1 Introduction
Urotensin-II (UII), a 12-amino-acid peptide, was initially discovered in fish neurosecretory cells located in the caudal portion of the spinal cord and which project to a neurohemal gland called the urophysis [1]. The presence of UII and of prepro-UII mRNA is not restricted to fish. The cDNAs encoding the UII precursor have been characterized and the corresponding mRNAs localized in mouse, rat and human [2,3]. Furthermore, among others, rat UII peptide was chemically synthesized on the basis of its peptide precursor sequence predicted from that of mRNA. This peptide displayed in vivo a potent vascular action in rats [4]. However, so far, nothing has been reported concerning an endogenous UII peptide isolated from rat. Only in pig, two molecular species of UII have been isolated from the spinal cord [5] and recognized as endogenous ligands of UII-receptor. This receptor was identified in mammals as the orphan receptor GPR14 [5–8]. A recent study reported the identification of a novel peptide considered as the endogenous ligand for UII-receptor [9]. This peptide, designated as UII-related peptide (URP), was isolated from a rat-brain extract. It is 8-amino-acid long and comprises a cyclic hexapeptide sequence, identical to that found in the C-terminal portion of UII, which is responsible for the majority of the UII biological activities [10,11]. On the other hand, both amino acids flanking the cycle are different between URP and UII. The presence of URP-mRNA has been detected in several peripheral or central tissues in rodents and humans, and recently by in situ hybridization (ISH) in motoneurons of the mouse thoracic spinal cord [12].
In our perspective to study possible relationships between two outputs of the central nervous system, the motoneurons on one hand, and the endocrine neurons of the hypothalamus on the other [13], we looked for the presence of this novel peptide in the mouse hypothalamus. First, we observed a URP immunoreactive pattern similar to that of the Gonadotropin-Releasing Hormone (GnRH) within the median eminence. This led us to co-localize URP and GnRH with confocal microscopy at the level of terminals and perikarya. To confirm immunohistochemistry results, we searched for the presence of URP-mRNA by ISH. Positive cell bodies were found mainly in the preoptic area where GnRH cells are localized. These positive results allow us to postulate that URP is a potential neuroendocrine peptide co-expressed and possibly co-released with GnRH.
2 Materials and methods
2.1 Animals
Eight adult male C57 black/6 mice (Janvier, Saint-Berthevin, France), six week-old and weighing 25–35 g, were housed under constant temperature and lighting (light on from 07.00 to 19.00) regimens. They had free access to standard animal food and water. All efforts were made to minimize animal suffering and to reduce the number of animals used. They were anesthetized and killed in accordance with the European Communities Council Directive of 24 November 1996 (86/609/EEC).
2.2 Immunohistochemistry
2.2.1 Immunofluorescence
Mice received an overdose of sodium pentobarbital (Sanofi–Santé animale, Libourne, France) and were perfused through the left ventricle with saline containing 0.1% sodium nitrite, followed by a solution of 4% paraformaldehyde in 0.1-M phosphate buffer (PB), pH 7.4. The brains were immediately removed, post-fixed in the same fixative for 4 h at 4 °C and stored in a 20% sucrose/0.05-M phosphate buffered saline (PBS) solution overnight, before being frozen in liquid nitrogen. Coronal 20-μm slices were cut on a cryostat at , mounted on gelatine-coated slides and used immediately for immunohistochemistry.
