [2], morphogenesis impacts on disease processes should be evaluated at each infection stage, such as, for example, colonisation, microbial adhesion and dissemination, tissue necrosis.
Comprehensive knowledge regarding mechanisms of microorganism adhesion is a prerequisite for the control of such potential colonizer on host surfaces. The elucidation of the delicate balance between the biological and/or physicochemical processes in relation with the initial physical interaction between the microorganism and the host surface is mostly complicated by the variation in surface properties, surface ultrastructures or specific synthesis with environmental changes. In this context, a generalized theory accounting for microbial adhesion to substratum is still lacking. One of the reasons is the complex fungal cell wall structure, as illustrated by the schematic representation in Fig. 1. Basically, the envelope is constituted of different layers mainly composed of polymers mechanically soft, as β-glucan, or mechanically hard, as chitin, but also of numerous specific proteins and glyco(manno)proteins that could exhibit receptor-like behaviours. The most external layer is usually considered with microfibrillar polymers, but could also present exopolymeric substances.
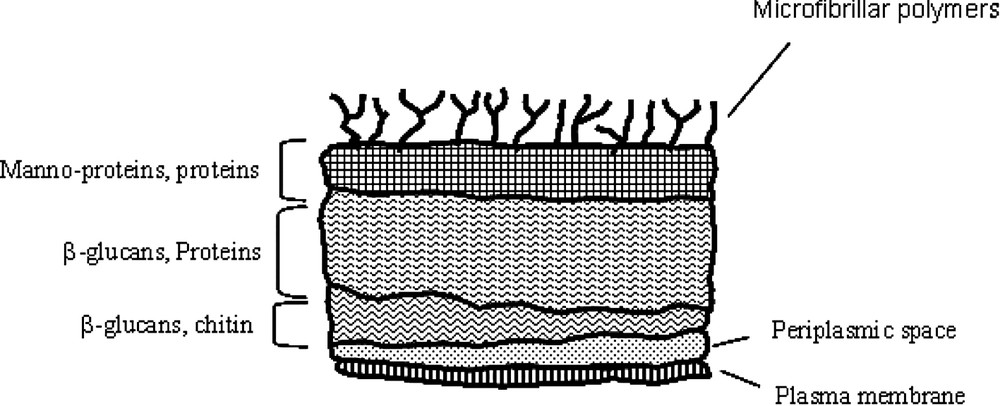
Schematic structure of the cell wall of Candida albicans (adapted from [17]).
Even though, it is widely accepted that the adhesion of microorganisms on host surfaces (colloidal or flat) occurs through an initial step in which the surface properties of both interacting surfaces are the main driving forces that lead to the subsequent attachment [3]. In the case of yeast, several studies mentioned that specific adhesin–ligand interactions strengthen this adhesion by lock-key bindings, but also by increasing the hydrophobic character of cell surfaces [4–7]. Consequently, surface properties of fungal cells play an important role in the pathogenecity of C. albicans.
The aim of the present work was to relate the macroscopic surface properties of C. albicans as derived from adhesion tests on human oral cells and octane droplets, and electrophoretic mobilities to their specific morphological state, i.e. yeast versus filamentous forms. To assist interpretation of the resulting macroscopic data, microscopic AFM force curves were recorded at different locations on the filamentous surfaces of C. albicans cells.
2 Materials and methods
2.1 Yeast strain and growth conditions
C. albicans (strain VW32 serotype A) was isolated from human renal candidiasis (Gift from CHU Lille, France) [8]. The yeast form was obtained after growth in Sabouraud medium (2% glucose and 1% peptone) at 25 °C during 24 h and the filamentous form after growth in medium 199 (Sigma) at 37 °C during 15 h to achieve 109 cells ml−1. After culture, cells were harvested by centrifugation (5 min at ) and washed three times in deionized water.
