1 Introduction
In mammals, leptin is a 16-kDa protein hormone, synthesised primarily by adipose tissues as a product of the obese gene [1]. It is also known as the obesity hormone, but this definition is too restrictive. Mammalian leptin is important for the regulation of food intake, controlling the amount of fat stores by inhibiting appetite [2–4]. This hormone also has a pleiotropic action in development and physiology. Some of the major actions of leptin include the promotion of linear growth through its influence on energy balance and the stimulation of secretion of the pituitary hormone [5–7].
Leptin acts via a feedback mechanism that operates at the level of the hypothalamus, where it binds to an OB-R receptor [1], but it may act peripherally as well for the presence of leptin receptors outside the central nervous system [8–10]. Mammalian leptin also plays an important role in promoting reproduction. Since a link exists between reproduction and energy balance, leptin could also indirectly affect reproduction, acting on peripheral metabolism [11]. The first indication of this role was the finding that female ob/ob mice are infertile [12] and that fertility can be rescued by leptin treatment [13]. In mammals, leptin controls gonadotropin secretion through stimulatory hypothalamic and pituitary actions [14,15]. The importance of leptin in regulating secretion from GnRH neurons in the hypothalamus is well established [16–18]. While vertebrate leptins show large divergence in their primary amino acid sequence, they form similar tertiary structures, and may have similar potencies when tested in vitro on heterologous leptin receptors (LepR) [19]. In non-mammalian vertebrates, leptin has been found in all major taxa with a wide array of functions [20,25–28]. However, the information on the role of leptin in ectotherms is scant and further investigations would be required to clarify the complex role of this hormone.
Several studies have been performed on fish, in which leptin appears to stimulate the reproductive axis [21–23]. According to a recent study, the interaction between leptin and the leptin receptor is conserved in terrestrial vertebrates, but with an increased variation in fish [24]. In reptiles, high levels of leptin have been evidenced in the brain of the lizard Sceloporus undulatus [25]. Leptin and a leptin receptor have been found in the stomach of a lizard and a snake [26], as well as in the thyroid gland and in the endocrine pancreas of a lizard [27,28], showing that it is involved in glucose metabolism as in mammals [28]. In Podarcis siculus, a lizard adapted to a temperate climate, leptin levels have been monitored in the plasma, liver and fat bodies throughout the year. During the period of maximum activity, the mass of fat bodies decreases, concomitantly with a decrease in leptin in both plasma and fat bodies. These data indicate that fat body depletion during the period of activity is due to its use in support of reproduction [29].
Given the above considerations, in this study we focused on the effects of leptin on FSH cells in the pituitary gland of P. siculus. The study was performed by different intraperitoneal injections of human recombinant leptin on female lizards in the month of November when they are in a quiescent reproductive period and their leptin levels in plasma are higher [29]. The hormone was injected at four different increasing concentrations based on the physiological levels of leptin in lizard plasma that are about 2.2 ng/mL in November [29]. Although in P. siculus two distinct cellular types of gonadotrope cells were revealed, the immunohistochemical studies were performed on FSH cells only because these have been reported elsewhere as the most expressed one [30–33].
2 Material and methods
2.1 Animals
The study was performed on five groups of adult females of P. siculus captured near Naples (Italy) and kept under controlled conditions of light and temperature. In each group, there were four specimens, making an overall total of twenty. Experiments were performed in accordance with the Guidelines for Animal Experimentation of the Italian Department of Health under the supervision of a veterinarian, and organised to minimise stress and the number of lizards used.
2.2 Experimental design
The treatment was performed in November, the period of reproductive quiescence, by intraperitoneal injection of recombinant human leptin (Sigma, USA) in 0.1 mL of saline solution for three consecutive days at four different concentrations (group A: 0.025 ug/0.1 mL; group B: 0.05 ug/0.1 mL; group C: 0.075 ug/0.1 mL and group D: 0.1 ug/0.1 mL). The concentrations were 10 to 50 times higher than the physiological levels of leptin in lizards in the quiescent period [29], and were chosen in accordance with previous reports [34]. As a control, one group of lizards was treated with an injection of 0.1 mL of physiological solution. No mortality or altered animal behaviour was recorded during the experiments. The treatments were made all at the same hour and the animals were sacrificed 2 h after the last injection.
2.3 Tissue sampling
All animals were killed under anaesthesia by a cervical cut. For the reasons reported in a previous work of ours [35], the gland was studied in toto within the brain: after removal of the skullcap, the brains were fixed in Bouin's solution for 48 hours at room temperature and then decalcified in a solution of 5% EDTA in 10% formalin for 25–30 days, dehydrated, and enclosed in paraffin.