Sections were first blocked with 1% bovine serum albumin (BSA) with 0.1% Triton X-100 in 0.05-M PBS for 1 h in a humid chamber at room temperature and incubated with URP-antiserum at a dilution of 1:1000 in the same solution during 48 h at 4 °C. The URP-antiserum was a rabbit polyclonal against mouse URP synthetic peptide complete sequence (lot # 00243, Phoenix Pharmaceuticals Inc., Belmont, CA). Sections were then washed three times for 10 min with the same BSA/Triton/PBS solution and incubated with biotinylated anti-rabbit immunoglobulin (IgG) obtained in goat (1:200 dilution in BSA/Triton/PBS; Vector Laboratories, Burlingame, USA) for 2 h at 20 °C. Following three washes for 10 min in 0.05-M PBS, sections were incubated with Streptavidin-Cy3 conjugate (1:200 dilution in PBS; Sigma, St Louis, MO, USA) for 1 h at 20 °C. Sections were washed four times for 10 min with 0.05-M PBS, mounted in Mowiol (Calbiochem–Novabiochem Corporation, La Jolla, CA) and coverslipped. Some sections were examined under a Leica TCS-SP confocal microscope and photographed. Cy3-labelled cells were identified by their fluorescence emission at 607–659 nm.
Other coronal sections were set aside for double-labelling experiments with URP-antiserum and GnRH-antiserum. The technique was the same as described above with the following modifications: the first incubation was with 1:1000 URP-antiserum and 1:1000 mouse monoclonal GnRH-antiserum (specific for mammalian GnRH C-terminal pentapeptide; Sternberger Monoclonals Incorporated, Lutherville, MD, USA); the revelation used different secondary antibodies, anti-rabbit IgG-Cy3 conjugate made in sheep (1:200 dilution in PBS; Sigma) and anti-mouse IgG-fluorescein isothiocyanate (FITC) conjugate made in horse (1:200 dilution in PBS; Vector). Cy3- and FITC-labelled cells were identified with confocal microscopy by their fluorescence emission, at 607–659 nm and 500–542 nm, respectively.
2.2.2 Peroxidase immunohistochemistry
Brain tissue from two mice was prepared by the same procedure as for immunofluorescence, except that the brains were cut with a vibratome and some coronal sections (40 μm) were transferred into 0.05-M PBS. In two serial consecutive sections, the first one was used for peroxidase immunohistochemistry and the next one for ISH. The technique was the same as described above for the simple URP-immunolabelling, with the following modifications: the last incubation was with peroxidase-labelled ABC complex (1:100 avidin and 1:100 biotin; Vector) and the peroxidase activity was developed by incubating the sections in a DAB-solution (-diaminobenzidine; Sigma) with H2O2. Finally, the sections were mounted in Permount (FisherChemicals, New Jersey, USA) for observation under a light microscope and photographed.
To study the specificity of the immunoreaction, the following controls were performed: (1) substitution of both the URP-antiserum and GnRH-antiserum with 0.05-M PBS, (2) pre-incubation of the URP-antiserum (diluted 1:1000) with mouse URP synthetic peptide (up to ; lot# 420956, Phoenix Pharmaceuticals Inc.), (3) pre-incubation of both the URP-antiserum (diluted 1:1000) with mammalian GnRH peptide (up to ; gift from Dr R. Counis) and GnRH-antiserum (diluted 1:1000) with mouse URP synthetic peptide (up to ).
2.3 In situ hybridization
The technique has been described in detail elsewhere [14]. Briefly, two oligonucleotide probes, complementary to mouse URP-mRNA (GenBank accession number NM_198166), were used in this study for in situ detection: -GCCGTAACCCAGGGAGGAGCAGGTTCACAAAGTGA- and -GGAAGGGCCTCTGTAAACCAGGATTTCGGGTCAAC-. These probes were labelled at the -end with biotin-16-dUTP. Serial vibratome sections adjacent to the immunoperoxidase treated ones were tested for ISH. These sections were incubated for 1 h at 37 °C in a pre-hybridization buffer. Then, they were incubated for 72 h at 37 °C with 2 nmol l−1 of each biotin-labelled URP probe in hybridization buffer. After hybridization, sections were rinsed then immersed for 1 h in 2% BSA with 0.1% Triton X-100 in 0.05-M PBS solution and incubated overnight at 4 °C in the same solution with alkaline-phosphatase conjugated anti-biotin F(ab) fragment (1:500; Roche, Mannheim, Germany). Alkaline-phosphatase activity was developed by incubating the sections during 4 h at room temperature with NBT/BCIP (nitro blue tetrazolium chloride/ 5-bromo-4-chloro-3-indolyl phosphate, toluidine salt; Roche). Sections were mounted on gelatine-coated slides in Mowiol, coverslipped for observation under a light microscope and camera digitalized. Control experiments were performed by omitting probes.