2.2 Adhesion of Candida albicans to the human epithelial cells
The human oral cells were obtained by light smear of the oral mucous membrane from healthy subject with a sterile spatula, and immediately suspended in sterile phosphate-buffered saline (PBS, 0.01 mol l−1, ). The resulting human epithelial cell suspension (105 cells ml−1) was mixed with washed yeast or filamentous suspension (107 cells ml−1) with equivalent volume ratio (100 μl each) and incubated 1 h at 37 °C. Then, a drop of the suspension (20 μl) was put on a glass slide and 10 μl of Calcofluor White solution (0.1 mg ml−1) was added. To evaluate the influence of hexanedithiol on this previous control sample, 5 μl of hexanedithiol prepared in DMSO solution was subsequently added for a new set of preparation at different hexanedithiol added concentrations (21 μM, 35 μM and 70 μM). Photomicrography was then performed using an MC80 camera mounted on an Axioscope UV microscope (Zeiss, Oberkochen, Germany). In order to evaluate the affinity of yeast or filamentous cells to human cell surfaces, the percentage of human cells covered by at least 15 fungal cells (HCC) was calculated using the formula:
2.3 Microbial adhesion to hydrocarbon droplets (MATH)
The microbial adhesion tests to octane droplets were carried out according to the procedure developed by Rosenberg et al. [9] for the two fungal C. albicans forms. The washed cells were suspended in potassium nitrate solution of different pH (4, 7 and 10) and ionic strength (0.1 mol l−1, 0.001 mol l−1). Next, 500 μl of octane was added to 2 ml of the fungal suspension, and the two-phase system was vortexed for 60 s. The percentage of bound cells to octane droplets was indirectly calculated from the optical density measurements of the aqueous phase at 600 nm using:
These tests have been also carried out for cells submitted before the tests to selective cell-wall protein extractions. Thus, the washed cells were mixed with lyticase enzyme (β-1,3 glucanase, Sigma) solution that lixiviate and release surface cell-wall proteins, as has been previously described [10].
2.4 Micro-electrophoresis
Electrophoretic mobilities were measured at 25 °C with a zetaphoremeter III (CAD Instrumentation, Les Essarts-le-Roi, France), equipped with laser illumination and video interface via CCD camera and image software analysis. Measurements were carried out in a potassium nitrate solution at constant ionic strength, of various pHs ranging from 3 to 10 by adding aliquot of acid or base to the yeast suspension (106 cells ml−1).
2.5 Atomic-force microscopy
A drop of yeast suspension (105 yeasts ml−1) was placed on the makrolon® surface (1 cm × 1 cm, Bayer, Germany) and left for 10 min at ambient temperature. None specifically adsorbed yeasts were removed by rinsing with 100 μl of MilliQ water. Finally, a drop of sodium nitrate solution (, 0.01 M) was placed and the AFM liquid scanner was immersed gently in the aqueous solution. AFM imaging and force distance measurements were made using a commercial microscope (Thermomicroscope Explorer Ecu+, Veeco instrument SAS). The same silicon nitride cantilever (Veeco, Ref MLCT-EXMT-BF) with a calibrated spring constant (0.02 N m−1 [±10%]) was used for all experiments. Force measurements were conduced by positioning the tip through AFM images over individual yeast at the rate of 1 μm s−1. The relative zero position on force curves was arbitrarily positioned at the onset of the linear compliance and the resulting curves were then averaged and standard deviations calculated.
3 Results
The surface properties of fungal cells have been evaluated indirectly through their adhesion to sub-micrometric human epithelial cells. Table 1 presents the percentage of human epithelial cells at least covered by 15 fungal cells. It appears clearly that, while filamentous form covered almost all of human cells, the yeast form presented significant opposite behaviour as the third of human epithelial cells were covered (column ‘Control’ in Table 1). These results are in agreement with previous work that demonstrated the increase of adherence to buccal epithelial cells for filamentous compared to yeast forms [11]. The addition of hexanedithiol greatly impaired the affinity of fungal cells onto human epithelial cells especially for filamentous forms (Table 1). The results demonstrated the effective action of hexanedithiol to decrease the adhesion of filamentous cells by a factor 3. Moreover, the rising of hexanedithiol concentration tends to merge percentage values of the two different forms above 5 μmol l−1 concentration.
Percentage of adherence of the two yeast forms on human oral epithelial cells with and without hexanedithiol
| Hexanedithiol | ||||
| Control | 3 μM | 5 μM | 10 μM | |
| C. albicans (Yeast form) | 33 (±7) | 22 (±6) | 16 (±3) | 10 (±3) |
| C. albicans (Filamentous form) | 95 (±10) | 36 (±5) | 14 (±7) | 6 (±2) |
However, those experiments combine specific and non-specific interactions between the two types of cells, i.e. human and fungal cells, which result of complex biological surfaces of both cells. To simplify such system, C. albicans adhesion assays to octane droplets were carried out for two ionic strengths, at different pH values (Fig. 2, top). In all cases, percentages of filamentous cells trapped by octane droplets were significantly higher than percentages of yeast form. In the case of the yeast form, slight variations of percentage were observed following the pH and ionic strength, which is consistent with electrostatic surface-charge dependence, i.e. the pH increase or ionic strength decrease rise surface charge that leads to affect adhesion. The same trend was observed according to the pH dependence for the filamentous form, with a higher drop from 90 to 60% of cells immobilised by octane droplets. To explore the role of surface proteins and cell-wall structure in fungal adhesion, cell-wall proteins were extracted before carrying out new sets of fungal adhesion assays (Fig. 2, bottom). The percentage ranges were dramatically reduced to non-significant adhesion of cells to octane droplets, i.e. lower than 4%, regardless the fungal forms and the physicochemical conditions (pH and ionic strength). However, these results demonstrated the efficiency of protein extraction and hexanedithiol addition to prevent fungal adhesion on hydrophobic substrates.