2.4 Immunohistochemistry
Sections of 5 μm of the pituitary glands were processed by immunohistochemical staining. For the immunohistochemical stain, the cells were revealed by the avidin–biotin–peroxidase complex technique using the antisera anti-human FSH (Signet Laboratories, MA) at a working dilution of 1:100 at 4 °C overnight. Visualization was carried out using the Vectastain Elite ABC kit (Vector Labs, Inc., Burlingame, CA, USA) and revealed by 3 mg of 3.3′-diaminobenzidine-tetrahydrochloride (Sigma, St. Louis, MO, USA) in 10 mL of a phosphate buffered saline (PBS) solution and 150 μL of 3% H2O2. The sections were then contrasted with haemalum for 1 minute. Antibody specificity was assessed by omitting the primary antiserum and absorbing it with the specific hormone. The images were examined and acquired by a Kontron Electronic Imaging System KS300 (Zeiss, Oberkochen, Germany). Quantification of the mean percentage number of FSH cells was carried out on at least 300 cells, with a visible nucleus, on serial sections for each animal and relative to the caudal region of the adenohypophysis in which these cells were mainly expressed. Data were expressed as the number of immunostained cells × 100/number of total cells. Statistical analysis was carried out by ANOVA test with a level of significance set to P = 0.01. Computations were made by the GraphPad Prism 6 package (GraphPad Software, San Diego, California).
3 Results
For the general morphology of the P. siculus pituitary gland, see our previous works [32,33,35]. In control lizards, immunostained FSH cells were mainly revealed in the caudal region (Fig. 1a) of the pituitary gland with a mean value of 43(± 3.05). They showed a variable shape and were organised into medium size cellular cordons, larger in the caudal area than in the other regions of the adenohypophysis (Fig. 1b and c). Rare immunopositive cells were visible in the rostral region (Fig. 1a). In these lizards, FSH cells showed a cytoplasm with well-immunostained secretory granules (Figs. 1b and c; Fig. 2a). In group A of lizards treated with 0.025 ug/0.1 mL, the mean was 41(± 2.14), which did not differ significantly from the control (Fig. 2b; Fig. 3). Indeed, in the lizards of groups B and C, treated respectively with 0.05 ug/0.1 mL and 0.075 ug/0.1 mL hormone, FSH immunoreactivity was weaker and the number of immunopositive cells decreased (Figs. 2c and d; Fig. 3). This significant decrease was higher in the lizard of group C in which few cells were weakly immunostained (Fig. 2d). Mean values of FSH cells were 36(± 3.79) for group B and 28(± 2.23) for group C. In group D, in which 0.1 ug/0.1 mL of leptin was injected, we revealed a considerable increase with respect to groups B and C, in their occurrence with a mean value of 46(± 1.40) and also a major intensity of immunostaining (Fig. 2e). These cells appeared full of strongly stained secretory granules such that they appeared even larger in size, evidencing the inhibition of the secretion. FSH cells in group D were also higher in number and more strongly immunostained in the rostral region (Fig. 2f), where they were otherwise rare. The histogram of Fig. 3 reports the relative frequencies of the FSH cells in the different groups of lizards and resumes the results of the statistical analysis.
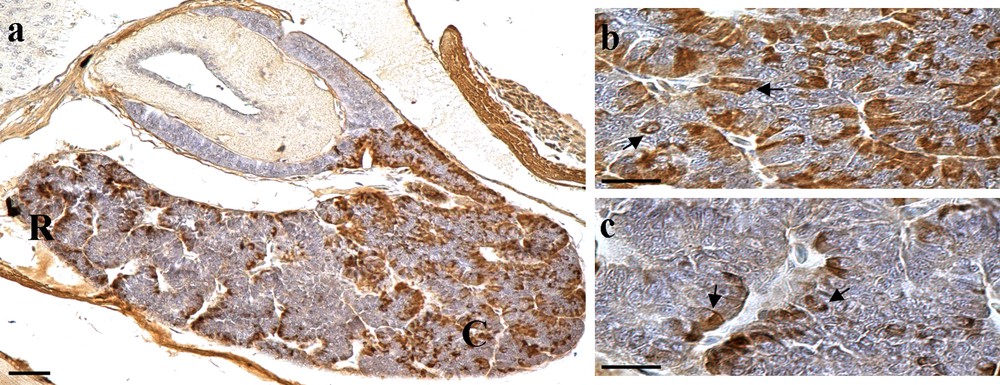
(Colour online.) a: sagittal section of the pituitary gland of the control lizard immunostained for FSH cells. Note the extension of the gland along the rostral–caudal direction and the distribution of FSH cells (in brown) in the adenohypophysis. FSH cells are more abundant in the caudal region (C) than in the rostral region (R); b: detail of the caudal region on Fig. 1a shows FSH cells (arrows) with a variable shape and organised into cellular cordons of medium size; c: detail of the rostral region of Fig. 1a, to be compared with (b) to see the differences of the immunostained FSH cells (arrows) in the two adenohypophyseal areas. In the rostral region there are clearly fewer FSH cells than those revealed in the caudal region. Scale bar: a: 50 μm; b and c: 20 μm. Masquer
(Colour online.) a: sagittal section of the pituitary gland of the control lizard immunostained for FSH cells. Note the extension of the gland along the rostral–caudal direction and the distribution of FSH cells (in brown) in the adenohypophysis. FSH cells ... Lire la suite

(Colour online.) a–f: details of the caudal region in lizard pituitary glands and FSH immunostaining (in brown) for: a: the control group; b: group A treated with 0.025 μg of leptin; c: group B treated with 0.05 μg of leptin; d: group C treated with 0.075 μg of leptin; e and f: group D treated with 0.1 μg of leptin, to show the significant differences. Note the decrease in FSH cells in (c) and especially in (d), the group of lizards treated with 0.075 μg of leptin in which the cells are rare and also less immunostained than those in the control lizard in (a). In (e), we can instead observe FSH cells of the group treated with 0.1 μg of leptin, full of secretory granules strongly immunostained (in dark brown) appearing even larger in size. In (f), FSH cells immunostained in the rostral region of lizard pituitary glands treated with 0.1 μg of leptin: note the increase in these cells also in this area. Scale bar: 20 μm. Masquer
(Colour online.) a–f: details of the caudal region in lizard pituitary glands and FSH immunostaining (in brown) for: a: the control group; b: group A treated with 0.025 μg of leptin; c: group B treated with 0.05 μg of leptin; d: group ... Lire la suite

FSH cells of different lizard groups expressed as mean ± SD; **P < 0.01.