3 Results
3.1 Immunohistochemistry
URP-immunoreactive axon terminals were principally located in the organum vasculosum laminae terminalis (OVLT) and in the median eminence (ME). A dense plexus of URP-positive fibres was present in the OVLT close to the capillary network. URP-immunoreactive axon terminals were found in the external layer of the ME rostral part (Fig. 1A). The ventral surface of the floor of the third ventricle and the ventricular walls also displayed URP-positive varicose fibres, right under the ventral surface of the brain and along the lateral infundibulum. More caudally, fibres were confined to lateral edges (Fig. 1B–D), with only few fibres in the medial area of ME. At retroinfundibular level, fibres were less abundant and occurred in the proximal part of the infundibular stalk, all around the structure, in the palisade layer (Fig. 1E).

Coronal sections of mouse brain at the level of hypothalamus, including the median eminence (A–D) up to the infundibular stalk of the pituitary gland (E), and medial preoptic nucleus (F) after treatment with anti-URP antibodies revealed by immunofluorescence. Labelled fibres of variable intensity occur at all levels of ME, especially in the outer palisade layer. Confocal microscopy shows conspicuous labelling of a fusiform cell body with a long positive process. Bars represent 100 μm (A–E) and 10 μm (F). Asterisk: third ventricle.
Most of URP-immunoreactive cell bodies were located rostrally to the anterior hypothalamus. They were dispersed in the medial septum nucleus, in the nucleus of the diagonal band of Broca and in the medial preoptic nucleus. Frequently, they were found in close apposition to blood vessels. The labelled cells were generally small (10 μm) and bipolar or unipolar fusiform neurons (Fig. 1F). Peroxidase immunohistochemistry confirmed the results of the fluorescence immunohistochemical studies by showing the same population of labelled neurons with respect to their number and localization.