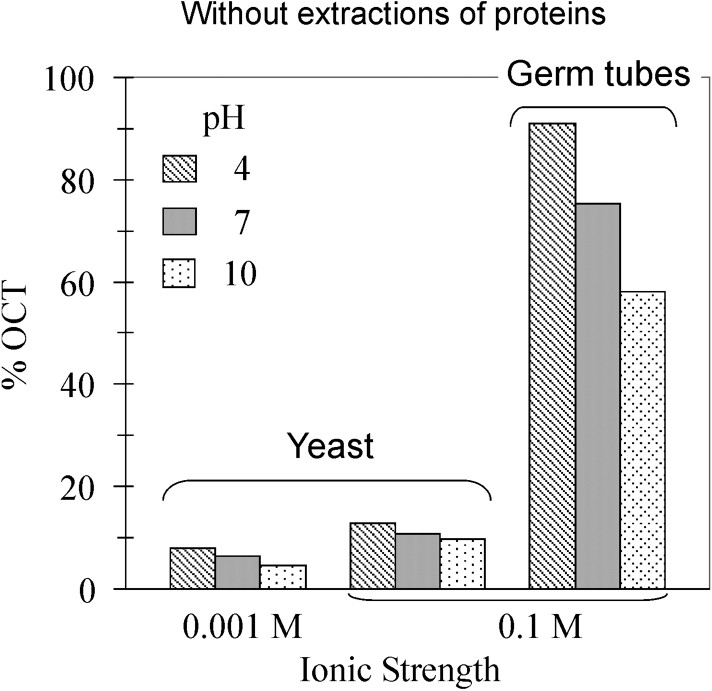
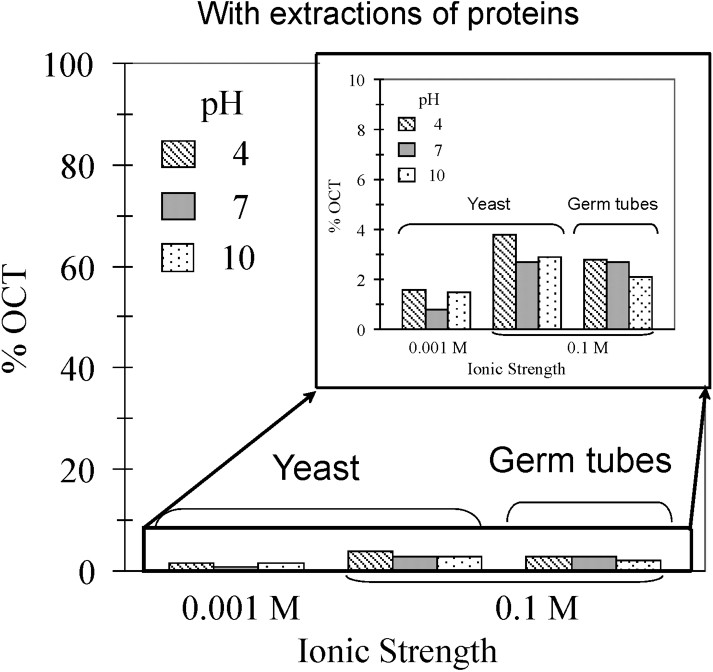
Effect of pH, ionic strength and phenotype (yeast vs germ tubes) on the percentage of cells trapped by the octane droplets. Top: experiments carried out on cells without extractions of proteins. Bottom: Proteins were extracted prior MATH tests.