4 Discussion
In this immunohistochemical study, we analysed the effects of the intraperitoneal injections of human recombinant leptin on FSH cells in females of P. siculus. The study was performed with four different, increasingly higher, concentrations of hormone than the normal plasmatic levels of this lizard. Our findings show that leptin acts on release of FSH in female lizards and these effects appear to be dose-dependent, in accordance with the evidence on adult and juvenile rats [14,36].
In mammals, the hypothalamus is the principal site of action for leptin. Leptin receptors have been identified in the different sites of the brain, but also in a variety of peripheral tissues such as kidney and lung but also pancreas, liver, adipose tissue and gonads, and it is an important protein with an endocrine and metabolic role [10]. In the few non-mammalian species studied, LepR mRNA is as highly expressed in the brain as it is in mammals [19,22]. As in mammals, LepR mRNA is found in many different tissues in ectotherms, indicating that the hormone has the potential to have diverse influences on development, signalling appropriate timing for developmental processes such as metamorphosis and the onset of reproductive maturity, and on physiology [19]. Despite the large divergence in their primary amino acid sequence observed especially in fish, in vertebrates structural analyses of leptin have suggested high functional homology and comparative studies are therefore important to gain insights into the action of this hormone.
Daily injections of recombinant murine leptin in fence lizards, S. undulates, produced phenotypic effects similar to those observed when leptin injections are given to mice [25]. A direct and in vitro action of human leptin on FSH and LH release has been reported in rainbow trout at different stages of its sexual cycle [37]. No clear physiological effect of human leptin has indeed been revealed in the immature coho salmon [38]. However, in male lizards, a regulatory role of leptin in reproduction has been shown by the effects on testis and epididymis during summer regression [34]. Leptin is evidently involved in the reproduction of a reptile also, the lizard P. siculus, but few works are available on the possible neuroendocrine role of leptin in these animals. In this work, we have demonstrated that leptin intakes the hormonal release of P. siculus FSH cells, the same involved in mammalian reproduction. The major input in performing this study is that in female lizards the levels of leptin in plasma, liver and fat bodies had just been determined, showing their fluctuations during the reproductive cycle [29], indicating a possible activity of this hormone in these animals. P. siculus is an oviparous reptile in which the leptin level in plasma is high in the quiescent period, when it is in a semi-hibernation state, and decreases during pre-vitellogenesis to the lowest levels. Similarly, the amount of lipid stored in the fat bodies increases during the winter months and undergoes depletion at the beginning of spring when sexual activity resumes. This could be attributed directly to gonadal action, through the hypothalamus–pituitary axis or both [29]. For this reason, we performed our study in female lizards in the month of November when they are in a quiescent period with high plasmatic leptin levels, by injecting the protein at four different concentrations from 10 to 50 times higher than the physiological values. The effects on the activity of FSH cells appear dose-dependent, with a significant activation of secretion of FSH cells in groups B and C. However, inhibition of this release in groups exposed to maximum concentration is indicative of control by a probable negative feedback that should be further investigated. It is also possible, as suggested by Paolucci et al. [29], to hypothesise that this hormone has an indirect action on vitellogenin. In this oviparous species, in which liver is the source of vitellogenin, the authors report that leptin is undectable in the liver of females during the quiescent period, but that it increases during the previtellogenic period and might be related to vitellogenin synthesis [29]. However, no leptin was found in the liver of the lizard S. undulatus [25], though in this species higher leptin titres were observed in a reproductive population [39]. Therefore, our data indicate a probable neuroendocrine role of leptin also in these oviparous lizards, which have a seasonal reproductive cycle. In this vertebrate, a functional receptor is evidently expressed, able to respond to a leptin-like ligand, suggesting the importance of investigate this protein also in ectotherms.