Similarly, GnRH-immunoreactivity was detected in the mouse brain in the OVLT and ME (Fig. 2A) as fibres and in the preoptic area as cell bodies located in the medial septum nucleus, in the nucleus of the diagonal band of Broca (Fig. 2B) and in the medial preoptic nucleus. Double-labelling studies revealed that nearly all URP-immunoreactive fibres (Fig. 2C) and cell bodies (Fig. 2D) were positive for GnRH (Figs. 2E and 2F), whereas numerous GnRH positive cell bodies and fibres were not URP-immunoreactive.
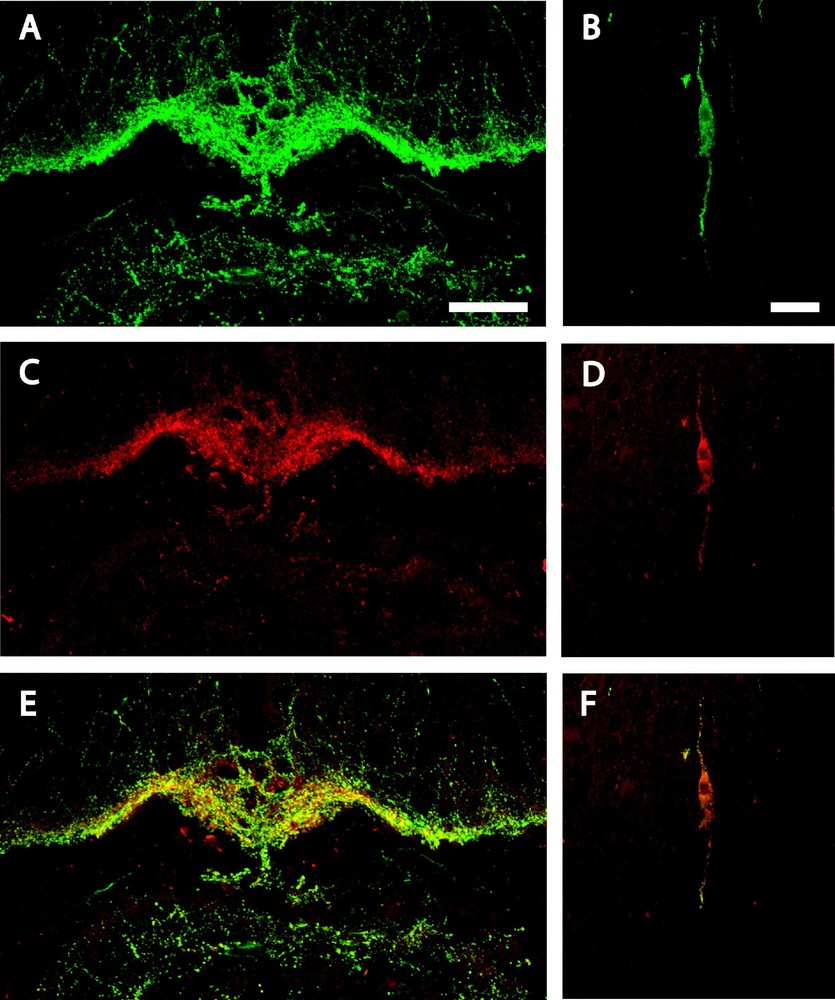
Confocal laser microscopy of a coronal section of mouse brain double-immunolabelled for GnRH and URP, at the retro-infundibular level (A, C & E) and in the nucleus of the diagonal band of Broca (B, D & F). GnRH-immunoreactive fibres (A) and cell body (B) are green while URP immunopositive fibres (C) and cell body (D) are red. Double-labelled fibres (E) and cell body (F) appear as yellow/orange on the merged projection of twenty successive 1 μm-thick optical sections. Bars represent 50 μm (A, C & E) and 20 μm (B, D & F).
When the primary antiserum was omitted, no immunofluorescence was observed. Preincubation of the URP-antiserum with mouse URP synthetic peptide resulted in a complete loss of the immunoreactions. In contrast, preincubation both of the URP-antiserum with mammalian GnRH peptide or GnRH-antiserum with mouse URP synthetic peptide did not reduce the intensity of the immunostaining.
3.2 In situ hybridization
In serial sections consecutive to immunoperoxidase labelled ones, ISH with probes for URP-mRNA showed a varying labelling of a few cell bodies in the medial preoptic nucleus (Fig. 3A), in the nucleus of the diagonal band of Broca (Fig. 3B) and in the medial septum nucleus. The positive cell bodies all displayed the same localization and neuronal characteristics as described in URP-immunoperoxidase. No signal was observed on control slides hybridized without probe.
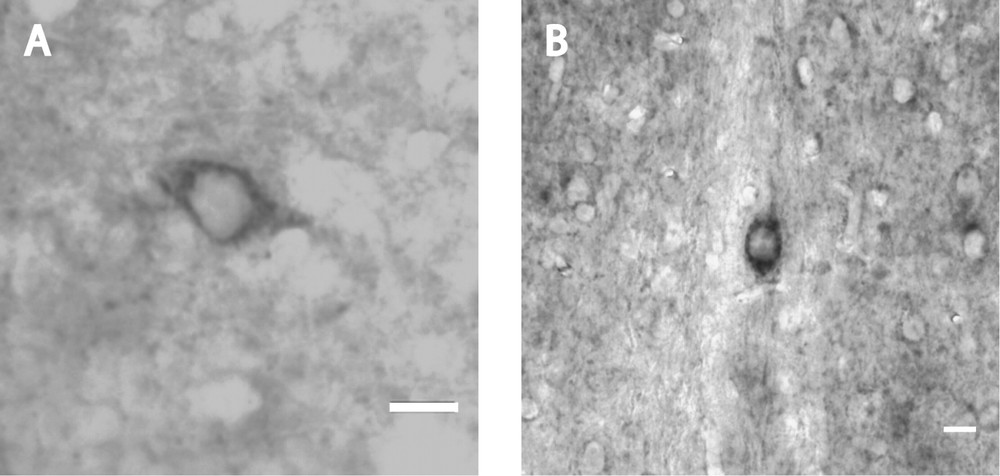
Coronal section of mouse brain after in situ hybridization of mouse URP–mRNA with biotin-labelled probes. Intensely reactive neuronal cell bodies with short labelled process occur in the medial preoptic nucleus (A) and in the nucleus of the diagonal band of Broca (B). Bars represent 10 μm.