Such global measurements of the fungal reactivity should be associated with the characterisation as much as possible of the fungal surface properties. First attempt has been done to quantify the electrophoretic mobilities of C. albicans cells with varying pH for the fungal forms. For each morphology and the ionic strength tested, the electrophoretic mobilities versus pH presented negative values on the wide pH range studied (Fig. 3). At high strength (0.1 mol l−1), the two fungal forms gave similar electrophoretic mobilities that tended to an isoelectric point below 2. As in the octane assays, the ionic-strength dependence of the electrophoretic mobilities is consistent with the double-layer theory for the yeast mobility profiles. Therefore, no significant difference in mobility profiles was found between the filamentous and the yeast cells.

Electrophoretic mobility of yeast and germ tubes of C. albicans as a function of pH and ionic strength.
To complement such macroscopic charge measurements, nano-mechanical investigations have been done by atomic-force microscopy experiences. Fig. 4 presents representative AFM images obtained for the mycelial Candida albicans in sodium nitrate solution. Notice that most of the AFM images showed cells preferentially organized as linear structure yielding apparent higher sizes compared to the classical dimension of the filamentous form, close to 10 μm. Even though, cells consistently showed the expected filamentous-shape cells. The morphological features observed alongside yeast cells, especially on deflection images (Fig. 3B), might be due to artefacts that result from the characteristic pyramidal morphology of AFM tip and also to specific electrostatic and mechanical interactions between the tip and the cell surface [12]. However, filamentous cells are sufficiently attached to makrolon© substrate when imaged by AFM in solution to pursue by force measurements. In contrast, the immobilization of the yeast form onto makrolon© substrate was unsuccessfully done.
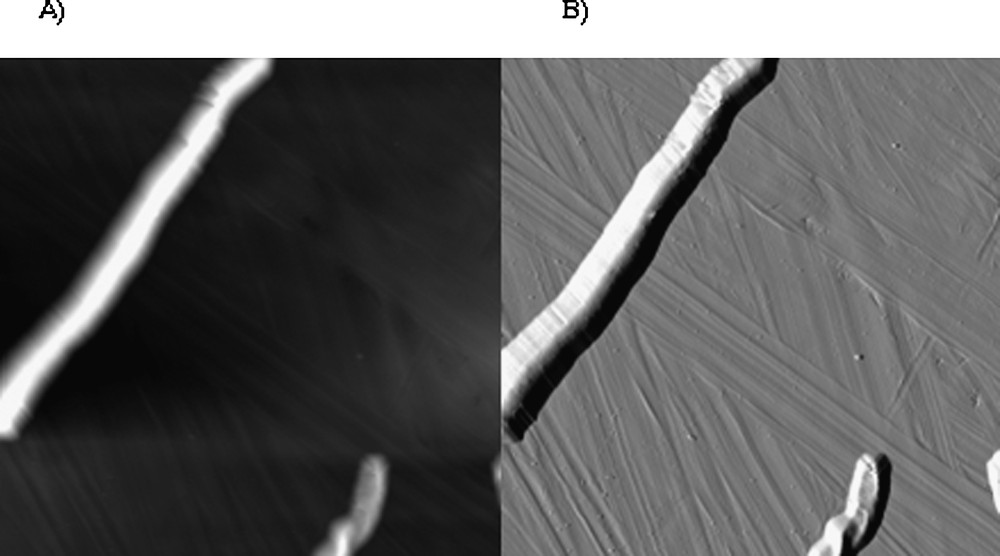
AFM grey-scale height (A; z-range = 1.2 μm) and deflection (B) images (28 μm × 28 μm) recorded for filamentous Candida albicans immersed in aqueous solution (pH 5, NaNO3 0.01 M).
Thus, force curves were recorded at different locations on the top of each filamentous yeast . To assist interpretation of the resulting curves, the data are compared to force curves recorded on the makrolon© and glass surfaces in the same conditions. Approach force curves demonstrated significant different behaviours (Fig. 5). Similar force curves were observed on glass and makrolon© surfaces, showing: (i) the absence of non-linear regimes indicated no long-range surface forces, (ii) wide linear regimes with slope characteristic of non-deformable stiff surfaces. On the other hand, force curves recorded on filamentous cells revealed non-linear regime from 130 to 0 nm piezo displacements and high-loading linear regime with lower slope than previous stiff surfaces. In this case, the relative zero position does not indicate the absolute contact between the AFM tip and the cell, but corresponds to the onset of the linear compliance. In fact, non-linear behaviour could result of electrostatic interactions and/or elastic deformation of the cell. Retraction curves gave additional insights concerning interactions between AFM tip and probed surfaces (Fig. 6). Upon retraction of the tip from yeast surface, multiple adhesion forces were observed with maximal adhesion ranging from 0.4 to 1.4 nN magnitudes. There have been numerous data reported in the literature that demonstrated such typical retraction curves due to the stretching of fibrillar macromolecules (e.g., [13–16]). In contrast, the retraction curves on makrolon© surfaces showed no adhesion or a single adhesion force of less than 0.4-nN magnitude.