4 Discussion
The pattern of immunoreactivity observed after GnRH antibodies closely corresponds to that previously described in mouse and rat for the GnRH system, with cell bodies dispersed in the preoptic area and high concentrations of fibres and nerve terminals in the neurohemal areas of OVLT and ME, where the peptide is released to control gonadotropic cells of the anterior pituitary [15,16].
The localization of URP-immunoreactivity is being described here for the first time in the hypothalamus. To make sure that the immunocytoreaction did correspond to peptide detection, several controls have been performed. First arguments in favour of the specificity of the reactions lie in the classical absorption controls, which show (1) extinction of the immunostaining after absorption with the homologous peptide, (2) absence of cross-reaction of the URP-antiserum with GnRH, and of the GnRH-antiserum with URP. Another important point is that, despite similarity of URP and UII structures, no immunoreactivity was observed for UII in the hypothalamus (Egginger, unpublished), whereas, in mouse spinal motoneurons, UII- and URP-mRNAs and peptides were found together [12, and in preparation]. But the essential argument for the presence of URP in the areas considered relies on ISH results. The specificity of each probe for the corresponding mouse URP-mRNA was confirmed by checking the DNA Database of Japan. For each probe used, the scores were 100% identity with Mus musculus URP-mRNA and Mus musculus UIIB-precursor mRNA complete sequence, UIIB being an another name for URP in mouse. The similar localization, on consecutive sections, of URP-immunoreactivity and URP-mRNA indicates that transcription and translation of the mRNA certainly occur in the same cells and that URP is expressed in these neurons. Furthermore, the varying labelling intensities both for URP-peptide and its mRNA could be correlated: the URP-mRNA concentration has been recently shown to be regulated by androgens in the motoneurons of the mouse thoracic spinal cord [12].
The similarities of immunoreactive patterns between URP and GnRH led us to perform immunofluorescence labelling for both peptides. Practically all brain URP-immunoreactive cell bodies and fibres did contain also GnRH. Thus it seems possible to assume an intraneuronal co-localization of both peptides. Other peptides have been already shown to coexist with GnRH like cholecystokinin and neurotensin [17].
Furthermore, the localization of URP in fibres of neurohemal organs at the vicinity of vessels suggest its possible release in the ME portal vascularization towards the anterior pituitary. Preliminary studies, performed in rat ME by immuno-electron microscopy, seem to confirm these possible neuroendocrine properties of URP.
5 Conclusion
Our results allow us to postulate that URP is a potential novel hypothalamic neuroendocrine peptide in mammals that is co-expressed in GnRH neurons. Possible interactions between these two peptides in regulation of gonadotropic function are currently under study. Furthermore, the presence of URP has been detected by immunohistochemistry in motoneurons at all levels of the mouse spinal cord (Egginger, unpublished), and its mRNA has been recently detected by ISH in motoneurons of the mouse thoracic spinal cord [12]. Taken together, these results support the assumption that URP, here characterized as a neuroendocrine peptide in the mouse hypothalamus, is also expressed within motoneurons and strengthen the concept of the existence of common properties between these two outputs of the central nervous system.
Acknowledgements
We are grateful to Dr P. Ciofi for his precious help and advice. We wish to thank R. Schwartzmann for his help in confocal microscopy. This work was supported by the Fondation Singer–Polignac (J.-G. Egginger).