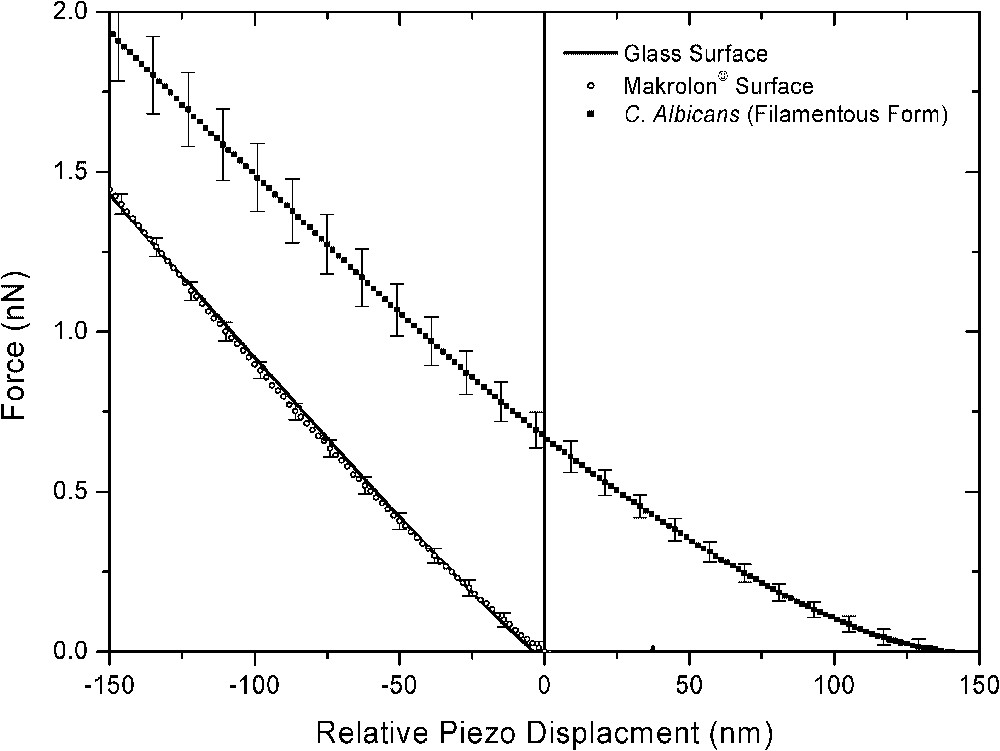
Mean and respective standard deviation of force curves recorded on the top of filamentous C. Albicans and stiff surfaces (makrolon© and glass) immersed in aqueous solution (pH 5, NaNO3 0.01 M).
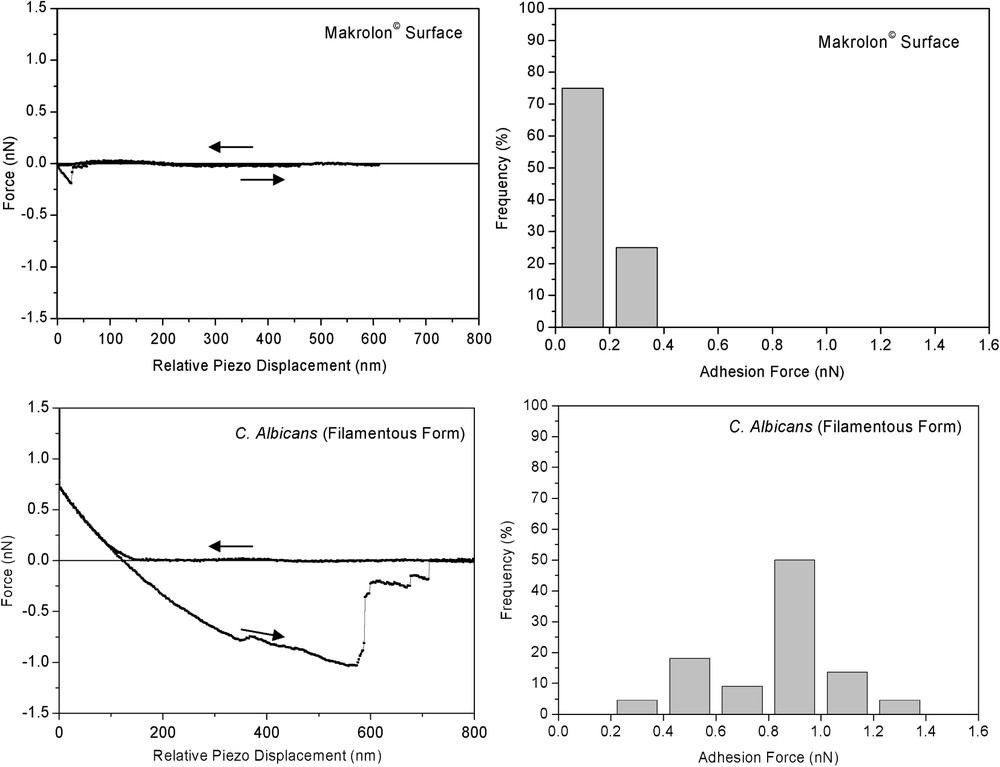
(Left) Representative single approach and retraction curves and (right) maximal adhesion force histogram (n=22) measured on makrolon© and mycelial C. albicans surfaces immersed in aqueous solution (pH 5, NaNO3 0.01 M).
With respect to the cell wall structure of filamentous yeast (Fig. 1), we hypothesised that non-linear regime result of complex interplay of electrosteric interactions due to the deformation of the outermost cell wall layer constituted of microfibrillar polymers of proteins and (manno)proteins. This assumption is consistent with multiple adhesion forces observed upon retraction of the tip (Fig. 6). Further indentation of the tip in this outermost layer yield linear regime, probably associated with compression of β-glycan-protein layers.
4 Discussion
The results presented here show a significant increase in the adhesion of C. albicans to epithelial cells and octane droplets for the filamentous form versus yeast form. In fact, one interesting finding of this study was such similar behaviour in the fungal adhesion to these two different host systems. While the epithelial cells are expected to present some specific protein receptors, octane droplets are supposed to enhance hydrophobic interactions. In any case, this target difference yielded to the same trend regarding the morphological specificity of filamentous form to highly adhere on these two substrates compared to yeast form. As demonstrated by the electrophoretic measurements, this specificity is not due to a difference in the apparent surface charge of cells between the two morphological forms. Notice that the mobilities of the cells is related to the surface charge of the cells but also to the softness of the biological interfaces, which complicate the absolute determination of the surface charge.
Actually, two main assumptions were discussed in the literature regarding Candida species adhesion. One view is that filamentous cell surfaces present specifically some surface proteins such as Hyphal wall protein (Hwp1), whose expression is induced when the differentiation process occurs [7]. This assumption implies lectinic interaction by specific recognition between such protein and the transglutaminase substrate covalently bonded to epithelial cells. Another view considers that specific synthesis of adhesion molecules such as Als1p, Ala1p expected to confer the attractive hydrophobic driving force leading to fungal adhesion [4,5]. To summarize, the driving force that leads to the predominant adhesion of filamentous form compared to yeast one is associated to different surface hydrophobicities, presumably due to specific hydrophobic proteins supposed inside the most external layers of the fungal wall. The results presented here corroborate such interpretations of specific hydrophobic domains for the filamentous forms, yielding to important adhesion on hydrophobic substrates. Moreover, force measurements by AFM performed on the filamentous form showed significant adhesion energy associated with microfibrillar polymeric structures. Therefore, we can hypothesize that such specific surface structure was responsible for the important capacity of filamentous form to stick on hydrophobic substrates.
However, it is important to point out that this morphological specificity is completely annealed by chemical treatment of cells either with hexanedithiol treatment or enzymatic lixiviation. Unfortunately, these treatments could change dramatically the structure of cell wall. Even if the occurrence of the significant decrease of adhesion was demonstrated, the interpretation based on the monitoring of adhesion by protein should be considered with caution.
To summarize, the theoretical aspect and sub-microscopic experimental quantification of cell surface hydrophobicity is still hotly debated. In the present study, it has been shown that the morphological form of Candida albicans plays a considerable role in the adhesion of cells to octane droplets or epithelial human cells. Such morphological specificity is not associated to differences in electrophoretic mobility and seems to be due to stronger hydrophobic domains, mainly supported by microfibrillar surface structures for the filamentous form. Therefore, it appears clearly that this approach should be systematically completed by force measurements for the different forms of yeast, but also after the different chemical or enzymatic treatment, to quantify microscopically the evolution of the surface structure.


